Mitochondrial Biogenesis as a Therapeutic Target for Rotator Cuff Tendon Tears
Armand N Yazdani, Arian Abdi, Parth Patel, Prathosh Velpuri, Vikrant Rai, Devendra K Agrawal*
Department of Translational Research, College of Osteopathic Medicine of the Pacific, Western University of Health Sciences, Pomona, California, United States
*Corresponding Author: Devendra K Agrawal, Department of Translational Research, College of Osteopathic Medicine of the Pacific, Western University of Health Sciences, Pomona, California, United States.
Received: 20 November 2023; Accepted: 27 November 2023 2023; Published: 04 December 2023
Article Information
Citation:
Armand N Yazdani, Arian Abdi, Parth Patel, Prathosh Velpuri, Vikrant Rai, Devendra K Agrawal. Mitochondrial Biogenesis as a Therapeutic Target for Rotator Cuff Tendon Tears. Journal of Orthopedics and Sports Medicine. 5 (2023): 442-449.
View / Download Pdf Share at FacebookAbstract
Rotator Cuff Injuries (RCI) are highly prevalent and characterized by shoulder pain, restricted shoulder movement, and difficulty with overhead activity, radiating pain in the deltoid muscle, and atrophy of the rotator cuff muscles. Increasing age, hand dominance, smoking, hypertension, hyperlipidemia, and obesity are common risk factors. Chronic inflammation plays a critical role in the underlying pathogenesis. RCI accounts for massive healthcare expenditure costing about $15,000 per repair, and over 4.5 million physician visits per year, however, there is still no therapeutic target to improve clinical outcomes. Mitochondrial biogenesis in response to inflammatory stimuli supports increased cellular energy requirements, cell proliferation, and differentiation. This suggests that mitochondrial biogenesis may play a role in healing RCI by serving as a protective factor against free oxygen species and promoting homeostasis within the rotator cuff. There is evidence highlighting the potential therapeutic benefits of mitochondrial biogenesis in various inflammatory diseases, but no study explored the role of mitochondrial biogenesis in rotator cuff tears. Since hypercholesterolemia is a risk factor for RCI, we investigated the effects of hypercholesterolemia on the expression of PGC-1α, a marker of mitochondrial biogenesis, in rotator cuff muscle. The findings revealed an increased gene and protein expression of inflammatory mediators and PGC-1α, suggesting enhanced inflammation and increased mitochondrial biogenesis due to hypercholesterolemia. Additional studies are warranted to further investigate the chronic effect of hyperlipidemia induced RCI to elucidate the cause of insufficient mitochondrial biogenesis unable to protect the rotator cuff and the therapeutic effect of promoting mitochondrial biogenesis.
Keywords
<p>Chronic inflammation; Hypercholesterolemia; Hyperlipidemia; Inflammation in the shoulder; Mitochondrial biogenesis; Rotator cuff injury; Therapeutic targets</p>
Article Details
1. Introduction
Rotator Cuff injury (RCI) is a prevalent cause of shoulder pain, with population studies suggesting that over 20% (16% to 34%) of the US population have a full-length tear throughout their lifetime [1-3]. RCI has many burdensome effects on activities of daily living due to shoulder pain, restricted shoulder movement to avoid pain, pain and difficulty with overhead activity, radiating pain in the deltoid muscle, and atrophy of the rotator cuff muscles (supraspinatus, subscapularis, infraspinatus, and teres minor) [4-6]. This can cause psychological and financial distress to patients affecting their earnings, missed workdays, and disability payments as more than two-thirds of RCI patients are of working age [7]. Overall, RCI accounts for massive healthcare expenditure costing about $15,000 per repair, and over 4.5 million physician visits per year [1].
Risk factors associated with RCI include age, hand dominance, smoking, hypertension, and body weight; obesity is of particular interest as its molecular association with RCI has not been defined [8-13]. Hyperlipidemia, directly related to obesity, is a metabolic disease characterized by increased blood lipids leading to inflammation, oxidative stress, and tissue degradation [3]. Hyperlipidemia interferes with the repair process via a variety of mechanisms involving inflammation, osteoclast migration, xanthoma accumulation, and extracellular matrix disorganization [13-15]. However, there is still no therapeutic target to improve clinical outcomes and thus, there is a need for a deeper understanding of the underlying molecular mechanism of RCI in hyperlipidemic patients.
