Quantification of Physical Activities Simulated Exercise Therapy in Ambulatory Inpatients Using Surface Electromyogram from the Vastus Medialis
Tomoaki Tsuji1,2, Chiaki Wada1,3, Makoto Kawanishi2, Yasuhisa Fujita1,2, Yoshi-ichiro Kamijo1,4, Yasunori Umemoto1, Ken Kouda1, Kazunari Nishiyama1,5, Fumihiro Tajima1, Yukihide Nishimura1,5*
1Department of Rehabilitation Medicine, Wakayama Medical University, Wakayama, Japan
2Division of Rehabilitation, Wakayama Medical University Hospital, Wakayama, Japan
3Department of Rehabilitation, Labour Health and Welfare Organization Wakayama Rosai Hospital, Kinomoto, Wakayama, Japan
4Department of Rehabilitation Medicine, Dokkyo Medical University Saitama Medical Center, Koshigaya, Japan
5Department of Rehabilitation Medicine, Iwate Medical University, Yahaba-cho, Iwate, Japan
*Corresponding Author: Yukihide Nishimura, Department of Rehabilitation Medicine, Wakayama Medical University, Wakayama, 641-8510, Japan
Received: 08 January 2023; Accepted: 16 January 2023; Published: 06 March 2023
Article Information
Citation:
Tomoaki Tsuji, Chiaki Wada, Makoto Kawanishi, Yasuhisa Fujita, Yoshi-ichiro Kamijo, Yasunori Umemoto, Ken Kouda, Kazunari Nishiyama, Fumihiro Tajima, Yukihide Nishimura. Quantification of Physical Activities Simulated Exercise Therapy in Ambulatory Inpatients Using Surface Electromyogram from the Vastus Medialis. Journal of Orthopedics and Sports Medicine. 5 (2023): 87-97.
View / Download Pdf Share at FacebookAbstract
The present study aimed to assess whether surface electromyogram (sEMG) signal from the vastus medialis could be a candidate method to quantify physical activities during combined activities in ambulatory persons (ergometer exercise, treadmill walking, and squatting). In the first trial, eleven healthy men performed a graded cycle ergometer exercise at 0%, 30%, 60%, and 80% of peak oxygen consumption rate (VO2peak), followed by treadmill walking at 0, 2, 4, and 6 km/h for 3 min of each, and each exercise was intermitted by 3 min of rest. sEMG from the Vastus Medialis Oblique Longus (VML) was collected, and the integrated amplitude of spikes (sEMGAMP) were calculated every minute. Positive correlations were observed between ΔVO2 and ΣsEMGAMP; data at sampling frequency of 250Hz in both exercise types were plotted (r=0.888; P<0.0001; y=339.04x+4.0267). In the second trial, thirteen healthy participants (three women) performed the combined exercise comprising 3 min each for optimal walking (3 km/h), fast walking (5 km/h and 6 km/h for women and men, respectively), squatting, second optimal walking, and ergometer exercise at 30% VO2peak, which were intermitted by 30 sec. Finally, they performed ergometer exercise at 100% VO2peak for 1 min followed by 3-min cool-down (0W). Changes (Δ) in VO2 from the resting value and sEMGAMP during exercise were summed throughout the exercise period (ΣΔVO2 and ΣsEMGAMP). ΣΔsEMGAMP was positively correlated with ΣΔVO2 (r=0.68, p=0.011, @250Hz). Monitoring sEMG from VML may be a candidate method for the evaluation of physical activities for exercise therapy in ambulatory persons.
Keywords
<p>Rehabilitation; Activities of daily living; Population health; Functional performance; Muscle fatigue</p>
Article Details
1. Introduction
In older adults, lower-limb muscle strength should be maintained by increasing physical activity to improve the quality of life. About one-half of Japanese people suffer from malignant neoplasm, and the number of patients who intend to return to their work after curative treatment is increasing. Furthermore, maintenance of skeletal muscle mass is now considered an essential strategy for successful chemotherapy [1]. In patients with cancer, improvement in cardiopulmonary function, such as physical endurance, before elective surgeries reduces the risk of postoperative complications, which results in shorter hospitalization [2]. To perform effective rehabilitation therapies, an evaluation of physical activities during exercise therapy needs to be considered for inpatients to encourage more activities.
Several methodologies can be used to quantify and assess physical activity in ambulatory persons, such as pedometers, triaxial accelerometers, monitoring heart rate, and questionnaires. Recently, wrist-worn smart devices and wearables have shown clinical utility [3]. However, the detection of physical activities remains a problem. A pedometer cannot distinguish between walking on level ground and climbing stairs, and between slow and fast walking [4]. A triaxial accelerometer cannot detect activities if there is no shift in the center of gravity and a change in posture, for example, a bicycle ergometer [5]. Moreover, squatting is also involved in the strengthening of muscles, which would be difficult to estimate using accelerometers [6]. The activPAL monitor, a thigh-worn device, can determine the start and end of each period spent in different activities, including sedentary behavior, such as sitting/lying, standing, and stepping, using accelerometer-derived information about thigh position [7]; however, users need over a week or more to achieve an acceptable repeatability of measuring sitting time and moderate-to-vigorous physical activity. In addition, monitoring heart rate for physical activity assessment has limitations as it can fluctuate depending on mental [8] and hydration states [9]. A short form of the International Physical Activity Questionnaire (IPAQ-SF), which was converted to metabolic equivalent minutes per week (MET-min/week) using the published formulation, is available for clinical study, but memory bias can occur [10]. The above methodologies have some limitations, which encompass lack of precise estimation of physical activities during exercise therapy, such as cycling, walking, and squatting.
Surface Electromyography (sEMG) can noninvasively detect skeletal muscle activity during contraction [11] and has been used to monitor and/or estimate muscle contraction during various human movements, including walking [12]. Electrical signals obtained by sEMG increase depending on the contraction intensity [13]. The signals in lower-limb ergometer exercise (the rectus femoris, vastus medialis, vastus lateralis, and biceps femoris) increased linearly with exercise intensity up to the maximum load [14]. Minoshima et al. [15] demonstrated that the fatigue characteristics of the vastus medialis, which stabilizes the knee joint when performing lower-limb exercise, during static contraction using a leg-press machine were highly reproducible within the same day and between days in healthy participants. If the sEMG signal in the agonist muscle and VO2 showed a positive correlation regardless of the exercise mode, the signal from the agonist muscle could be one of the methods for quantifying physical activity in ambulatory persons. In this case, even the signal from other sites, e.g., upper limbs, would need to be ignored during lower-limb exercise. Although this method would not estimate precise energy expenditure, it could quantify physical activity more precisely.
