Activities of Metal-Organic Frameworks for the Adsorption of Clofibric Acid from Water
Naz Fathma1, Yeon-Koo Jeong1*
1Kumoh National Institute of Technology, Department of Environmental Engineering, Gumi, Gyeongbuk, South Korea
*Corresponding author:Yeon-Koo Jeong, Kumoh National Institute of Technology, Department of Environmental Engineering, Gumi, Gyeongbuk, 39177, South Korea
Received: 2nd May-2019; Revised: 25th May-2019; Accepted: 26th May-2019
Copyright: ©2019 Yeon-Koo Jeong. This is an open-access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Article Information
Citation:
Naz Fathma, Yeon-Koo Jeong. ACTIVITIES OF METAL-ORGANIC FRAMEWORKS FOR THE ADSORPTION OF CLOFIBRIC ACID FROM WATER. International Journal of Plant, Animal and Environmental Sciences 9 (2019): 63-73.
View / Download Pdf Share at FacebookAbstract
Pharmaceutical and personal care products (PCPPS) are a newly emerging pollutant and are not well managed in traditional water treatment processes. In addition, metal-organic frameworks (MOFs) are being studied for application to various fields such as catalysts, energy storage, adsorption, etc. due to their structural characteristics such as porosity, high specific surface area, some metal organic structures are undergoing basic research to be applied to the treatment of environmental pollutants in water and atmospheric environment. Recently distinguished types of metal-organic frameworks (MOFs) have dynamic applications for clofibric acid (PPCPs pharmaceutical and personal care product) removal through the adsorption process from the aqueous phase. In this study, we investigated the adsorption and removal characteristics of clofibric acid belonging to medicinal materials and personal care products by synthesizing metal organic structure. The metal organic structure used in the study is MIL-101(Cr) and MIL-101-OH, modified by attaching the hydroxyl functional groups (-OH) to MIL-101(Cr). The metal organic structure was prepared by hydrothermal reaction and its structural characteristics were investigated by FTIR, XRD and specific surface area analysis. Batch adsorption experiments were carried out and the results were determined by kinetic analysis and optimum isothermal adsorption. Adsorption reaction rate analysis showed that both adsorption of clofibric acid to MIL-101 (Cr) and MIL-101-OH was followed by pseudo-second-order kinetic equation. All adsorption experiments were found to be more consistent with Langmuir isotherms. Therefore, the maximum adsorption amount of MIL-101-OH with -OH radicals was higher in the pollutant, which is somewhat different from the result of structural characterization.
Keywords
<p>Metal-organic frameworks, Contaminant, Harmful, Pharmaceuticals</p>
Article Details
INTRODUCTION
Water pollution has been gathering worldwide attention as now-a-days it is finding harmful for life, and mostly for human life. Concerning about health effects on human, the World Health Organization (WHO) issued the maximum contaminant level (MCL) of various contaminants in drinking water [1]. Recently, because of the necessity in daily life and for the generation/expenditure globally, Pharmaceuticals and personal care products (PPCPs) have been affectionate much attention. Pharmaceuticals and personal care products (PPCPs) as pollutant commonly include a group of chemical contaminants that can come off from the use of dictation medicines, cosmetics, perfumes, veterinary drugs, fungicides, disinfectant and agricultural practices [2,3]. To fulfill buyers’ need, and as PPCPs have long self-existence that’s why PPCPs mostly exist in the environment since they have been outspent entirely. For instance, several PPCPs have lately been found in surface water, ground water, tissues of fish and vegetables [4-13]; hence, PPCPs are examples of reputed emerging contaminants [8, 9].
Reports said that hormonal mode can be changed due to the endocrine disruptions which may able to cause by ‘Pharmaceuticals and personal care products (PPCPs)’ [4-13]; though the discrepant influence on the environment and on human body and health by PPCPs, is completely understand yet. Because of the unfavorable effects of unwavering PPCPs’ and even their remaining metabolites in hygrophyte systems will be a subsequent environmental intimidation. Consequently, the dismissal of PPCPs from water (i.e. ground and surface water) has lately been tilling numerous considerations [4-15]. Chlorination, biodegradation, (AOPs) i.e. advanced oxidation processes or ozonation, were some small number of processes which have been practiced for the dismissal of PPCPs [4-17]; more elaboration need, as PPCPs dismissal from water not been proven very practically. For instance, remaining byproducts composition and exalted energy expenditure were some of the inconveniences of AOP and ozonation[16,17]. Whereas it’s a facile procedure that can diverge PPCPs with expenditure of low energy and with the inferiority of byproducts, numerous attention being achieved by adsorption [18]. Up to expectation for the withdrawal of PPCPs mesoporous materials (transition metal-grafted) [14,15] and carbonaceous materials (together with activated carbon, carbon nanotubes, and bone char)[6,19], extensively been practiced as a potential adsorbents. For the adsorption of PPCPs activated carbons (ACs) are nowadays maximal extensively applied.Among research tremendous betterment been acquired in period of synthesis and applications, both during work with nanoporous materials, for example metal-organic frameworks (MOFs) [20-28] and mesoporous materials [29-31].