Mitochondrial biogenesis typically increases in response to acute stressors within cells, serving as a protective factor against free oxygen radicals and promoting homeostasis within the rotator cuff [16]. However, chronic hyperlipidemia may cause persistent low-grade inflammation within rotator cuff tendons, leading to stiffness, and oxidative stress and ultimately overwhelming the ability of cells to effectively generate mitochondria and their biological activity and response to stress [17,18]. Mitochondrial biogenesis has an acute role in protecting cells from ischemic damage following oxidative stress. Within the brain, markers for mitochondrial biogenesis (PGC-1 α, NRF- 1, and mitochondrial transcription Factor A) are elevated within a week following an ischemia-inducing events. This serves as a neuroprotective mechanism that may be beneficial for brain recovery following ischemia [19]. Data also reveal that mitochondrial biogenesis is also activated in diverse inhalation-induced lung injuries and oxidative stress [20]. Furthermore, there has been evidence for implementing mitochondrial biogenesis as a protective strategy for preventing multiple organ dysfunction syndrome [21]. Impairment of mitochondrial biogenesis has been linked to a variety of adverse effects including right ventricular hypertrophy in congenital heart disease [22]. Mitochondrial biogenesis is increased in skeletal muscle in exercised patients and patients experiencing mitochondrial myopathy [23].
While these studies exist highlighting the potential therapeutic benefits of mitochondrial biogenesis, there are no studies on its role in rotator cuff tears. Since hypercholesterolemia is a risk factor for RCI, we investigated the effects of hypercholesterolemia on the expression of PGC-1α, a marker of mitochondrial biogenesis, in rotator cuff muscle.
2. Material and Methods
Tissue collection and processing: This study used collected muscle tissues from the rotator cuff of hypercholesterolemic (blood cholesterol level 480-1100 mg/dL) Yucatan microswine (n=7) and control muscle tissues collected from Yucatan miniswine (n=7) on a normal diet (blood cholesterol level <120 mg/dL) to compare the effect of hypercholesterolemia on rotator cuff muscles. We did the power analysis with an α value of 0.05, the sample size necessary to have at least 90% power to detect a change of at least 30% between the groups is 7 in each group. Yucatan miniswine and microswine are involved in other ongoing studies in the lab and tissues were collected following euthanasia after completion of the experiments. The protocols were approved by the institutional animal care and use committee (IACUC) at the Western University of Health Sciences (Protocols No. R20IACUC038 and R19IACUC026) [24,25]. The collected tissues were processed using a tissue processor following standard protocol in our laboratory and 5μm thin sections were used for all experiments.
Hematoxylin and eosin (H&E) and Trichrome staining. H&E staining was done following the standard protocol in our lab. Briefly, after deparaffinization and rehydration of the slides through a series of xylene, alcohol, and distilled water, the tissue sections were stained with hematoxylin (45 seconds) followed by eosin (8-10 dips). The stained slides were mounted with xylene-based mounting media. Masson Trichrome staining was done using a modified Trichrome kit (HT-15 Sigma Aldrich) following the manufacturer's protocol and following standard lab procedure. Stained tissue sections were scanned at 100µm using a light microscope (Leica DM6). All the scanned images were blindly reviewed by at least two observers with less than 5% variability between the observers.
Quantitative Real-Time Polymerase Chain Reaction: Total RNA was extracted using TRIZOL reagent (#T9424, Sigma, St. Louis, MO, USA) following manufacturer’s instructions, and RNA yield was measured using Nanodrop 2000. The cDNA was prepared using an iScript kit (#1708891, BioRad, USA) following the manufacturer’s instructions. Real-time PCR (RT-qPCR) was performed in triplicate using SYBR Green (# 1708884, BioRad, USA) using the CFX96 RT-PCR system (BioRad Laboratories, Hercules, CA, USA). The forward and reverse primers were obtained from Integrated DNA Technologies (Coralville, IA, USA) (Table 1). The PCR cycling conditions were 5 min at 95°C for initial denaturation, 40 cycles of 30s each at 95°C (denaturation), 30s at 55–600C (according to the primer annealing temperatures), and 30s at 72°C (extension) followed by melting curve analysis. Fold change in mRNA expression relative to controls was analyzed using 2-^^ct after normalization with housekeeping gene 18S. Each experiment was repeated for three biological replicates (n = 3).
