Song Dialects of Wrens (Troglodytes troglodytes) in Three Districts in Japan
Hisataka Fujimoto1*, Taku Hasegawa2
1Department of Anatomy, Kawasaki Medical School, 577 Matsushima, Kurashiki, 701-0192 Okayama, Japan
2Laboratory for Imagination and Executive functions, RIKEN Center for Brain Science, 2-1 Hirosawa, Wakoshi, Saitama 351-0198, Japan
*Corresponding Author: Hisataka Fujimoto, Department of Anatomy, Kawasaki Medical School, 577 Matsushima, Kurashiki, 701-0192 Okayama, Japan.
Received: 22 June 2022; Accepted: 28 June2022; Published: 30 June 2022
Article Information
Citation:
Hisataka Fujimoto, Taku Hasegawa. Song Dialects of Wrens (Troglodytes troglodytes) in Three Districts in Japan. International Journal of Plant, Animal and Environmental Sciences 112 (2022): 096-105.
View / Download Pdf Share at FacebookAbstract
Vocal signals, including spoken languages and birdsongs, are composed of a finite number of acoustic elements, including repetition, which is composed of a combination of these elements linked together by syntactic rules. While songbirds follow specie-specific syntactical rules, they often show regional dialects presumably due to the acquisition process of their songs. Many previous studies have examined migratory birds that seasonally traveled abroad; thus, it is unclear whether the dialects are restricted within the geometric regions. Here, we examined the songbird wren (Troglodytes troglodytes), which is a sedentary songbird with one of the most complex songs, in three areas: Mount Aso (Kumamoto Prefecture, Japan), Mount Ishizuchi (Ehime Prefecture, Japan), and Mount Daisen (Tottori Prefecture, Japan). We examined ten birds in each area and identified each bird using binoculars, and syntactic differences associated with geometric regions, or dialects, were investigated. The male wrens in the Mount Daisen dialect sang with little or no continuation of the different types of trills. On the other hand, male wrens in Mount Aso and Mount Ishiduchi frequently and continuously sang two or more different types of trills. All birds used trills and whistles. Our results suggest that sedentary songbirds, in addition to migratory birds, have dialects in song syntactic structure.
Keywords
<p>Songbird; Dialect, Repetition; Wren; Troglodytes troglodytes; Syllable; Whistle; Trill</p>
Article Details
1. Introduction
Vocalization of complex and stereotyped phonons requires precise neural control, which is mediated by the modulation of excitatory and inhibitory activities in higher-order neural circuits. The phenomenon of "dialect" variation in birdsongs, appearing as a transient or persistent difference in the predominant song type between populations of the same species, serves as a focus for attention in the discussion of diverse topics such as speciation [1,2], learning [3], and mechanisms of social communication [4]. The wren (Troglodytes troglodytes), which is a sedentary Japanese bird that remains in Japan for all seasons without emigrating, affords suitable example cases of such "dialect" variation among Japanese birds. Such vocalizations are regulated by activity in higher cortical nuclei. HVC (used as a proper name) [5,6] is analogous to the supplementary motor cortex in mammals, whereas AreaX is homologous to the supplementary striatum in mammals [7-9]. Similar to the cortico-basal gangalia pathway in mammals, HVC-AreaX in songbirds constitute the anterior forebrain pathway, necessary for birdsong acquisition [10]. HVCX neurons, namely AreaX projection HVC neurons, which innervate the basal ganglia area X [11], are involved in vocal plasticity and syntactical motor signals for song production [12,13]. To the best of our knowledge, not many species have been proven to have “dialects” in their songs [14,15]. To determine whether the difference in the dialect of songs is due to acquired learning or congenital imprinting, investigations are needed to examine what kind of song a a young bird sings after being raised in isolation from the parent bird. In our current hypothesis, regional differences in songs are mostly due to acquired learning [16,17]. Previous studies that examined the differences in songs of white-crowned sparrow across populations showed various differences in phrases, clauses, and their order [18,19]. These previously examined songbird species are migratory birds because the majority of their population, including the songbirds, are migratory. Currently, it is unclear whether dialects are restricted within geometric regions. Here, we examined the songbird wren (Troglodytes troglodytes), a sedentary songbird with one of the most complex songs. We examined wrens in three areas: Mount Aso (Kumamoto Prefecture, Japan), Mount Ishizuchi (Ehime Prefecture, Japan), and Mount Daisen (Tottori Prefecture, Japan).