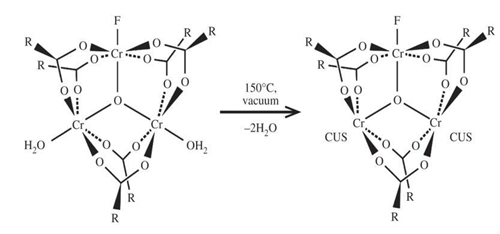
On account of high porosity among the portion of mesoporous and microporous; tremendous considerations being bearing through metal-organic frameworks (MOFs) because the metallic and organic groups formation in the structure of MOFs. Facile functionalization or modifications are able to do with MOFs physiochemical properties, conducting organic linkers or coordinatively unsaturated sites (CUSs)[32] that enhance consummately the use proficiency of MOFs in miscellaneous appeals, separation [33], catalysis [34], adsorption or storage[35-37] are some example of MOFs applications. In case the adsorptive removal of hazardous materials [23-25, 38-41] diverse functionalized and virgin MOFs been used which was based on numerous interaction mechanisms [38-41], for example acid-base interactions, electrostatic interactions, coordination etc.
Another significant area of MOFs application is water purification [42-43]. Generally, MOFs show hydrophilic and ineffective characteristics when it was being used for water purification besides special interactions and incorporating functionalities hence the functionalization of MOFs is anticipated to be necessary for water purification [44]. MOFs such as MIL-100 and MIL-101 for the adsorptive removal of PPCPs with and without functionalization with acidic a basic site being reported as an effective application in the present time [45, 46], were suggested that electrostatic and acid base interactions of MOFs structure capable for the adsorptive removal.
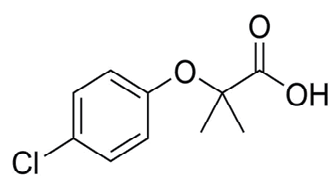
So after analyzing the previous research reports about MOFs, in this thesis we used MIL-101(Cr) (with and without functionalization) as delegate MOF and oxybenzone and clofibric acid as delegate PPCPs. MOFs which have the large pore size and extensive porosity i.e. MIL-101, CrO3(F/OH)(H2O)2[C6H4(CO2)2] consider as one of the extensively studied MOFs[47],being contained CUSs MIL-101(Cr) is compatible for modification[32] and after functionalization MIL-101(Cr) can be used for numerous applications such as adsorption[48] and catalysis[49]. Clofibric acid is bioactive metabolite of various lipids regulating pro-drugs and is a metabolite of the cholesterol lowering pharmaceutical drug known as clofibrate. PPCP has functional groups such as ketone, carboxylic acid and phenol groups that effectively can interlude with adsorbents (i.e. modified MOFs). The chemical structure of clofibric acid is given below in Figure 1 along with their functional groups.
2–2-methylpropanoic acid [C10H11ClO3] (Wikipedia)
MATERIALS AND METHODS
All chemicals had been used without any kind of purification and all chemicals were obtained commercially from different chemical companies. Chromium (III) nitrate nonahydrate (98.0%) (Cr(NO3)3.9H2O, N,N-Dimethylformamide HCON(CH3)2 (DMF, above 99.8%), acetone C3H6O (99.5%), methanol CH3OH (99.5%), toluene C6H5CH3 (Above 99.7%) were purchased from DAEJUNG chemicals. On the other hand, terephthalic acid (TPA, 99.0%) C6H4(COOH)2 was purchased from JUNSEI chemicals and ethanolamine (ETA) C2H7NO (98+%) was obtained from Alfa Aesar. Other chemicals such as ethanol C2H6O (99.9%), Oxybenzone C14H12O3 (98+%) Clofibric acid C10H11ClO3 (99.0%) were purchased from J.T. Baker, Alfa Aesar, SIGMA-ALDRICH respectively.
MIL-101(Cr) was synthesized by using chromium(III) nitrate nonahydrate Cr(NO3)3.9H2O, terephtalic acid (TPA) and deionized water following the previous works [50,51]. Teflon-lined autoclave bomb having working values of 250 ml was used in the synthesis of MIL-101(Cr). At the beginning of the synthesis method, 4.0 g or 10mmol chromium(III) nitrate nonahydrate [Cr(NO3)3.9H2O] and 1.66 g (10mmol) terephtalic acid (TPA) was compounded with 40 mL deionized water. Then the mixture was sonicated shortly where dark blue color suspension was its consequence. After that the suspension was poured into a Teflon-lined autoclave bomb. Then the autoclave bomb was placed in an electric oven at 220°C temperature for 17 hours. After cooling down to the room temperature green-color MOF solids were separated from water (15 minute, 3134xg). Then MOF solids were washed with water, methanol and by contribution acetone in serial. MOF suspension in acetone was separated by centrifugation. Then the solids were entrusted in 40 mL N, N-dimethylformamide to remove the unreacted terepthalic acid (TPA). Then the suspension was sonicated and then the mixture was kept at 100°C overnight [52, 53]. Then the resulting solids were separated and the separated solids were washed again with methanol and then with acetone. After that the solids were dried overnight at 100°C temperature. After being dried the obtained solids were called MIL-101(Cr).