|
Nucleotide |
Sequence |
|
18S F |
5'-CCCACGGAATCGAGAAAGAG-3' |
|
18S R |
5'-TTGACGGAAGGGCACCA-3' |
|
TRAF6 F |
5'-ATGCATCTGGACGCCCTAAG-3' |
|
TRAF6 R |
5'-CCCGAGTCTGTACTTCGTGG-3' |
|
TNF-α F |
5'-CATCTACCTGGGAGGGGTCT-3' |
|
TNF-α R |
5'-CCAGATAGTCGGGCAGGTTG-3' |
|
MyD88 F |
5'-CCATTCGAGATGACCCCCTG-3' |
|
MyD88 R |
5'-TGCACAAACTGGGTATCGCT-3' |
|
NRF2 F |
5'-AAACCAGTGGATCTGCCGAC-3' |
|
NRF2 R |
5'-GGGAATGTCTCTGCCAAAAGC-3' |
|
PGC-1α F |
5'-AGCTTGACGAGCGTCATTCAG-3' |
|
PGC-1α R |
5'-AGCACACTCGATGTCAGTCC-3' |
|
F, Forward; R, Reverse; MyD88, Myeloid differentiation primary response 88; NRF2, NF-E2–related factor 2; PGC-1α, peroxisome proliferator-activated receptor-gamma coactivator-1α; TNF-α, Tumor necrosis factor-α; TRAF6, tumor necrosis factor receptor- associated factor 6. |
|
Table 1: Nucleotide sequence of genes evaluated by polymerase chain reaction.
Immunohistochemistry (IHC): IHC was performed using the peroxidase anti- peroxidase method using a secondary antibody conjugated to horseradish peroxidase. The paraffin fixed sections were deparaffinized, rehydrated, and antigen retrieved using 1% citrate buffer (Sigma Aldrich # C9999) before immunostaining as per the standard protocol in our laboratory. Briefly, the slides were washed with 1X phosphate-buffered saline (PBS) after antigen retrieval. The tissue was encircled using a Pap Pen. The tissue samples were incubated with 3% hydrogen peroxide (Sigma Aldrich # H1009) for 15 minutes and washed with PBS for 5 minutes each three times. Blocking was done using the blocking solution from Vectastain kit (PK-6102 or PK-6101) and the tissues were incubated for 1 hour at room temperature. After tipping off the blocking solution, the tissue sections were incubated overnight at 4°C with the primary antibodies including tumor necrosis factor (TNF)-α (ab1793), c-Jun N-terminal kinases (JNK), myeloid differentiation primary response 88 (MyD88; sc-136970), peroxisome proliferator-activated receptor-gamma coactivator (PGC)-1α (sc-517380), and NF-E2–related factor 2 (NRF2; ab89443) after titrating for dilution. A dilution of 1:50 to 1:200 was used for various antibodies. After overnight incubation, the slides were washed 3 times 5 minutes each with 1X PBS and then incubated with the secondary antibody for 1 hour at room temperature. The slides were rinsed 3 times with 1X PBS, followed by incubation with the ABC solution for 30 minutes at room temperature. The tissue sections were then rinsed with 1X PBS followed by incubation with 3,3′-diaminobenzidine (DAB) (Thermo Scientific, Cat # 34002) for 2 to 5 minutes until the development of the brown color of the DAB. Tissue sections were washed with water once and then stained with hematoxylin for 20-30 seconds. The slides were rinsed in running tap water for 5 minutes and mounted with a xylene-based mounting medium. The stained slides were imaged with a Leica DM6 microscope at a scale of 100 µm. The high-magnification images from each tissue section were manually analyzed for average stained intensity and percent-stained area using Fiji Image J. Three sections from each swine and three random images from each stained section were used for statistical analysis.
Statistical analysis: Data are presented as the mean ± SEM. Data were analyzed using GraphPad Prism 9. The comparison between the two groups for the expression of the protein of interest was performed using Student's t-test and more than two groups using One-way ANOVA with Bonferroni’s post-hoc correction. A probability (p) value of < 0.05 was accepted as statistically significant.