There are two methods to evaluate the sEMG signal, the Root Mean Square (RMS) and amplitude integration of firing signals. Since the summation of RMS can increase with time independent of muscle contraction and relaxation, the amplitude integrated value is suggested to better reflect the muscle contraction activity [16]. Moreover, because the number of motor units in the active muscle increases due to muscle fatigue [17], the rise in the amplitude of sEMG during contraction could be enhanced at a higher intensity, especially over 80% of maximal workload [18]. The sEMG signal could be altered after heavy exercise because of fatigue [17], suggesting that sEMG signal may include fatigue components during the combined exercise. However, no study has tested the analysis method that would better estimate the physical activity during the combined exercise mentioned above.
The purpose of this study was to assess whether integrations of sEMG from the vastus medialis could be a candidate method to quantify physical activity in ambulatory persons during a combined exercise comprising a lower-limb ergometer, treadmill walking, and squatting (as a simulation of rehabilitation therapy for cancer patients before surgery). In the first trial, participants performed a graded cycle ergometer exercise and treadmill walking while measuring sEMG signals from the vastus medialis and VO2. We examined whether sEMG and VO2 would show a positive correlation, regardless of exercise modalities. In the second trial, participants performed the combined exercise while monitoring sEMG and VO2, and we tested whether summation of the sEMG during the combined exercise significantly correlated with that of VO2. As there were at least two methods for evaluating sEMG, the RMS and amplitude integration of each detected activity, we also compared which method showed better correlation. Furthermore, we examined whether the fatigue component would affect the summation of sEMG during exercise by comparing the values of the sEMG during walking before and after heavy exercise of the combined exercise program.
2. Materials and Methods
2.1 Participants
Twelve healthy adult men and thirteen healthy adults (3 women) participated in the first and second trials, respectively. One person in the first trial also participated in the second trial. These trials were performed intermittently for over six months. Table 1 presents the physical characteristics of the participants in both trials. The first and second trials were conducted in accordance with the Declaration of Helsinki and the Ethical Guidelines for Medical Research Involving Human Subjects and were approved by the Human Ethics Committee of Wakayama Medical University (#2101 and #2814, respectively). The purpose and content of each trial were fully explained using consent forms prior to the measurements. None of the participants had cardiopulmonary or orthopedic diseases. The participants refrained from consuming alcohol and caffeine above 4 mg/kg [19] on the previous day and during the measurement in both trials.
|
1st |
2nd |
|
|
Sex women/men |
0/12 |
3/10 |
|
Age, years old |
26 (3) |
31 (6) |
|
Height, cm |
176 (5) |
169 (6) |
|
Body weight, kg |
67.7 (5.9) |
65.6 (13.8) |
|
BMI, kg/m2 |
21.7 (1.5) |
22.8 (3.7) |
|
VO2peak, mL/kg/min |
48.6 (6.2) |
41.3 (8.5) |
|
HRpeak, beats/min |
185 (16) |
169 (6) |
|
The characteristics of the first (1st) and second trials (2nd) are shown as mean (standard deviation). BMI, body mass index; VO2peak, peak oxygen consumption rate; HRpeak, peak heart rate. VO2peak and HRpeak were obtained from a graded exercise test before each experiment. |
||
Table 1: Characteristics of participants in the 1st and 2nd trials.
2.2 Protocol
A cardiopulmonary exercise test using a lower-extremity ergometer (915E, MONARK, Varberg, Sweden) was performed in an upright position for at least four days prior to each trial. On the day of the preliminary measurement, participants changed their shirts and shorts. After voiding and measuring height and body weight, they sat on the ergometer and were taken to a rest room controlled at ~25°C, while electrocardiographic electrodes were placed on the chest. A mask for expiratory gas sampling was attached while avoiding leakage. After measurements at rest for 3 min, participants started pedaling at 60 revolutions/min (rpm) without loading, that is, 0 W. After 3 min of rest, the workload was increased by 50 W until it reached 100 W every 3 min for men, and above this intensity, it was increased by 20 W every minute until they could not maintain the rhythm due to exhaustion. For women, the workload was increased by 30 W until 90 W every 3 min, and then increased by 10 W every minute above the intensity. As the maximal power output in women is lower than that in men, the workload needs to be raised by a smaller increment than that of men for precise measurement of VO2. Exhaled gas was collected by the breath-by-breath method using a portable breath gas analyzer (MetaMax 3B, CORTEX, Leipzig, Germany) to calculate VO2. VO2 was averaged every 15 s, and then a moving average of three points was taken until the end of the exercise. Peak VO2 (VO2peak) was determined as the largest value. Heart Rate (HR) was also recorded every minute using an electrocardiogram monitor (BMS-2401; Nihon Kohden, Tokyo) to determine peak HR (HRpeak).
2.3 The first trial
On the day of the first trial, the electrodes for sEMG measurements were attached to the right vastus medialis of participants after changing clothes to shirts and shorts and voiding. Then, a mask to collect expired gas was put on the face, covering the nose and mouth. Electrocardiographic electrodes were also put on the chest. The exercise protocol involved an ergometer exercise (Ergo) and treadmill walking (Tread) (STM -2000, Nippon Koden, Tokyo). Ergometer exercise was performed after 3 min of rest in a sitting position and then exercise loads of 0%, 30%, 60%, and 80% VO2peak for 3 min at a pedal speed of 60 rpm. Treadmill walking was performed after a 20–60 min recovery period. Participants walked 2 km/h, followed by 4 km/h and 6 km/h for 3 min at each stage after moving on a treadmill and standing for 3 min (Figure 1).
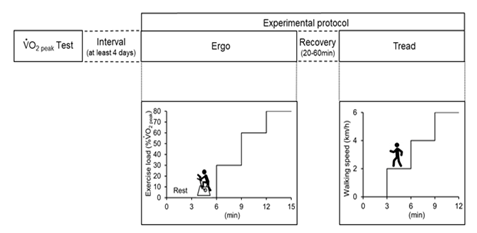
Figure 1: Protocol of the 1st trial. VO2peak, peak oxygen consumption rate.
2.4 The second trial
On the day of the second trial, body weight and subcutaneous fat thickness on the thigh were measured after voiding. The electrodes for sEMG measurements were then attached to the right vastus medialis, and a mask was put on the face, as described above. The protocol of the second trial is shown in Figure 2, which comprised the contents of cancer rehabilitation in our hospital [2]. Briefly, after a 3-min seated rest, participants walked at 3 km/h (optimal speed) with 5 and 6 km/h (fast walk) on a treadmill for women and men, respectively, performed a squat exercise (half squat with approximately 90° hip and knee flexion; 60 squats/min), walked again at 3 km/h, and then performed ergometer exercise at 30% of VO2peak (60 rpm) for 3 min of each session. The interval between each session was 30 s. Finally, participants performed ergometer exercise at 100% of VO2peak for a minute followed by a 3-minute cool down at 0 W. The period from the onset of exercise to the end of the cool down was 21’30”. All measurements were continued even during the intervals. After cooling down, the participants rested in a seated position for 40 min until VO2 returned to baseline before the onset of exercise.