2. Materials and Methods
2.1. Songbird animals and data collection
All procedures were approved (approval number: 16-080) by the Kawasaki Medical School Animal Care and Use Committee in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals for experiments. We examined 10 identical wrens (Troglodytes troglodytes) at each fieldwork location. We recorded songs of 10 wrens each on Mount Aso (Kumamoto Prefecture), Mount Ishizuchi (Ehime Prefecture), and Mount Daisen (Tottori Prefecture). Each bird per area was identified using binoculars (10 × 42 LISWP; Canon, Japan). Sound source localization was possible using four microphones (MPM-1000, Marantz Professional, Japan) and by analyzing the differences among the four recordings. To avoid the possible seasonal change in syntax, all of the recordings were performed between June 4th to 13th 2022. The recorded data were computerized, and a sound-activated recording system was used to detect and digitize the song (OCTA-CAPTURE, Roland, Japan). Recorded songs were digitally filtered at 0.3–2 kHz for offline analysis.
2.2. Song analysis
Wrens’ (Troglodytes troglodytes) songs consist of acoustic elements arranged in specific sequences. For description and analysis, syllable, or individual acoustic elements were separated from each other by at least 1 ms of silence [21-23]. Labeling and analysis of syllables were identical to those in previously published studies [20,23,24]. All syllables were initially identified using a linear support vector machine [20] and confirmed and corrected as needed by researchers in a blinded manner. Briefly, following amplitude-based syllable segmentation, syllables were manually labeled based on visual inspection of spectrograms using custom-written MATLAB software (T.H.). Acoustic features (syllable duration, mean frequency, mean amplitude, spectral entropy, spectrotemporal entropy, and amplitude entropy) [21, 25-27] for each syllable in the birds’ songs were analyzed and used to determine the syllable type using a linear support vector machine. Following automatic syllable identification, all results were blinded by researchers and used to quantify the observed changes in syllable structure. Trills were defined as more than three repetitions of the same syllable structure.
3. Results
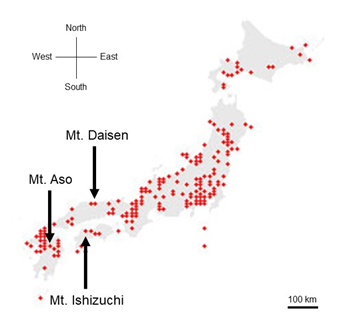
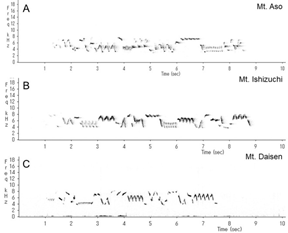
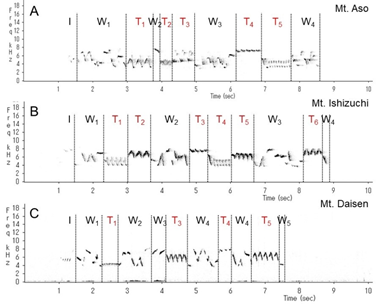
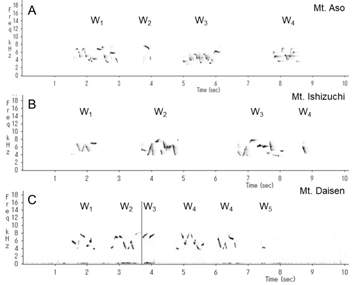
This wren (Troglodytes troglodytes) species was distributed throughout almost all of Japan (Figure 1). In each of the three regions recorded, the song began with an introductory note succeeded by the whistle verse (Figures 2 and 3). The next verse was another whistle or trill, and the phrases in this verse were distinctly different between dialects (Figures 2 and 3). Furthermore, depending on the region, some dialects had both trills and up to four to six whistles.