3. Results
Hematoxylin and Eosin (H&E) and trichrome staining: H&E staining demonstrated a marked increase in muscle and tendon tissue loss and disorganization of the extracellular membrane (ECM) and matrix in the hypercholesterolemic rotator cuff tissues (Figure 1 (i) panels C-F) compared to the control (non-hypercholesterolemic) rotator cuff tissues (Figure 1 (i) panels A and B). In control tissues, there were clear borders that define the muscular fibers from the fascia compared to the irregular tissues in the hypercholesterolemic rotator cuff tissues with disorganized fascia infiltrated with fat (Figure 1 (i) panels C-F). Hypercholesterolemic rotator cuff tissues showed increased adipose tissue infiltrate in the muscle and fascia region (Figure 1(i) panels C-F). There was minimal fatty infiltration in non- hypercholesterolemic swine (Figure 1 (i) panels A and B). Trichrome staining demonstrated increased collagen disorganization, and fatty infiltration in the hypercholesterolemic group (Figure 1(i) panels C-F) compared to the non-hypercholesterolemic group (Figure 1(ii) panels A and B).
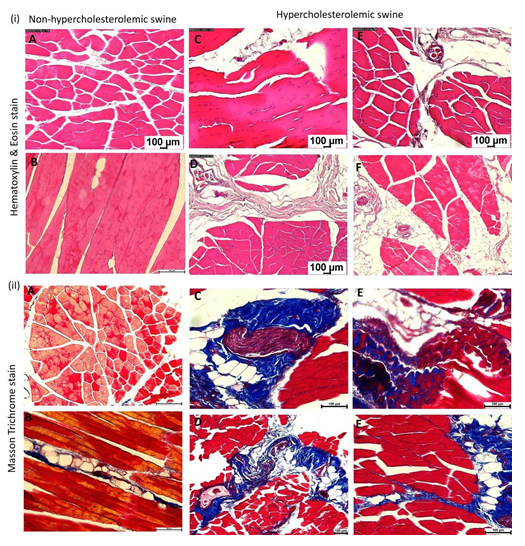
Figure 1: Hematoxylin and Eosin (H&E) and Masson’s trichrome staining in rotator cuff muscle tissues. (i) H&E staining: panels A and B in non-hypercholesterolemic swine and panels C-F in hypercholesterolemic swine. (ii) Masson’s trichrome staining: panels A and B in non- hypercholesterolemic swine and panels C-F in hypercholesterolemic swine. All images were scanned with a scale of 100μm. These are representative images from all swine.
Immunohistochemistry: Immunohistochemistry (IHC) revealed immunopositivity for JNK, MyD88, PGC-1α, TNF-α, and NRF2 and the immunoreactivity was significantly higher in muscle tissues from hypercholesterolemic swine (Figure 2 panels B, D, F, H, and J) compared to control swine (on normal diet) (Figure 2 panels A, C, E, G, and I). Image analyses showed significantly increased average stained intensity and average stained area (percent area) for JNK, MyD88, PGC-1α, TNF-α, and NRF2 in hypercholesterolemic swine compared to control swine (Figure 2 panels K-T).
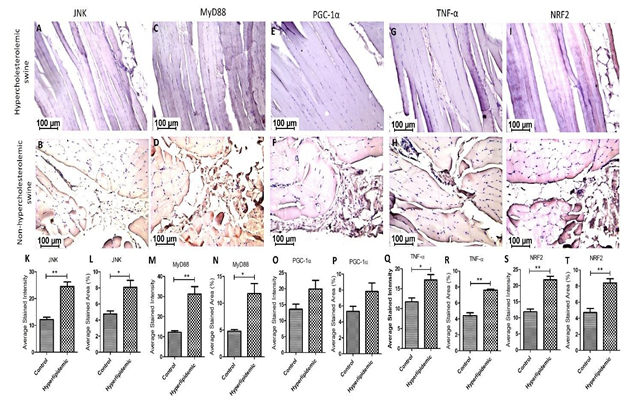
Figure 2: Immunohistochemistry (IHC) for c-Jun N-terminal kinases (JNK), myeloid differentiation primary response 88 (MyD88), peroxisome proliferator-activated receptor- gamma coactivator (PGC-1α), tumor necrosis factor (TNF)-α, and NF-E2–related factor 2 (NRF2) in hypercholesterolemic (panels B, D, F, H, and J) and control (panels A, C, E, G, and I) swine. Average stained intensity (panels K, M, O, Q, and S) and average stained area (percent) (panels L, N, P, R, and T). Data are presented as the mean ± SEM. *p< 0.05 and **p< 0.01. These are representative images from all swine.