2.5 Measurements
The activity of muscle contraction was evaluated using sEMG with a cordless sensor (MQ Air, KISEEI COMTEC, Matsumoto, Japan) on the right medial vastus longitudinal muscle fiber (the vastus medialis oblique longus: VML). Before attaching electrodes to the measurement site, the skin was wiped with alcohol cotton swabs and shaved to reduce skin resistance. Electrodes were placed at 80% on the line between the anterior spina iliaca superior and the joint space in front of the anterior border of the medial ligament, according to the international standard SENIAN [20]. Three Ag-Ag chloride electrodes with 10-mm diameter were placed as plus, minus, and ground in a relevant area 20 mm away from each other. Band-pass filters were set 8–500 Hz and 20–500 Hz in the first and second trials, respectively. A low cutoff frequency of 8 Hz was determined in a previous study by Minoshima et al. [15] from our research team. A cutoff frequency of 20 Hz for high-pass filters was used to remove movement-induced artefacts [21]. A high cutoff frequency of 500 Hz was determined by considering the Nyquist frequency [22]. The filtered signal was recorded at a sampling frequency of 1000 Hz by a software (Vital Revorder2, KISSEI COMTEC) through a 16-bit analog-to-digital converter (AIO-163202FX-USB, CONTEC, Osaka, Japan). VO2, carbon dioxide excretion rate (VCO2), and ventilation were measured or calculated and averaged every 15 s after removing obvious artifacts. The Respiratory Exchange Ratio (RER) was calculated by dividing VCO2 by VO2. HR was recorded every minute on an electrocardiogram monitor (BMS-2401, Nihon Kohden, Tokyo).
Subcutaneous fat thickness on the right VML was measured using the caliper method (Eiken subcutaneous fat meter, Yagami, Nagoya, Japan). Prior to measurement, the skin and subcutaneous fat were pinched with the thumb and index finger, and the tip of a caliper was placed on a right angle at a distance of 1–2 cm from the pinched area and then released for 1–2 seconds. Measurements were taken twice, and the values were averaged.
The number of steps during walking was counted from a raw sEMG signal stored in a computer after the present measurement.
2.6 Analyses of sEMG signals and VO2
The first trial: First, the sEMG signal was resampled at 250 Hz and 500 Hz, and then the RMS during the exercise was calculated every minute (sEMGRMS [mV/min]) using a numerical analysis software (BIMTAS, KISSEI COMTEC). The calculation at each sampling frequency included in 1000 Hz was as follows:
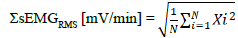
(N, sample number; X, EMG signal; i, time index of X)
EMG amplitudes over the average value of each stage ± 3 SDs were excluded from the analysis to distinguish it from background noise. Changes in sEMGRMS (ΔsEMGRMS) were shown as subtraction of resting values from sEMGRMS during each exercise session and summed every minute in both Ergo and Tread trials.
The integrated spike amplitude method was obtained after the sEMG signals were filtered with 8–500Hz of band-pass filter, rectified, and then resampled at 250 Hz and 500 Hz. The amplitudes of the detectable spikes were summed during the last minute at each stage and divided by the number of spikes (sEMGAMP [mV/points/min]):
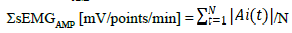
(N, sample number; A, amplitude of signal detected; t, time index)
The average value of VO2 was calculated every minute. The baseline value for rest was defined as the minimum moving average value for 15 consecutive seconds while sitting on the ergometer for 3 min. Based on this, the changes in VO2 (ΔVO2) from the baseline (averaged resting value) at each stage during Ergo and Tread were calculated. The changes in ΔVO2 and ΔsEMGRMS and ΔsEMGAMP for the last 1 min were representative for each stage and were used for a simple regression analysis.
The second trial: The two methods mentioned above were also used. Both ΔsEMGRMS and ΔsEMGAMP were calculated at sampling frequencies of 250 Hz, 500 Hz, and 1000 Hz throughout the exercise (21’30”) and until the end of the cooldown exercise (61’30”). ΔVO2 from the baseline values, which were averaged from 75 to 150 sec of resting period, were integrated for the corresponding period (SΔVO2 [mL/kg/21’30”] and [mL/kg/61’30”]). The time series of sEMG (32768 points) were analyzed by the Fast-Fourier transforms every 30 sec during each exercise period. The Fast-Fourier transforms were implemented within each Hanning-windowed data segment to calculate the autospectra of sEMG. A Median Frequency (MF) of the autospectra was calculated for every segment. The two consecutive MFs were averaged as minute data.
2.7 Statistical analyses
In the first trial, linear regression analyses between SsEMG [mV/min] and ΔVO2 [mL/kg/min] were performed for each participant at 250 Hz, 500 Hz, and 1000 Hz of sampling frequencies. Furthermore, all participant data (Ergo 48 points [4 levels: 0%, 30%, 60% and 80%, × 12 participants], Tread 36 points [3 levels: 2, 4, and 6 km/h, × 12 participants], 84 points in total) were plotted for each exercise phase for Pearson's correlation, and the 95% confidence intervals are shown.
In the second trial, the correlation between ΣΔVO2 and ΣsEMGRMS or ΣsEMGAMP at each sampling frequency was assessed using Pearson's correlation. The paired t-test was applied to compare ΣsEMGAMP and the numbers of steps during the first and second optimal walking.
The significance level was set at less than 5%. Values are represented as mean (SD), unless specified otherwise. The post hoc power analysis of the present simple regression was performed by multiple linear regression of the F test using G*Power 3.1.9.2 (Heinrich-Heine University, Düsseldorf, Germany). Effects sizes, with an error of probability assumed as 0.05, are shown in each table.