By the functionalization method –OH group been added with MIL-101(Cr). MIL-101(Cr) which been functionalized with –OH group, was named MIL-101-OH. MIL-101-OH was synthesized following the grafting utilizing method which already been reported previously [32,46]. At first MIL-101(Cr) was dehydrated (-H2O) at 150°C temperature for 12 hour in a vacuum oven.
After the specific time 0.6 g of dehydrated MIL-101(Cr) was suspended in 60mL anhydrous toluene. Then the suspension was placed in a round-bottom flask which was embellished with a reflux condenser and a magnetic stirrer. Then 2mmol of ethanolamine (ETA) was put into the suspension. Then the mixture was incessantly stirred and refluence (reflux) for 12 hours. After that the acquired solids were cooled to room temperature. After being cold the solids were separated and washed either with deionized water or with ethanol. Then the solids were dried at room temperature. After being dried the obtained solids were called modified MIL-101(Cr) i.e. MIL-101-OH.
XRD or X-ray powder diffraction [SWXD (D-MAX/2500-PC), Cu-Kα radiation] has been used to determine X-ray powder diffraction pattern, nitrogen adsorption of synthesized MOFs had been done at 77 K (-196, 15°C) with surface area/porosity analyzer [BELSORP- max] after evacuation for 12 hours at 150°C and BET equation had been used to calculate the surface area and pore size distributionof MOFs. FTIR or Fourier-transform infrared spectroscopy analysis of synthesized MOFs had been done with FTIR spectroscopy [Vertex 80v, Hyperion 2000, ATR].The intended concentrations of clofibric acid solutions were prepared by dissolving clofibric acid in deionized water. Standard solutions of clofibric acid were made and the concentrations of clofibric acid’s standard solutions were 10ppm-50ppm and calibration curve was made. The concentrations of clofibric acid’s solutions were delimited at 227 nm wavelength using a spectrophotometer.
Due to the bluffy solubility of clofibric acid in water; so for adsorption experiment the initial concentrations of clofibric acid’s solutions were 30ppm-150ppm and PH of each solution were between 4.7~4.9. At the starting of adsorption experiment, the specific adsorbents (i.e. MIL-101(Cr) and MIL-101-OH) which were selected for this research work were dried at 100°C in an oven overnight and then the adsorbents were stored in a desiccator for further using. For adsorption experiment 5.0 mg of adsorbents (MIL-101(Cr)) were put in an exact concentration of 50mL clofibric acid solution. After that the clofibric acid solution bearing MIL-101(Cr) (adsorbent) was mixed together with a magnetic stirring for a stable time, i.e. 30min-12hours at the temperature (20-25°C). The solution of clofibric acid was separated with a syringe filter with 0.45µ, pore size (PTFE, hydrophobic); after the adsorption experiment for a pre- defined time (fixed time). After the filtration of the clofibric acid solution from MIL-101(Cr) (during adsorption experiment) with syringe filter, clofibric acid concentration was measured with the UV spectrophotometer. For the confirmation of the initial concentration of clofibric acid before adsorption experiment, UV assessment was wielded after the dilution of the clofibric acid solution.To calculate the amount of clofibric acid adsorbed over MIL-101(Cr), on the duration of times the equation (1) been used for each adsorption experiments.

Where, C0 was the initial concentration of pollutant solution, C t was concentration of pollutant in liquid-phase at different time or when time is t, v volume of the solution which has been used in adsorption experiment, w(g) was weight of the MOF or adsorbents, qt was the amount of adsorbed (mg/g).