Real-Time Polymerase Chain Reaction: PCR analysis reveals significantly increased fold changes in mRNA expression of TNF-α, TRAF6, NRF2, and PGC-1α in hypercholesterolemic swine compared to control swine (Figure 3 panels A-D).
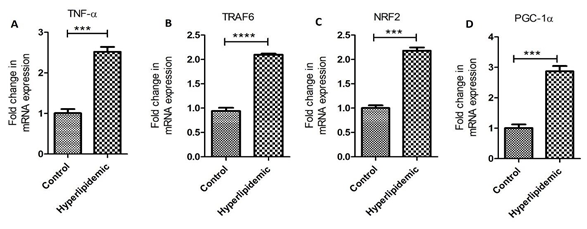
Figure 3: Real-Time Polymerase chain reaction (RT-PCR) for mRNA transcript. Tumor necrosis factor (TNF)-α (panel A), tumor necrosis factor receptor (TNFR)-associated factor (TRAF) 6 (panel B), NF-E2–related factor (NRF)2 (panel C), and peroxisome proliferator-activated receptor-gamma coactivator (PGC)-1α (panel D). Data are presented as the mean ± SD. ***p < 0.001 and ****p < 0.0001.
4. Discussion
The results of this study revealed increased immunopositivity for TNF-α suggesting the presence of chronic inflammation in hypercholesterolemia swine compared to non-hypercholesterolemic control swine. The persistent low-grade inflammation in the hypercholesterolemic samples may be associated with increased activation of nuclear factor kappa beta (NF-κB) followed by subsequent activation of proinflammatory cytokines TNF-α and IL-6 [26,27]. Increased expression of TNF-α in hypercholesterolemic rotator cuff muscle tissues is supported by our previous in-vivo and in-vitro reports that hypercholesterolemia increases the expression of TLR-4, TIRAP, TRAF6, pIkB, RelA, IL-6, and TNF-α and contributes to inflammation [25]. Further, increased expression of pattern recognition receptors within hypercholesterolemic tendon fibroblast ultimately contributes to tendinopathy. Moreover, the production of IL-6 has been reported to stimulate the recruitment of T-cells and macrophages.
The presence of TNF-α further triggers the activation of additional inflammatory pathways. The association of an increase in TNF-α with myocyte apoptosis, disorganized ECM, and increased nonorganized collagens is supported by previous reports that inflammation is associated with catabolism of intramyocellular proteins and dysregulation of regeneration pathways [28,29]. The presence of chronic inflammation reinforces the idea that hypercholesterolemia diminishes the levels of IκBα, which is an anti-inflammatory agent inhibiting the action of NF-κB and is prominently found in type II muscle fibers [26]. Within our study, the heightened expression of TNF-α and TRAF6 coupled with the loss of muscle fibers within supraspinatus muscles suggests that inflammation significantly impacts the development of rotator cuff tears [26,30-32].
Hypercholesterolemia-mediated chronic inflammation may be mediated by multiple factors involving TLRs, DAMPs, and TREM-1 signaling [33], and increased expression of MyD88, a downstream signaling molecule in TLR4 signaling, suggests the involvement of TLR-4 in hypercholesterolemia-mediated chronic inflammation in rotator cuff muscles causing rotator cuff tear [34]. The involvement of TLR-4 signaling in rotator cuff inflammation is further supported by increased mRNA expression of TRAF6, a downstream signaling molecule in TLR4 signaling, and of TNF-α in RT-PCR studies.