3. Results
VO2 and sEMG signals increased with increase in workload during the first trial (Tables 2 and 3). High positive correlations between workload (watts in ergometer exercise and km/h in treadmill walking) and SΔsEMGRMS, SΔsEMGAMP, or ΔVO2 (r = 0.886–1.000; all P <0.001) were observed for each participant in both the Ergo and Tread exercise (not shown).
|
%Work load |
Ergo |
Work load |
Tread |
|||
|
Means |
(SD) |
Means |
(SD) |
|||
|
Rest |
3.9 |
(0.7) |
Rest |
5.1 |
(1.2) |
|
|
VO2peak |
0 |
8.4 |
(1.3) |
2 km/h |
9.3 |
(1.3) |
|
0.3 |
17.7 |
(2.0) |
4 km/h |
12.1 |
(1.3) |
|
|
0.6 |
32 |
(3.5) |
6 km/h |
19.8 |
(2.7) |
|
|
0.8 |
42.4 |
(3.5) |
||||
|
Values at %work load of the peak VO2 (VO2peak) and walking velocity are shown as means (standard deviation, SD) for eleven participants. Ergo, ergometer exercise; Tread, treadmill walking. |
||||||
Table 2: Oxygen consumption rate (VO2) during each type of exercise and stage of the 1st trials.
|
Mode |
Type |
Work load |
250Hz |
500Hz |
1000Hz |
|||
|
Means |
SD |
Means |
SD |
Means |
SD |
|||
|
Ergo |
RMS |
Rest |
0.0026 |
(0.0011) |
0.0026 |
(0.0011) |
0.0026 |
(0.0012) |
|
0% |
0.0163 |
(0.0143) |
0.0163 |
(0.0143) |
0.0164 |
(0.0144) |
||
|
30% |
0.0552 |
(0.0147) |
0.0530 |
(0.0139) |
0.0530 |
(0.0139) |
||
|
60% |
0.0962 |
(0.0273) |
0.0972 |
(0.0282) |
0.0972 |
(0.0282) |
||
|
80% |
0.1397 |
(0.0459) |
0.1444 |
(0.0495) |
0.1444 |
(0.0495) |
||
|
AMP |
Rest |
0.0062 |
(0.0010) |
0.0063 |
(0.0010) |
0.0063 |
(0.0010) |
|
|
0% |
0.0161 |
(0.0089) |
0.0163 |
(0.0087) |
0.0163 |
(0.0087) |
||
|
30% |
0.0448 |
(0.0119) |
0.0436 |
(0.0113) |
0.0436 |
(0.0113) |
||
|
60% |
0.0709 |
(0.0213) |
0.0714 |
(0.0217) |
0.0714 |
(0.0218) |
||
|
80% |
0.0937 |
(0.0272) |
0.0965 |
(0.0294) |
0.0966 |
(0.0295) |
||
|
Tread
|
RMS |
Rest |
0.0063 |
(0.0048) |
0.0063 |
(0.0048) |
0.0066 |
(0.0048) |
|
2km/h |
0.0059 |
(0.0030) |
0.0060 |
(0.0030) |
0.0060 |
(0.0030) |
||
|
4km/h |
0.0126 |
(0.0076) |
0.0127 |
(0.0076) |
0.0127 |
(0.0076) |
||
|
6km/h |
0.0227 |
(0.0102) |
0.0227 |
(0.0103) |
0.0227 |
(0.0102) |
||
|
AMP
|
Rest |
0.0078 |
(0.0020) |
0.0078 |
(0.0020) |
0.0078 |
(0.0020) |
|
|
2km/h |
0.0123 |
(0.0039) |
0.0122 |
(0.0039) |
0.0122 |
(0.0039) |
||
|
4km/h |
0.0199 |
(0.0074) |
0.0199 |
(0.0074) |
0.0199 |
(0.0074) |
||
|
6km/h |
0.0351 |
(0.0110) |
0.0351 |
(0.0110) |
0.0351 |
(0.0110) |
||
|
Values at %work load of the peak VO2 (VO2peak) and walking velocity are shown as means (standard deviation, SD) for eleven participants. AMP, the integrated amplitudes of sEMG; Ergo, ergometer exercise; RMS, the root mean square; Tread, treadmill walking. |
||||||||
Table 3: Values of surface electromyogram during the 1st trial.
Table 4 shows the results of the simple regression analysis in the first trial between SsEMG and ΔVO2 for each participant during both the Ergo and Tread trials at every sampling frequency, and significant correlations (r = 0.884 – 0.896; all P <0.015) were observed in all participants. The correlate coefficients (r) were relatively lower in Tread than Ergo, but they were similar between RMS and AMP and among sampling frequencies. Figure 3 shows the relationship between SsEMGAMP at 250Hz and ΔVO2 at 30%, 60%, and 80% of the VO2peak during Ergo and at 2, 4, and 6 km/h during Tread for all participants. SsEMG was highly correlated with ΔVO2 (r = 0.888; P <0.0001; y = 339.04 × + 4.0267).
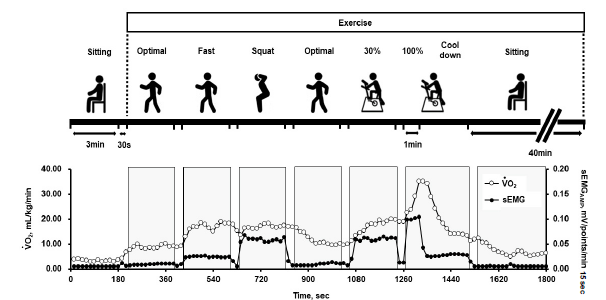
Figure 2: Measurement protocol in the 2nd trial and typical examples of oxygen uptake (VO2) and sEMGAMP,mV/points/15 Sec to change of the vastus medialis. Participant ID8 was a man measuring 171 cm height and 65 kg body weight).
Figure 3: The relationship between increases in the integrated electromyograph (EMG) of the vastus medialis (SsEMG, mV/points/min) and changes in oxygen consumption rate from the resting value (DVO2) during lower-leg exercise in the 1st trial. ? indicates data during lower-leg ergometer exercise and ? indicates data during treadmill walking. The dotted line indicates the regression equation y = 339.04 × + 4.0267 (r = 0.888; P <0.0001) obtained from the data of eleven participants. The gray line shows the confidence interval of 95%. r, correlation coefficient.
Figure 4 shows metabolic and sEMG responses during the second trial. VO2 and VCO2 increased with an increase in exercise intensity, but these values were still high during the second optimal walking just after squatting. The RER tended to be over 1.0 during and just after squatting and/or ergometer exercise at 100% of VO2peak. RER was above 1.0 in 11 out of 13 participants during the study period.