RESULTS AND DISCUSSION
Characterization
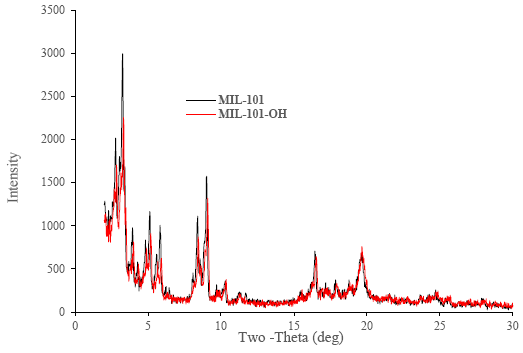
The XRD or powder diffractometer patterns show that MIL-101(Cr) and MIL-101-OH were successfully prepared [47, 54]. Again, as MIL-101-OH has been synthesized by the funtionalization of MIL-101(Cr) but XRD patterns also showed that the crystal structure of MIL-101(Cr) and MIL-101-OH also does not show variation. Figure 4 containing MIL-101s (i.e. MIL-101 and MIl-101-OH) XRD spectra results together. FTIR result in Figure 5, band at 1189 cm-1 and at 1115 cm-1 were obtained, which were corresponded with C-O bond stretching. Again from the FTIR result of MIL-101-OH band at 1165 cm-1 was obtained because of C-N bond stretching [75] which indicated that the synthesis of MIL-101s were successful. Nitrogen adsorption isotherms and BET surface area and pore size distribution of synthesized MOFs (MIL-10(Cr) and MIL-101-OH) was on the order of MIL-101(Cr) > MIL-101-OH. The averaged pore size of MIL-101(Cr) & MIL-101-OH were 2.0533 nm and 2.221 nm respectively. BET surface area of MIL-101(Cr) & MIL-101-OH were 2234.4 m2 g-1 and 1551.5 m2 g-1 respectively. The surface area, pore size distribution and porosity of MIL-101-OH have been decreasing because additional volumes of organic minutiae had been used for funtionalization.
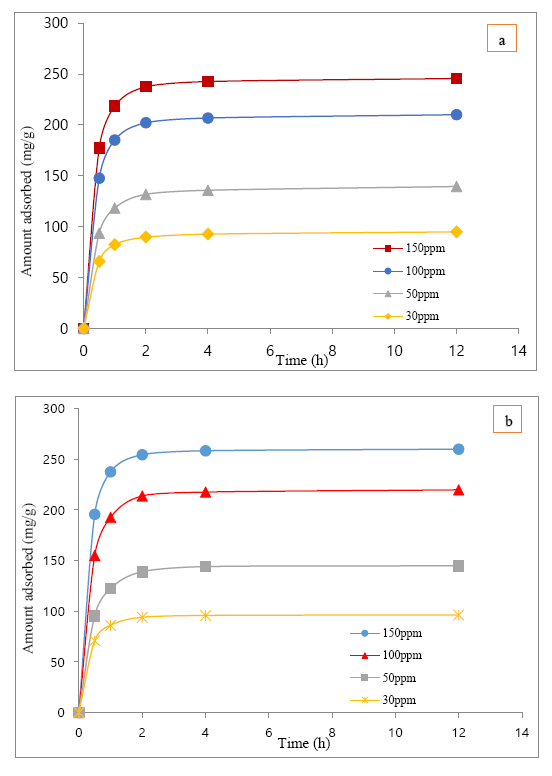
The adsorption of clofibric acid over MIL-101(Cr) and MIL-101-OH were obtained on the basis of time at a variable concentrations (30-150ppm) and for each points 2-3 experiment had been performed, during all the batch experiment the PH of clofibric acid was 4.7~4.9, and in Figure 6 (a, b) the average value of that adsorption results over MIL-101(Cr) and MIL-101-OH were delineated.
[The adsorbed amount of clofibric acid was based on the unit weight of MIL-101(Cr) & MIL-101-OH (adsorbent) and the initial concentrations of clofibric acid’s solution during this experiment were 150ppm, 100ppm, 50ppm, 30ppm]
The adsorption kinetics of clofibric acid over MIL-101(Cr) and MIL-101-OH at different initial concentrations (i.e. 30ppm-150ppm) had been calculated and from both Pseudo-first-order & Pseudo-second-order kinetics plot the the best-fitting kinetic model for experimental data was Pseudo-second-order determined by the value of correlation coefficients i.e. r². So the value of qe, k2 and r² (i.e. correlation factor) obtained from the plots t/qt against t (time) from pseudo-second-order kinetics in Table 1 for different initial concentration clofibric acid’s adsorption over MIL-101(Cr) & MIL-101-OH have been given.
|
MOFs |
Initial concentration |
qe (mg/g) |
k2(g/mg,hour) |
r2 |
|
MIL-101(Cr) |
30ppm |
96.15 |
0.041 |
0.999 |
|
50ppm |
140.85 |
0.043 |
0.9994 |
|
|
100ppm |
212.77 |
0.053 |
0.9996 |
|
|
150ppm |
250 |
0.055 |
0.9997 |
|
|
MIL-101-OH |
30ppm |
97.09 |
0.051 |
0.9995 |
|
50ppm |
147.06 |
0.056 |
0.9996 |
|
|
100ppm |
222.22 |
0.057 |
0.9997 |
|
|
150ppm |
263.16 |
0.065 |
0.9998 |
Table 1: Parameters of pseudo-second-order kinetics for clofibric acid adsorption over MIL-101(Cr) & MIL-101-OH
After the adsorption experiment for 12 hours the adsorption isotherms Figure 7 (a,b) of clofibric acid adsorption over MIL-101(Cr) and MIL-101-OH were obtained. Two adsorption isotherms: Langmuirand Freundlich isotherms were applied in this study in order to fit equilibrium for the adsorption resultsof clofibric acid over MOFs. Linearized form of Langmuir and Freundlich model equation respectivelyto justify best fitted model for the adsorption results of clofibric acid over MIL-101(Cr) and MIL-101-OH.