Rotator cuff tendonitis, an inflammation of the rotator cuff tendons is frequently seen along with shoulder impingement and can occur due to chronic repetitive overuse activities or following an injury [5]. Increased expression of TNF-α, TRAF6, and MyD88 in hypercholesterolemic muscle tissues suggests the presence of chronic inflammation. Chronicity is because the tissues were collected after 12 months of a hypercholesterolemic diet. Further, significantly elevated oxidative stress (reactive oxygen species; ROS) is associated with both acute and chronic tendon injuries [35]. Furthermore, hypercholesterolemia is associated with oxidative stress [36] and increased oxidative stress is associated with mitochondrial biogenesis and mitochondrial DNA maintenance [37]. Our study revealed an increased gene and protein expression of PGC-1α, a marker of mitochondrial biogenesis. Increased expression of PGC-1α suggests the presence of oxidative stress in the muscle tissues and sustainably increased oxidative stress may lead to RCI [35]. The study demonstrates the deleterious role of sustained oxidative stress and its implications in tendon fibrosis, adhesions, and scarring leading to acute tendon injuries, as revealed by histological studies. The reactive oxygen species generated because of foam cells aggregates within tendon xanthoma which can trigger an inflammatory response, causing tendon damage. Hypercholesterolemia promotes ROS activation of the mTOR pathway leading to fatty infiltration, metaplasia, and heterotopic ossification. mTOR signaling is worsened by increased expression of nesfatin-1, a neuropeptide involved in suppressing the autophagy-lysosomal pathway used to suppress oxidative stress and tendon degeneration. High cholesterol also triggers ROS production, histopathological abnormalities, apoptosis, and autophagy within tendons via activation of the AKT/ FOXO1 pathway and the NF-kB pathway [38]. Additionally, peritendinous adhesions due to oxidative stress exacerbate inflammation involving TNF-α, IL-1β, TGF-β, SOD1, SOD2, COL1, and HIF1α [13]. The chronic inflammation in tendons leads to macrophage recruitment triggered by TREM-1, which results in disorganization of the extracellular matrix. This disorganization, in turn, further upregulates inflammatory cytokines, causing increased oxidative stress [13] and contributing to rotator cuff injury.
Cholesterol is an important determinant of muscle atrophy [39]. We recently reported that hyperlipidemia lowers the biomechanical properties of rotator cuff tendon [40]. In the rotator cuff tendon injury following repair, we found large amount of fibrosis, increased water content, and significant fatty infiltration [41]. It is very likely that the disorganization of extracellular matrix and oxidative stress could have resulted in altered biomechanical properties of the rotator cuff tendons in hyperlipidemic swine. Thus, the strategies to reduce oxidative stress and enhance re-organization of extracellular matrix would support the regeneration of tendon tissue following injury [42].
The NLRP3 pathway, involving IL-1β, plays a role in inducing the degradation of connective tissue in tendons and bones. Hypoxia within the tenocytes can lead to the persistent activation of the NLRP3 pathways, perpetuating inflammation, mitochondrial dysregulation, and ECM degradation within the rotator cuff [17,33]. Furthermore, following rotator cuff tears, mechanical unloading of the tissue can shift muscle cell metabolism from anabolic to catabolic, a process expedited by mitochondrial dysfunction. The presence of damage-associated molecular patterns and pro- inflammatory recruitment (e.g., TNF-α, IL-1, IL-6) stimulates catabolic processes within the intramyocellular proteins of the rotator cuff muscle [28]. This metabolic alteration may be due to mitochondrial dysfunction ultimately leading to mitochondrial biogenesis as a compensatory mechanism. NRF2 controls the cellular oxidant level and oxidative signaling and is activated in response to oxidative stress [43,44]. This suggests that NRF2-mediated oxidative signaling may be an attractive therapeutic target [45,46] in pathologies involving inflammation and oxidative stress. An increased expression of NRF2 in hypercholesterolemic muscle tissues in association with increased PGC-1α, TNF- α, and TRAF6 suggests that targeting NRF2-mediated oxidative signaling and mitochondrial biogenesis may be attractive therapeutic strategies in rotator cuff treatment. However, this hypothesis warrants investigation.
5. Conclusion
Hypercholesterolemia is associated with rotator cuff muscle inflammation, fibrosis, oxidative stress, and mitochondrial biogenesis which in turn is associated with rotator cuff tendon tear. Thus, oxidative stress and mitochondrial biogenesis may be attractive therapeutic targets, however, this association warrants further research. The focus should be on the chronicity of inflammation because in acute settings, mitochondrial biogenesis is beneficial, but we need to investigate the effect of chronicity.
Funding:
The research work of DKA is supported by the R01 HL144125 and R01 HL147662 grants from the National Institutes of Health, USA. The content of this original research article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Competing interest: All the authors have read the manuscript and declare no conflict of interest. No writing assistance was utilized in the production of this manuscript.
Consent for publication: All the authors have read the manuscript and consented for publication.
References
- Yeranosian MG, Terrell RD, Wang JC, et al. The costs associated with the evaluation of rotator cuff tears before surgical repair. J Shoulder Elbow Surg 22 (2013): 1662-1666.
- Yamamoto A, Takagishi K, Osawa T, et al. Prevalence and risk factors of a rotator cuff tear in the general population. J Shoulder Elbow Surg 19 (2010): 116-120.