|
Type |
f, Hz |
N |
Slope |
Intercept |
r |
r2 |
Effect size |
Power |
|
|
Whole |
RMS |
250 |
84 |
217.51 |
5.27 |
0.8961 |
0.8029 |
4.0743 |
1 |
|
500 |
84 |
209.09 |
5.58 |
0.891 |
0.7939 |
3.8512 |
1 |
||
|
1000 |
84 |
209.06 |
5.58 |
0.891 |
0.7939 |
3.8523 |
1 |
||
|
AMP |
250 |
84 |
339.04 |
4.03 |
0.8877 |
0.788 |
3.7164 |
1 |
|
|
500 |
84 |
328.21 |
4.3 |
0.8845 |
0.7823 |
3.5931 |
1 |
||
|
1000 |
84 |
327.93 |
4.31 |
0.8844 |
0.7822 |
3.5905 |
1 |
||
|
Ergo |
RMS |
250 |
48 |
218.98 |
5.04 |
0.8741 |
0.7641 |
3.2392 |
1 |
|
500 |
48 |
207.46 |
5.72 |
0.8664 |
0.7507 |
3.0114 |
1 |
||
|
1000 |
48 |
207.48 |
5.71 |
0.8665 |
0.7508 |
3.0124 |
1 |
||
|
AMP |
250 |
48 |
339.83 |
4.27 |
0.8649 |
0.7481 |
2.9697 |
1 |
|
|
500 |
48 |
325.27 |
4.82 |
0.8596 |
0.739 |
2.8311 |
1 |
||
|
1000 |
48 |
324.95 |
4.83 |
0.8595 |
0.7388 |
2.8278 |
1 |
||
|
Tread |
RMS |
250 |
36 |
245.2 |
5.03 |
0.6478 |
0.4197 |
0.7232 |
0.9986 |
|
500 |
36 |
245.75 |
5.02 |
0.6496 |
0.422 |
0.7302 |
0.9987 |
||
|
1000 |
36 |
245.81 |
5.02 |
0.6497 |
0.4221 |
0.7304 |
0.9987 |
||
|
AMP |
250 |
36 |
245.2 |
5.03 |
0.6478 |
0.4197 |
0.7231 |
0.9986 |
|
|
500 |
36 |
245.75 |
5.02 |
0.6496 |
0.422 |
0.7302 |
0.9987 |
||
|
1000 |
36 |
245.81 |
5.02 |
0.6497 |
0.4221 |
0.7304 |
0.9987 |
||
|
Results of a simple regression analysis of the 1st trial in twelve participants are shown. f, sampling frequency; Slope and Intercept, slope and intercept of the present regression analysis between changes (D) in oxygen consumption rate (VO2) and integrated surface myography (sEMG) on the vastus medialis oblique longus (SsEMG) during graded ergometer exercise (Ergo) and treadmill walking (Tread); r, correlation coefficient; RMS, the root mean square; AMP, the integrated amplitudes of sEMG. Post hoc power analysis of a simple regression was performed by multiple linear regression on the F test using G*Power 3.1.9.2. |
|||||||||
Table 4: A simple regression analysis in the 1st trial.
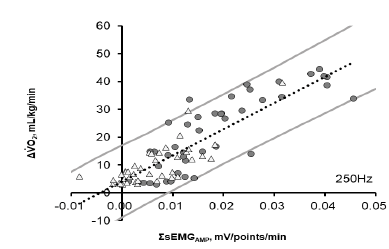
Figure 4: Oxygen consumption rate (VO2), carbon dioxide excretion (VCO2), ventilation (VE), Respiratory Exchange Ratio (RER), and sEMGAMP,mV/points throughout the experiment in the 2nd trial. Values are shown as means ± standard deviation for 13 participants.
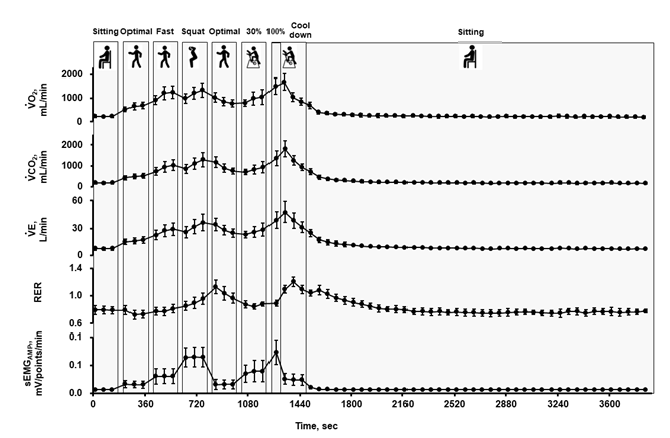
Figure 5: Correlations between ΣΔVO2 (mL/kg) and ΣsEMGRMS (mV) or ΣsEMGAMP (mV), ΣΔVO2, summation of changes (Δ) in oxygen consumption rate (VO2) from the baseline during the whole exercise period (21`30”) in the 2nd trial; ΣsEMGRMS (mV) and ΣsEMGAMP (mV) were integrated at each sampling frequency over the entire measurement period. Data are shown for 13 participants.
ΣsEMGRMS and ΣsEMGAMP showed a significant positive correlation with ΣΔVO2 at each sampling frequency, as shown in Figure 5 and Table 5. The correlation coefficient between ΣsEMGAMP and ΣΔVO2 was slightly higher than that of ΣsEMGRMS. For ΣsEMGRMS, the correlation was lower at a sampling frequency of 250 Hz and 500 Hz than at 1000 Hz. However, the correlation was independent of the sampling frequency for ΣsEMGAMP.
No significant correlation was found between ΣsEMGAMP and thigh subcutaneous fat thickness (r = -0.254; p = 0.402; effect size = 0.069; power = 0.139). The comparison of the step counts and the ΣsEMGAMP value between the first and second optimal walks showed that the second value was not significantly different in the step counts (effect size = 0.490; power = 0.671; p = 0.102) but was significantly higher in ΣsEMGAMP than that of the second one (p = 0.025; effect size =0.709, power = 0.935; Figure 6). The MFs at the third minute of the first optimal and fast walking periods were lower than those at the first minute (p < 0.05; Table 6). In contrast, the MF at the third minute of squat was greater than that at the first minute (p < 0.05; Table 6).
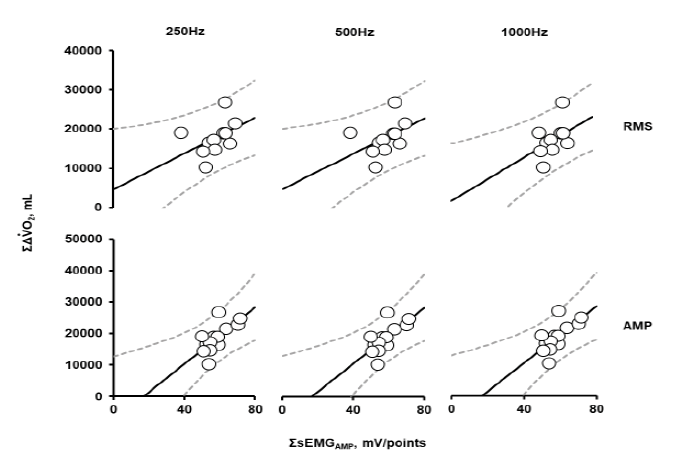
Figure 6: Comparison of individual differences in number of steps and ΣsEMGAMP (mV/points) during the first and second optimal walks in the 2nd trial. #, significant difference between the first and second optimal walk periods at the level of p < 0.05.