Where, Ce (mg/L) is the equilibrium concentration of adsorbate solution, qe (mg/g) is amount of adsorbate which been adsorbed, Q0 (mg/g) is the maximum adsorption capacity of adsorbent (Langmuir constant) and b (L/mg) or (L/mol) is known as Langmuir constant, therefore Q0 can be achieved from the integrand of the slop of a Langmuir plot of Ce/qe against Ce.

Where KF and nFreundlich constants andtheabsorptioncapacity ofthe adsorbentisKF(mg/g(L/mg)1/n). A measure of surface heterogeneity or adsorption intensity is being 1/n slope which ranging between 0 to 1 and if the value gets closer to 0 then it became more heterogeneous [55]. While 1/n is above 1 then it indicates cooperative adsorption and if 1/n is below 1 then it indicative of normal Langmuir isotherms.
The correlation coefficient i.e. R² value from Langmuir and Freundlich plot had been obtained using Equation-2 and Equation-3. The R² value of Langmiur plot for clofibric acid adsorption with both MOFs (i.e. MIL-101(Cr) and MIL-101-OH) were higher (0.9998 and 0.9995 respectively) than the R² value (0.9864 and 0.9846 respectively) of Freundlich plot. So, according to the previous work [55] higher R² value of Langmuir plot indicated that the adsorption results of clofibric acid over MIL-101(Cr) and MIL-101-OH can best describe by Langmuir model. Then a Langmuir isotherm was used to calculate the maximum clofibric acid adsorption ability of MIL-101(Cr) and MIL-101-OH.
The results of adsorption experiments with clofibric acid over MIL-101(Cr) and MIL-101-OH in Table 2, which been obtained from Langmuir plot have been presented.
Table 2: Langmuir parameters (plot’s result) of clofibric acid over MOFs (MIL-101(Cr) and MIL-101-OH).
|
MOF(adsorbent) |
b (L/mg) |
Q0 (mg/g) |
r2 |
|
MIL-101(Cr) |
0.018 |
357 |
0.9998 |
|
MIL-101-OH |
0.023 |
385 |
0.9995 |
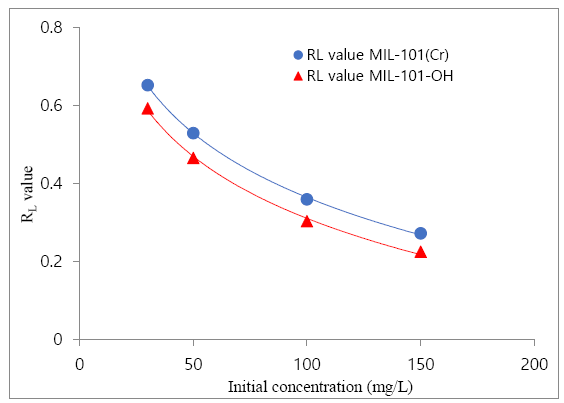
The above table contains clofibric acid adsorption result over MIL-101(Cr) & MIL-101-OH, which has been obtained from the Langmuir plot of clofibric acid’s adsorption result. Langmuir plot was more suitable for the result of clofibric acid adsorption. Maximum adsorption capacity (Q0) of absorption (MIL-101 (Cr) & MIL-101-OH), Langmuir constant (i.e. b) and the r2 value was obtained from the Langmuir plot which has been obtained by using clofibric acid adsorption results (isotherm). For adsorption results further understanding, a dimensionless constant, i.e. RLvalue which known as equilibrium parameter or separation factor [56], was investigated, followed by Equation 4.

The shape of isotherm indicates by the parameter RL, according to the previous work [56] the table about the parameter RL has been given below.
|
RL Value |
Isotherms types |
|
RL>1 |
Unfavorable |
|
RL=1 |
Linear |
|
0<RL<1 |
Favorable |
|
RL=0 |
Irreversible |
The shape of isotherm indicates by the parameter RL, according to the previous work [56]. The obtained RLvalues from the Figure 8 showed that the RLvalue decreased when the initial concentration increased and all RLvalues were from 0 to 1 i.e. 0<RL<1 which indicating the favorable clofibric acid adsorption over MIL-101(Cr) and MIL-101-OH.