- Lin TT, Lin CH, Chang CL, et al. The effect of diabetes, hyperlipidemia, and statins on the development of rotator cuff disease: a nationwide, 11-year, longitudinal, population-based follow-up study. Am J Sports Med 43 (2015): 2126-2132.
- May T, Garmel GM. Rotator cuff injury (2019).
- Varacallo M, El Bitar Y, Mair SD. Rotator cuff syndrome. In StatPearls [Internet]; StatPearls Publishing (2022).
- Maruvada S, Madrazo-Ibarra A, Varacallo M. Anatomy, rotator cuff (2017).
- Mather RC, 3rd; Koenig L, Acevedo D, Dall TM, Gallo P, Romeo A, Tongue J, Williams G, Jr. The societal and economic value of rotator cuff repair. J Bone Joint Surg Am 95 (2013): 1993-2000.
- Gumina S; Arceri V, Carbone S, et al. The association between arterial hypertension and rotator cuff tear: the influence on rotator cuff tear sizes. J Shoulder Elbow Surg 22 (2013): 229-232.
- Bishop JY, Santiago-Torres JE, Rimmke N, et al. Smoking Predisposes to Rotator Cuff Pathology and Shoulder Dysfunction: A Systematic Review. Arthroscopy 31 (2015): 1598-1605.
- Hattrup SJ. Rotator cuff repair: relevance of patient age. J Shoulder Elbow Surg 4 (1995): 95-100.
- Sayampanathan AA, Andrew TH. Systematic review on risk factors of rotator cuff tears. J Orthop Surg (Hong Kong) 25 (2017): 2309499016684318.
- Zhao J, Pan J, Zeng LF, et al. Risk factors for full- thickness rotator cuff tears: a systematic review and meta-analysis. EFORT Open Rev 6 (2021): 1087-1096.
- Yazdani AN, Rai V, Agrawal DK. Rotator Cuff Health, Pathology, and Repair in the Perspective of Hyperlipidemia. J Orthop Sports Med 4 (2022): 263-275.
- Burkhardt R. Hyperlipidemia and cardiovascular disease: new insights on lipoprotein (a). Curr Opin Lipidol 30 (2019): 260-261.
- Keuroghlian A, Barroso AD, Kirikian G, et al. The effects of hyperlipidemia on implant osseointegration in the mouse femur. J Oral Implantol 41 (2015): e7-e11.
- Popov LD. Mitochondrial biogenesis: An update. J Cell Mol Med 24 (2020): 4892-4899.
- Thankam FG, Roesch ZK, Dilisio MF, et al. Association of Inflammatory Responses and ECM Disorganization with HMGB1 Upregulation and NLRP3 Inflammasome Activation in the Injured Rotator Cuff Tendon. Sci Rep 8 (2018): 8918.
- Lai J, Gagnier JJ. The Effect of Lipid Disorders on the Risk of Rotator Cuff Disease: A Systematic Review and Meta-Analysis. JB JS Open Access 3 (2018): e0018.
- Zhang Q, Wu Y, Zhang P, et al. Exercise induces mitochondrial biogenesis after brain ischemia in rats. Neuroscience 205 (2012): 10-17.
- Carraway MS, Suliman HB, Kliment C, et al. Mitochondrial biogenesis in the pulmonary vasculature during inhalational lung injury and fibrosis. Antioxid Redox Signal 10 (2008): 269-275.
- Piantadosi CA, Carraway MS, Haden DW, et al. Protecting the permeability pore and mitochondrial biogenesis. Novartis Found Symp 280 (2007): 266-276, discussion 276-280.
- Karamanlidis G, Bautista-Hernandez V, Fynn-Thompson F, et al. Impaired mitochondrial biogenesis precedes heart failure in right ventricular hypertrophy in congenital heart disease. Circ Heart Fail 4 (2011): 707-713.
- Adhihetty PJ, Taivassalo T, Haller RG, et al. The effect of training on the expression of mitochondrial biogenesis- and apoptosis- related proteins in skeletal muscle of patients with mtDNA defects. Am J Physiol Endocrinol Metab 293 (2007): E672-680.
- Rai V, Radwan MM, Nooti S, et al. TLR- 4 inhibition attenuates inflammation, thrombosis, and stenosis in arteriovenous fistula in Yucatan miniswine. Cardiol Cardiovasc Med 6 (2022): 432-450.