|
f, Hz |
Regression equation |
r |
P |
Effect size |
Power |
|
|
RMS |
250 |
y = 188.7 x + 6666 |
0.598 |
0.031 |
0.555 |
0.687 |
|
500 |
y = 186.5 x + 6788 |
0.595 |
0.032 |
0.548 |
0.6814 |
|
|
1000 |
y = 208.7 x + 5355 |
0.658 |
0.015 |
0.763 |
0.8173 |
|
|
AMP |
250 |
y = 353.1 x + 1650 |
0.675 |
0.011 |
0.838 |
0.8512 |
|
500 |
y = 349.7 x + 1788 |
0.674 |
0.012 |
0.833 |
0.8493 |
|
|
1000 |
y = 353.2 x + 1655 |
0.676 |
0.011 |
0.842 |
0.8528 |
|
|
The integrated value of the increase in the surface electromyogram (sEMG) during all physical activities was calculated using the root mean squares (RMS) or the integrated amplitudes of sEMG (AMP); ΣΔVO2, the integrated value of the increase in oxygen consumption rate (VO2) from the baseline before the onset of exercise; f, sampling frequency; r, Pearson's correlation coefficient. |
||||||
Table 5: Correlations between ΣsEMG and ΣΔVO2 in the 2nd trial.
|
Hz |
Effect size |
Power |
P |
|||
|
1 min |
2 min |
3 min |
||||
|
1st Optimal walking |
36.9 |
31.5 |
26.2# |
0.553 |
0.852 |
0.041 |
|
(21.9) |
(19) |
(19.8) |
||||
|
Fast walking |
60.9 |
58.2 |
57.8# |
0.612 |
0.917 |
0.022 |
|
(7.2) |
(7.6) |
(7.6) |
||||
|
Squat |
6.9 |
9.4 |
11.0# |
0.595 |
0.901 |
0.026 |
|
(5.4) |
(9) |
(11.4) |
||||
|
2nd Optimal walking |
48 |
50.1 |
49.9 |
0.363 |
0.481 |
0.226 |
|
(24.7) |
(24.1) |
(23.6) |
||||
|
The median frequencies are shown as means (standard deviation) for 13 participants at every minute of each period. #, a significant difference from the first minute of each period at the level of p < 0.05. sEMG, surface electromyogram. |
||||||
Table 6: Median Frequency (MF) of autospectra of sEMG during the 1st and 2nd optimal walking, fast walking, and squat in the second trial.
The comparison of the Coefficient of Variation (CV) values among participants for each exercise showed that ΣsEMGAMP had a higher CV value than ΣΔVO2, with the highest value during the second optimal walk (Table 7).
VO2 response tended to delay from the EMG signal during high intensity exercise. Thus, an inclusion of oxygen deficit during the 40-min recovery in ΣΔVO2 did not improve the correlation between ΣsEMGAMP and ΣΔVO2 (data not shown).
|
ΣVO2, mL/kg |
ΣVO2, mL |
ΣsEMGAMP, mV/points |
|||||||
|
Mean |
SD |
CV |
Mean |
SD |
CV |
Mean |
SD |
CV |
|
|
1st Optimal walking |
18.2 |
4.1 |
0.227 |
1186 |
340 |
0.287 |
3.159 |
0.994 |
0.315 |
|
Fast walking |
40.2 |
5.1 |
0.127 |
2632 |
616 |
0.234 |
6.134 |
2.309 |
0.376 |
|
Squat |
43.3 |
10.0 |
0.230 |
2812 |
715 |
0.254 |
11.454 |
2.730 |
0.238 |
|
2nd Optimal walking |
30.5 |
5.7 |
0.188 |
1981 |
472 |
0.238 |
4.125 |
1.721 |
0.417 |
|
Ergo 30% |
33.0 |
10.0 |
0.305 |
2130 |
638 |
0.299 |
6.659 |
1.823 |
0.274 |
|
Ergo 100% |
19.5 |
4.3 |
0.218 |
1269 |
313 |
0.246 |
4.193 |
0.951 |
0.227 |
|
Cool down |
44.5 |
8.3 |
0.187 |
2864 |
547 |
0.190 |
4.264 |
1.061 |
0.248 |
|
Variability of index for physical activities during each period are shown. ΣVO2, cumulative oxygen consumption rate (VO2); ΣsEMGAMP, summations of the integrated amplitudes of surface electromyograms from the vastus medialis during each period. Ergo 30% and 100%, ergometer exercises at 30% and 100% of peak VO2, respectively. All durations are 3 min except for Ergo 100% (1 min). |
|||||||||
Table 7: Coefficient of Variation (CV) between participants for each exercise in the 2nd trial is not reflected in the text.
4. Discussion
The main finding of the first trial was the positive correlation between ΣsEMG and ΔVO2, which was evident even when all data points for the different types of lower-leg exercises (cycling ergometer exercise and treadmill walking) were plotted. The results suggest that EMG recording of the VML is a potential candidate method for assessing physical activity regardless of exercise modalities; however, the advantage of using ΣsEMGAMP remains unknown. In the second trial, the results suggest that ΣΔVO2 correlated with both ΣsEMGRMS and ΣsEMGAMP, and ΣsEMGAMP showed a stronger positive correlation with ΣΔVO2 than ΣsEMGRMS. Furthermore, ΣsEMGAMP was less affected by the sampling frequency. The inclusion of oxygen deficit during the 40-min recovery in ΣΔVO2 did not improve the correlation between ΣsEMGAMP and ΣΔVO2. Finally, ΣsEMGAMP during the optimal walking was higher after squatting, which might be related to fatigue. Although VO2 is the standard metabolic index from a nutritional point of view and sEMG signal cannot precisely estimate energy expenditure during physical activity, sEMGAMP is a potential index of physical activity, including fatigue component, for assessing the effects of exercise therapy.
4.1 The first trial
VO2 and sEMG signal from the quadriceps increased linearly with an increase in workload during aerobic ergometer exercises [23]. In addition, sEMG signal from the quadriceps correlated with an increase in VO2 up to 75–90% of VO2max [18]. The slope of the rise in the sEMG signal with the increase in VO2 was enhanced over the intensity range [18,24]. This enhancement was observed simultaneously with respiratory compensation, which may have been caused by lactate production [24] and/or increased recruitment of type II fibers during high-intensity contractions [18,25]. The workload in the present study was determined to be up to 80% of VO2peak. Thus, significant correlation between ΣΔsEMG and ΔVO2 was observed.