The obtained RLvalues showed that all RLvalues were from 0 to 1 i.e. 0<RL<1 which indicating the favorable clofibric acid adsorption over MIL-101(Cr) and MIL-101-OH.The RLvalues of clofibric acid adsorption over MOFs were indicating the adsorption of clofibric acid over MOFs were favorable. Again, after reviewing some previous works of some selected MOFs which has been applied for clofibric acid adsorption from aqueous solution, these results Table 3 had been compared with this research work results.
Table 3: Comparison of clofibric acid previous adsorption results with present research results (This research).
|
MOFs (adsorbent) |
PPCPs (Pharmaceuticals and personal care products) |
Maximum adsorption capacity Q0 (mg/g) |
Previous works (References) |
|
MIL-101(Cr) |
315 (unit weight) |
46 (Hasan, Z. et al.) |
|
|
ED-MIL-101(-NH2) |
347(unit weight) |
46 (Hasan, Z. et al.) |
|
|
AMSA-MIL-101(-SO3H) |
Clofibric acid |
105(unit weight) |
46 (Hasan, Z. et al.) |
|
MIL-101(Cr) |
357(unit weight) |
This research |
|
|
MIL-101-OH |
385 (unit weight) |
This research |
|
|
No research work has been found for clofibric acid adsorption over MIL-101-OH |
|||
Adsorption possible mechanism understanding is important, for chemical separation and efficient removal. The adsorbate, clofibric acid has a functional group such as carboxylic and ether. From the adsorption results study, even though the surface area and pore size of MIL-101-OH was not highest but MIL-101-OH showed highest clofibric acid adsorption capacities compare to MIL-101(Cr). Favorable H-bonding between H of -OH (on MOFs) O of adsorbents (PPCP) and the presence of – COOH group can clearly explain clofibric acid adsorption. This can be explained by the presence of oxygen and carboxyl (–COOH) group in clofibric acid structure. As from a previous work [46], due to the presence of H-bounding (MOFs) and oxygen molecule and carboxyl (–COOH) group (clofibric acid) acid-base interaction or π-complexation (π-π stacking between MIL-101s and clofibric acid bezene ring) took place during the adsorption process. Therefore, MIL-101-OH has higher clofibic acid adsorption capacity, then MIL-101(Cr).
CONCLUSION
In order to remove the pharmaceuticals and personal care products, i.e. clofibric acid from water by the adsorption process with MOFs; MIL-101 (Cr) and its functionalized version (MIL-101-OH) had been used. At first MIL-101(Cr) was synthesized and then it had been functioning successfully by added (-OH) group with MIL-101(Cr) and the functionalized MIL-101(Cr) was called MIL-101-OH. Even though the surface area of MIL-101(Cr) was higher than MIL-101-OH but the adsorption results of MIL-101-OH with clofibric acid were remarkably higher compare to MIL-101(Cr) (on the basis of unit weight). The Q0 value of clofibric acid adsorption over MIL-101-OH was 1.65 times and 1.08 times higher than that of MIL-101(Cr) respectively, and during all the batch experiment the PH of clofibric acid was 4.7~4.9. The adsorption results of clofibric acid over MIL-101(Cr) and MIL-101-OH showed that the PPCPs adsorption capacity of MIL-101-OH was competitively higher than MIL-101(Cr) and MIL-101-OH can be disclosed as an effective MOF, which can be used for the removal of PPCPs as its rapid and higher adsorption capacity compared to other carbonaceous materials and virgin MIL-101(Cr).
ACKNOWLEDGEMENT
This research work was supported by Kumoh National Institute of Technology research fund.
REFERENCES
- United States Environmental Protection Agency, Drinking Water Contaminants.
- Daughton CG, Ternes TA. 1999. Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ. Health Perspect 107: 907-938.
- Mestre AS, PiresJ, NogueiraJMF, 2007. Activated carbons for the adsorption of ibuprofen, Carbon 45: 1979-1988.
- Bulloch DN, 2015. Occurrence of Halogenated Transformation Products of Selected Pharmaceuticals and Personal Care Products in Secondary and Tertiary Treated Wastewaters from Southern California. Environ. Sci. Technol 49: 2044-2051.
- Bu Q, Wang B, Huang J, 2013. Pharmaceuticals and personal care products in the aquatic environment in China: A review. J.Hazard. Mater 262: 189-211.
- Jung C, 2015. Removal of endocrine disrupting compounds, pharmaceuticals, and personal care products in water using carbon nanotubes: A review. J. Ind. Eng. Chem 27: 1-11.
- Dong S, 2015. Recent developments in heterogeneous photocatalytic water treatment using visible light-responsive photocatalysts: a review. RSC Adv 5: 14610-14630 .
- Richardson SD, Ternes TA. 2014. Water Analysis: Emerging Contaminants and Current Issues. Anal. Chem. 86: 2813-2848.
- Gadipelly C, 2014. Pharmaceutical Industry Wastewater: Review of the Technologies for Water Treatment and Reuse. Ind. Eng. Chem Res 53: 11571-11592.