- Nooti S, Rai V, Radwan MM, et al. Oxidized low-density lipoproteins and lipopolysaccharides augment carotid artery plaque vulnerability in hypercholesterolemic microswine. Cardiol Cardiovasc Med 7 (2023): 273-294.
- Bhatt BA, Dube JJ, Dedousis N, et al. Diet-induced obesity and acute hyperlipidemia reduce IkappaBalpha levels in rat skeletal muscle in a fiber-type dependent manner. Am J Physiol Regul Integr Comp Physiol 290 (2006): R233-240.
- Clarke MC, Talib S, Figg NL, et al. Vascular smooth muscle cell apoptosis induces interleukin-1-directed inflammation: effects of hyperlipidemia- mediated inhibition of phagocytosis. Circ Res 106 (2010): 363-372.
- Krieger JR, Tellier LE, Ollukaren MT, et al. Quantitative analysis of immune cell subset infiltration of supraspinatus muscle after severe rotator cuff injury. Regen Eng Transl Med 3 (2017): 82-93.
- Zumstein MA, Ladermann A, Raniga S, et al. The biology of rotator cuff healing. Orthop Traumatol Surg Res 103 (2017): S1-S10.
- Yang Y, Qu J. The effects of hyperlipidemia on rotator cuff diseases: a systematic review. J Orthop Surg Res 13 (2018): 204.
- Beason DP, Tucker JJ, Lee CS, et al. Rat rotator cuff tendon-to-bone healing properties are adversely affected by hypercholesterolemia. J Shoulder Elbow Surg 23 (2014): 867-872.
- Gatto AP, Hu DA, Feeley BT, et al. Dyslipidemia is associated with risk for rotator cuff repair failure: a systematic review and meta-analysis. JSES Rev Rep Tech 2 (2022): 302-309.
- Thankam FG, Dilisio MF, Dietz NE, et al. TREM-1, HMGB1 and RAGE in the shoulder tendon: Dual mechanisms for inflammation based on the coincidence of glenohumeral arthritis. PLoS One 11 (2016): e0165492.
- Abboud JA, Kim JS. The effect of hypercholesterolemia on rotator cuff disease. Clin Orthop Relat Res 468 (2010): 1493-1497.
- Prasetia R, Purwana SZB, Lesmana R, et al. The pathology of oxidative stress-induced autophagy in a chronic rotator cuff enthesis tear. Front Physiol 14 (2023): 1222099.
- Singh UN, Kumar S, Dhakal S. Study of oxidative stress in hypercholesterolemia. International Journal of Contemporary Medical Research 4 (2017): 1204-1207.
- Lee HC, Wei YH. Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress. Int J Biochem Cell Biol 37 (2005): 822-834.
- Lui PPY, Zhang X, Yao S, et al. Roles of oxidative stress in acute tendon injury and degenerative tendinopathy- A target for intervention. Int J Mol Sci 23 (2022): 3571.
- Le Hoangvi, Rai V, Agrawal DK. Cholesterol: An important determinant of muscle atrophy in astronauts. J Biotechnol Biomed 6 (2023): 67-79.
- Merlin Rajesh Lal LP, Agrawal DK. Hyperlipidemia lowers the biomechanical properties of rotator cuff tendon. J Orthop Sports Med 5 (2023): 391-400.
- Merlin Rajesh Lal LP, Radwan MM, Thankam FG, et al. Rotator cuff tendon repair after injury in hyperlipidemic swine decreases biomechanical properties. J Orthop Sports Med 5 (2023): 414-425.
- Merlin Rajesh Lal LP, Agrawal DK. Biomechanical forces in the tissue engineering and regeneration of shoulder, hip, knee, and ankle joints. J Biotech Biomed 6 (2023): 409-429.
- Ngo V, Duennwald ML. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants (Basel) 11 (2022): 2345.
- Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 53 (2013): 401-426.
- Ruiz S, Pergola PE, Zager RA, et al. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int 83 (2013): 1029-1041.
- Hammad M, Raftari M, Cesario R, et al. Roles of Oxidative Stress and Nrf2 Signaling in Pathogenic and Non-Pathogenic Cells: A Possible General Mechanism of Resistance to Therapy. Antioxidants (Basel) 12 (2023): 1371.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 73.64%
Acceptance Rate: 73.64%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 