Type I fibers are smaller in diameter than type II fibers and are activated first during muscle contraction. As contraction strength increases, larger type IIa and IIb fibers are activated [23]. The Vastus Medialis Comprises VML and Vastus Medialis Obliquus (VMO), which act primarily on the phase of the final knee extension and enhance patella stability [26]. The ratio of type IIb fibers in the rectus femoris, vastus lateralis, and VMO is relatively high [27]; on the other hand, type II fibers are the main component of VML [27]. The energy metabolism of type II fibers is mainly anaerobic and is likely to produce more lactic acid than that of type I fibers [28]. Therefore, the measurement of the VML was suitable for EMG during the cycling exercise using an ergometer and walking using a treadmill. The less variability contributes to the observed correlation.
Furthermore, ΣsEMG of the VML and ΔVO2 had comparable relationship between the cycling and walking exercises (Figure 3), and this suggests that the ΣsEMG of the VML can reflect the activity of exercise therapy in ambulatory persons, which is usually difficult to estimate. However, the correlations are limited to ergometer and treadmill exercises.
4.2 The second trial
As shown in Table 5, for ΣsEMGRMS, the correlation coefficient was slightly lower at 500 Hz and 250 Hz than at 1000Hz. However, for ΣsEMGAMP, no difference was observed at sampling frequencies among 1000 Hz, 500 Hz, and 250 Hz. This might be because the components of the detected firing peaks comprised components within 100 Hz in frequency [29]; therefore, ΣsEMGAMP had not affected the sampling frequency as much as ΣsEMGRMS. The advantage of ΣsEMGAMP compared with ΣsEMGRMS was not clear in the first trial and was suggested in the second trial.
We found that the sEMG signal of some participants increased during the treadmill walking exercise after squatting. As the walking speed and step counts were similar for a participant, the width of gait was the same between the first and second optimal walking (Figures 2 and 6). The RER data indicated that anaerobic metabolism may have been enhanced during the second optimal walking (Figure 4). Therefore, the higher ΣsEMGAMP in the second optimal walking suggests involvement of a fatigue component, as well as physical activities, dependent on exercise intensity. On the other hand, responses of MFs during the first and second optimal walking, fast walking, and squatting were puzzled (Table 6). Although MF is a well-established fatigue index during isometric contraction, it could not be an index of fatigue in the present study [15].
In addition, the CV values of the sEMGAMP among participants in each optimal walk were also higher in the second one (Table 7), suggesting a variation of physical fitness among participants. Participants with a lower fitness level might show a higher fatigue level. Regarding the effects of muscle fatigue, recruitment of more fibers, such as type II, [28] and increasing the intramuscular temperature [30] should be considered.
Considering the effect of subcutaneous fat thickness at the sEMG measurement site, as previously reported, the sEMG is affected by the thickness of the subcutaneous fat because the impedance between the muscle fibers and the skin could vary [31]. The sEMG amplitude was attenuated by up to 62% by subcutaneous fat between the electrode and muscle [32]. In the present study, there was no significant correlation between subcutaneous fat thickness and the integrated value of ΣsEMGAMP (P = 0.402), which could be due to less variation in our study. At least, it is unlikely that subcutaneous fat thickness affected the results of this study.
There is a significant linear relationship between workload and VO2 during endurance exercise with an ergometer, as well as the sEMG integral values of the quadriceps muscle [23]. Action potentials during muscle contraction appear when exercise is performed via excitation-contraction coupling [33]. Therefore, the sEMG signal rose and fell immediately when the exercise was ongoing or stopped (Figure 4). However, the increase in VO2 delayed as compared to the sEMG signal, and even after the exercise was stopped, the VO2 was still higher than the resting value (Figure 4). It would be appropriate to monitor the VO2 during the whole period or day. However, measuring VO2 daily using a mask and a device has many barriers. Furthermore, if we included the summation of VO2 during the 40-min recovery, the correlation did not improve in the present study. Therefore, oxygen deficit may not be related to physical activity measured by sEMG but to energy expenditure. It can be inferred that sEMG is possibly superior to VO2 from the point of assessing effects of exercise therapy, although it involves stress due to fatigue.
5. Limitations
Our study has several limitations. First, the present study was conducted among healthy and relatively young persons. For greater versatility, individuals with relatively different body weights and patients with hemiplegia and gait disturbance should be included in the future study. Second, our findings suggested sex differences in muscle fatigue [34]. The endurance time of a fatiguing contraction with the elbow flexor muscles was less in women than in men, and the difference was related to the absolute contraction intensity [35]. The sex difference in endurance performance of isometric trunk extension was mediated by the muscle mass and strength hypothesis [36]. Third, it will be necessary to verify the measurements of quadriceps muscles other than the vastus medialis muscle. In addition, the physical activities of the upper limbs could not be detected as the electrodes were placed on the thigh.
6. Perspectives
The sEMG signal on the agonist muscle can quantify physical activities during exercise therapy, even for patients with lower activity regardless of their posture: standing, sitting, or lying on the bed. The agonist muscle is VML in ambulatory persons, but can be the deltoid or trapezius muscles in persons with spinal cord injuries.
7. Conclusions
An increase in the sEMG signal of the VML was positively correlated with VO2 during lower leg exercises, regardless of cycling exercise and treadmill walking. Furthermore, the cumulative sEMG signal of the vastus medialis was related with the cumulative VO2 in ambulatory persons during a combined exercise of lower-limb ergometer, treadmill walking, and squatting. The sEMG signal is a potential index for evaluating the amount of physical activity in exercise therapy.
Acknowledgments
The authors thank the medical staff at the Wakayama Medical University Hospital for their assistance.
Financial Disclosure
WHO Kobe Centre‘s Budget (ERC.0002978) in 2018 "Project title: Development of new assistive technologies to enhance quality of life of older people (PI: Prof. Fumihiro Tajima)"
Competing Interests
The authors have declared that no competing interests exist
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
The study was designed by YK, FT, and YN. The measurements were performed by TT, CW, MK, YF, KK, and YiK. The collected data were analyzed by TT, CW, YF, and YiK. This manuscript was drafted by TT, CW, YF, and YiK. All authors have approved the final manuscript submitted for publication and agree to be accountable for all work and interpretations and revised the manuscript.
References
- Pin F, Couch ME, Bonetto A. Preservation of Muscle Mass as a Strategy to Reduce the Toxic Effects of Cancer Chemotherapy on Body Composition. Curr Opin Support Palliat Care 12 (2018): 420-426.
- Mikami Y, Kouda K, Kawasaki S, et al. Preoperative In-Hospital Rehabilitation Improves Physical Function in Patients with Pancreatic Cancer Scheduled for Surgery. Tohoku J Exp Med 251 (2020): 279-285.
- Sergio V, Manlio V. Smart Devices and Healthy Aging. Nutr Healthy Aging 5 (2019): 13-19.