- Wu X, Conkle JL, Erns F, 2014. Treated Wastewater Irrigation: Uptake of Pharmaceutical and Personal care Products by Common vegetables under field Conditions. Environ. Sci. Technol 48: 11286-11293.
- Miller EL, Nason SL, Karthikeyan KG, 2016. Root Uptake of Pharmaceutical and Personal Care Product Ingredients. Environ. Sci. Technol 50: 525-541.
- Tanoue R, 2015. Uptake and Tissue Distribution of Pharmaceuticals and Personal Care products in Wild Fish from Treated-Wastewater-Impacted Streams. Environ. Sci. Technol 49: 11649-11658.
- Subedi B, 2012. Occurrence of Pharmaceuticals and Personal Care Products in German fish tissue: A national study. Environ. Sci. Technol 46: 9047-9054.
- Rivera-Jimenez SM, Mendez-Gonzalez S, Hernandez-Maldonado A. 2010. Metal (M = Co2+, Ni2+ and Cu2+) grafted mesoporous SBA-15: Effect of transition metal incorporation and pH conditions on the adsorption of Naproxen from water. Microporous Mesoporous Mater 132: 470-479.
- Rivera-Jimenez SM, Hernandez-Maldonado AJ. 2008. Nickel (II) grafted MCM-41: A novel sorbent for the removal of naproxen from water. Microporous Mesoporous Mater. 116: 246-252.
- Esplugas S, Bila DM, Krause LGT, 2007. Ozonation and advanced oxidation technologies to remove endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) in water effluents. J. Hazard. Mater 149: 631-642.
- Klavarioti M, Mantzavinos D, Kassinos D. 2009. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Env. Int 35: 402-417.
- HasanZ, JeonJ, JhungSH. 2012. Adsorptive removal of naproxen and clofibric acid from water using metal-organic frameworks, J. Hazard Mater 210: 151-157.
- Reynel-Avila HE, Mendoza-Castillo DI, Bonilla-Petriciolet A, 2015. Assessment of naproxen adsorption on bone char in aqueous solutions using batch and fixed-bed processes. J. Mol. Liq 209: 187.
- Furukawa H, Cordova KE, O’Keeffe M, 2013. The Chemistry and Applications of Metal-Organic Frameworks. Science 341: 1230444.
- Wu H, Gong Q, Olson DH, 2012. Commensurate Adsorption Of Hydrocarbons and Alcohols in Microporous Metal Organic Frameworks. Chem. Rev. 112: 836-868.
- Yang Q, Liu D, Zhong C, 2013. Development of Computational Methodologies for Metal-Organic Frameworks and Their Application in Gas Separations. Chem. Rev. 113: 8261-8323.
- DeCoste JB, Peterson GW. 2014. Metal-Organic Frameworks for Air Purification of Toxic Chemicals. Chem. Rev. 114: 5695-5727.
- Barea E, Montoro C, Navarro JAR. 2014. Toxic Gas Removal Metal-Organic Frameworks for the Capture and Degradation of Toxic Gases and Vapours. Chem. Soc. Rev 43: 5419-5430.
- Jhung SH, Khan NA, Hasan Z. 2012. Analogous Porous Metal-Organic Frameworks: Synthesis, Stability and Application in Adsorption. CrystEngComm 14: 7099-7109.
- Van de Voorde B, Bueken B, Denayer J, 2014. Adsorptive Separation on Metal-Organic Frameworks in the Liquid Phase.Chem. Soc. Rev 43: 5766-5788.
- Silva P, Vilela SMF, Tome JPC, 2015. Multifunctional metal-organic frameworks: from academia to industrial applications. Chem. Soc. Rev 44: 6774-6803.
- Canivet J, Fateeva A, Guo Y, 2014. Water adsorption in MOFs: fundamentals and applications. Chem. Soc. Rev 43: 5594-5617.
- Zhou Z, Hartmann M. 2013. Progress in enzyme immobilization in ordered mesoporous materials and related applications. Chem. Soc. Rev 42: 3894-3912.
- Zhang S, 2015. Totally Phospholipidic Mesoporous Particles. J. Phys. Chem. C 119: 7255- 7263.
- Vinu, A. & Ariga, K. 2013. New ideas for mesoporous materials. Adv. Porous Mater 1: 63-71.
- Hwang YK, 2008. Amine grafting on coordinatively unsaturated metal centers of MOFs: consequences for catalysis and metal encapsulation. Angew. Chem. Int. Ed 47: 4144-4148.
- de VoordeBV, Bueken B, Denayer J, 2014. Adsorptive separation on metal-organi frameworks in the liquid phase, Chem. Soc. Rev 43: 5766-5788.
- HasanZ, Jun JW, Jhung SH. 2015. Sulfonic acid-functionalized MIL-101(Cr):Anefficient catalyst for esterification of oleic acid and vapor- phase dehydration of butanol. Chem. Eng. J 278: 265-271.