- Tudor-Locke C, Craig CL, Aoyagi Y, et al. How Many Steps/Day are Enough? For Older Adults and Special Populations. Int J Behav Nutr Phys Act 8 (2011): 80.
- Yamazaki T, Gen-no H, Kamijo Y, et al. A New Device to Estimate VO2 During Incline Walking by Accelerometry and Barometry. Med Sci Sports Exerc 41 (2009): 2213-2219.
- Sallis JF. Measuring Physical Activity: Practical Approaches for Program Evaluation in Native American Communities. J Public Health Manag Pract 16 (2010): 404-410.
- Edwardson CL, Winkler EAH, Bodicoat DH, et al. Considerations When Using the ActivPAL Monitor in Field-Based Research with Adult Populations. J Sport Health Sci 6 (2017): 162-178.
- Brouwer AM, van Dam E, van Erp JBF, et al. Improving Real-Life Estimates of Emotion Based on Heart Rate: A Perspective on Taking Metabolic Heart Rate into Account. Front Hum Neurosci 12 (2018): 284.
- Moreno IL, VanderLei LC, Pastre CM, et al. Cardiorespiratory Effects of Water Ingestion During and After Exercise. Int Arch Med 6 (2013): 35.
- Kurtze N, Rangul V, Hustvedt BE. Reliability and Validity of the International Physical Activity Questionnaire in the Nord-Trøndelag Health Study (HUNT) Population of Men. BMC Med Res Methodol 8 (2008): 63.
- Merletti R, Rainoldi A, Farina D. Surface Electromyography for Noninvasive Characterization of Muscle. Exerc Sport Sci Rev 29 (2001): 20-25.
- Hof AL, Elzinga H, Grimmius W, et al. Speed Dependence of Averaged EMG Profiles in Walking. Gait Posture 16 (2002): 77-86.
- Basmajian JV, DeLuca CJ. Muscle Alive, Their Functions Revealed by Electromyography, The 5th edition, Williams and Wilkins, Baltimore (1985): 187-200.
- Torres-Peralta R, Losa-Reyna J, González-Izal M, et al. Muscle Activation During Exercise in Severe Acute Hypoxia: Role of Absolute and Relative Intensity. High Alt Med Biol 15 (2014): 472-482.
- Minoshima Y, Nishimura Y, Tsuboi H, et al. Reliability of Power Spectral Analysis of Surface Electromyogram Recorded During Sustained Vastus Medialis Isometric Contraction in Assessment of Muscle Fatigability. Open J Ther Rehabil 05 (2017): 43-52.
- Gordon G, Holbourn AH. The Sounds from Single Motor Units in a Contracting Muscle. J Physiol 107 (1948): 456-464.
- Masuda K, Masuda T, Sadoyama T, et al. Changes in Surface EMG parameters During Static and Dynamic Fatiguing Contractions. J Electromyogr Kinesiol 9 (1999): 39-46.
- Lannetta D, Qahtani A, Millet GY, et al. Quadriceps Muscles O2 Extraction and EMG Breakpoints during a Ramp Incremental Test. Front Physiol 9 (2017): 1-9.
- Killer SC, Blannin AK, Jeukendrup AE. No Evidence of Dehydration with Moderate Daily Coffee Intake: A Counterbalanced Cross-Over Study in a Free-Living Population. PLOS ONE 9 (2014): e84154.
- Hermens HJ, Freriks B, Disselhorst-Klug C, et al. Development of Recommendations for SEMG Sensors and Sensor Placement Procedures. J Electromyogr Kinesiol 10 (2000): 361-374.
- Lu G, Brittain JS, Holland P, et al. Removing ECG noise from surface EMG signals using adaptive filtering. Neurosci Lett 462 (2009): 14-19.
- Millette P. The Heisenberg Uncertainty Principle and the Nyquist-Shannon Sampling Theorem. Prog Phys 3 (2013): 1-14.
- Bigland-Ritchie B, Woods JJ. Integrated EMG and Oxygen Uptake During Dynamic Contractions of Human Muscles. J Appl Physiol 36 (1974): 475-479.
- Latasa I, Cordova A, Quintana-Ortí G, et al. Evaluation of the Electromyography Test for the Analysis of the Aerobic–Anaerobic Transition in Elite Cyclists during Incremental Exercise. Appl Sci 9 (2019).
- Chin LM, Kowalchuk JM, Barstow TJ, et al. The Relationship between Muscle Deoxygenation and Activation in Different Muscles of the Quadriceps during Cycle Ramp Exercise. J Appl Physiol (1985) 111 (2011): 1259-1265.
- Lieb FJ, Perry J. Quadriceps Function. An Anatomical and Mechanical Study Using Amputated Limbs. J Bone Joint Surg Am 50 (1968): 1535-1548.
- Travnik L, Pernus F, Erzen I. Histochemical and Morphometric Characteristics of the Normal Human Vastus Medialis longus and Vastus Medialis Obliquus Muscles. J Anat 187 (1995): 403-411.
- Burke RE. Motor Unit Properties and Selective Involvement in Movement. Exerc Sport Sci Rev 3 (1975): 31-81.
- Tupa O, Prochazka A, Vysata O. Multiscale Peak Detection in EMG Signal Analysis. In Proc. of the 19th International Conference Technical Computing Prague 121 (2011): 1-6.
- Petrofsky J; Laymon M. Muscle Temperature and EMG Amplitude and Frequency During Isometric Exercise. Aviat Space Environ Med 76 (2005):1024-1030.
- Kuiken TA, Lowery MM, Stoykov NS. The Effect of Subcutaneous Fat on Myoelectric Signal Amplitude and Cross-Talk. Prosthet Orthot Int 27 (2003): 48-54.
- Keenan KG, Farina D, Maluf KS, et al. Influence of Amplitude Cancellation on the Simulated Surface Electromyogram. J Appl Physiol 9 (2005): 120-131.
- Barrett KE, Barman SM, Boitano S, et al. Ganongs Review of Medical Physiology, 24 Edition (2012): 107-108.
- Fulco CS, Rock PB, Muza SR, et al. Slower Fatigue and Faster Recovery of the Adductor Pollicis Muscle in Women Matched for Strength with Men. Acta Physiol Scand 167 (1999): 233-239.
- Hunter SK, Enoka RM. Sex Differences in the Fatigability of Arm Muscles Depends on Absolute Force During Isometric Contractions. J Appl Physiol 91 (2001): 2686-2694.
- Clark BC, Manini TM, The DJ, et al. Gender Differences in Skeletal Muscle Fatigability are related to Contraction Type and EMG Spectral Compression. J Appl Physiol 94 (2003): 2263-2272.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 73.64%
Acceptance Rate: 73.64%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 