- KhanNA, HasanZ, JhungSH. 2013. Adsorptive removal of hazardous materials using metal- organic frameworks (MOFs): a review, J. Hazard. Mater: 444-456.
- WuH, GongQ, OlsonDH, 2012. Commensurate adsorption of hydrocarbons and alcohols in microporous metal-organic frameworks, Chem. Rev 112: 836-868.
- BareaE, MontoroC, NavarroJAR. 2014. Toxic gas removal- metal-organic frameworks for the capture and degradation of toxic gases and vapours, Chem. Soc. Rev 43: 5419-5430.
- Khan NA, Hasan Z, Jhung SH. 2013. Adsorptive removal of hazardous materials using metal-organic frameworks (MOFs): A review. J. Hazard. Mater 244: 444-456.
- Hasan Z, Jhung SH. 2015. Removal of hazardous organics from water using metal-organic frameworks (MOFs): Plausible mechanisms for selective adsorptions. J. Hazard. Mater 283: 329-339.
- Ahmed I, Jhung SH. 2016. Adsorptive desulfurization and denitrogenation using metal-organic frameworks. J. Hazard. Mater. 301: 259-276.
- JiangJQ, YangCX, YanXP.Zeolitic 2013. Imidazolate Framework-8 for Fast Adsorption and Removal of Benzotriazoles from Aqueous Solution.ACS Appl. Mater. Interfaces 5: 9837-9842.
- LinKYA,LeeWD. 2016. Self-assembled magnetic graphene supported ZIF-67asa recoverable and efficient adsorbent for benzotriazole.Chem.Eng. J284: 1017-1027.
- Jun JW, 2015. Effect of Central Metal Ions of Analogous Metal-Organic Frame works on Adsorption of Organoarsenic Compounds from Water: Plausible Mechanism of Adsorption and Water Purification.Chem.Eur.J21: 347-354.
- Bhadra BN, Cho KH, Khan NA, 2015. Liquid-Phase Adsorption of Aromatics over a Metal-Organic Framework and Activated Carbon: Effects of Hydrophobicity/Hydrophilicity of Adsorbents and Solvent Polarity. J. Phys. Chem C 119: 26620-26627.
- Hasan Z, Jeon J, Jhung SH. 2012. Adsorptive removal of naproxen and clofibric acid from water nusing metal-organic frame works. J. Hazard. Mater. 209: 151-157.
- Hasan Z, Choi EJ, Jhung SH. 2013. Adsorption of naproxen and clofibric acid over a metal-organic frame work MIL-101 functionalized with acidic and basic groups. Chem. Eng. J. 219: 537-544.
- Ferey G, 2005. A chromium terephthalate-based solid with unusually large pore volumes and surfacearea. Science 309: 2040-2042.
- HaqueE, 2010. Adsorptive removal of methyl orange from aqueous solution with metal-organic frameworks, porous chromium benzenedicarboxylates. J. Hazard. Mater 181: 535-542.
- Hasan Z, Jun JW, Jhung SH. 2015. Sulfonic acid-functionalized MIL-101(Cr): An efficient catalyst forest erification of oleic acid and vapor-phase dehydration of butanol. Chem. Eng. J. 278: 265-271.
- LevB, YingD., HuimengWV, 2012. Chromium (III) erephthalate metalorganic frame work (MIL-101): HK-Free Synthesis, Structure, and catalytic properties, Chem. Mater 24: 1664-1675.
- KhanNA, Jhung SH, 2012. Low-temperature loading ofCu+species over porous metal-organic frame works (MOFs) and adsorptive desulfurization with Cu+ loaded MOFs, J. Hazard.238: 180-185.
- HaqueE, KhanNA, Lee JE, 2009. Facile purification of porous metaltere phthalates with ultrasonic treatment in the presence of amides, Chem. Eur. J 15: 11730-11736.
- Khan NA, Kang IJ, Seok HY, et al. Jhung, S. H. 2011. Facilesynthesisofnano-sizedmetal-organic Framework, chromium-benzenedicarboxylate, MIL-101, Chem. Eng. J 166: 1152-1157.
- Khan NA, Jhung SH. 2010. Phase-Transition and Phase-Selective Synthesis of Porous Chromium Benzenedicarboxylates. Cryst. Growth Des10: 1860-1865.
- Tan IAW, Ahmad AL, Hameed BH. 2009. Adsorption isotherms, kinetics, desorption studies of 2,4,6-trichlorophenol on oil palm empty fruit bunch-based activated carbon, J.Hazard. Mater 164: 473-482.
- Weber TW, Chakkravorti RK. 1974. Poreand diffusion models for fixed-bed adsorbers AlChE J.20: 228-238.




 Impact Factor: * 4.1
Impact Factor: * 4.1 Acceptance Rate: 75.32%
Acceptance Rate: 75.32%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 