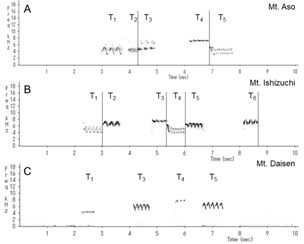
We identified and classified all the syllables (Figure 3) in the songs of 10 wrens in Mount Aso (Kumamoto Prefecture), 10 wrens in Mount Ishizuchi (Ehime Prefecture), and 10 wrens in Mount Daisen (Tottori Prefecture). We analyzed more than 20 bouts of each wren. Wrens in Mount Daisen rarely sang consecutive trill syntactical sequences. On the other hand, wrens in Mount Aso and Mount Ishizuchi, both located far west of Mount Daisen, sang consecutive trill syntactical sequences (Figure 3). We verified the identified syllable sequences using acoustic features of syllables (Figures 4 and 5). All trills consisted of more than three repetitions of the same syllable structure (Figure 4). Almost all other elements contained whistles with large frequency moderations during bouts that were relatively longer than trill syllable elements (Figure 5).
Figure1: The locations of three recorded locations. The songs of 10 wrens in Mount Aso (in Kumamoto Prefecture), the song of 10 wrens in Mount Ishizuchi (in Ehime Prefecture) and the song of 10 wrens in Mount Daisen (in Tottori Prefecture) were recorded. The red points were locations where wren activities were identified between 2016 and 2021 (from the Ministry of the Environment of Japan). The Scale bar, 100km.
4. Discussion
The fact that song types vary among the western regions of Japanese populations of the wrens (Troglodytes troglodytes) was suggested in this work. The wren (Troglodytes troglodytes) is a sedentary songbird with one of the most complex songs. We examined song dialects in wrens in three areas: Mount Aso (Kumamoto Prefecture), Mount Ishizuchi (Ehime Prefecture), and Mount Daisen (Tottori Prefecture). Many songbird species are regarded to have dialects in syntactic rules [1-3, 14-16], which are supposedly based on the characteristics of these songbirds acquiring learning of their song [4-8, 11-13]. Many previous studies have examined migratory birds who seasonally traveled abroad; thus, it is unclear whether the dialects are restricted within geometric regions [16-18]. It was not until the advent of the sound spectrogram that an unambiguous analysis of these interpopulation variations could be examined. Using this technique, Marler and Tamura [1] found that each male's song type and syntax remain constant over time and that there are constant and distinctive differences among wrens in different regions in California. Therefore, they regarded songs of different populations as dialects. Japan is almost the same size as California, and the regional distance examined in this study resembles that in their reports. It is fundamentally important to determine whether dialects are confirmed in a non-immigratory sedentary songbird. In mammals, brain regions, including the basal ganglia, are believed to play a role in habitual syntactical movements. In rodents, striatal neurons encode the initiation and termination of well-trained repetitive behaviors [28,29]. In addition, abnormal function in basal ganglia disorders induces repeated movements. Basal ganglia impairment results in motor or speech disorders in humans, including Parkinson’s disease [30]. These previous studies indicate that the basal ganglia are responsible for executing repetitive movements, and GABAergic intervention induces unintended repetition [31]. Our study demonstrated that syntactic rules in songbird vocalization might be acquired by learning, considering that it is suggested in a domestic sedentary songbird such as wren (Troglodytes troglodytes). Earlier work suggests that a major function of songs in wrens is territorial defense. A territorial male counters the song of the other male and is sometimes accompanied by chase and attack in response to visual or auditory presentation of another male [2,3,17,18]. A study of vocal responses of captive male white-crowned sparrows that analyzed songs of the same and another species revealed a high probability of a hostile response [32]. The main limitations of the present study were its retrospective design and small sample size. Our results require verification in a future prospective randomized blinded study in a wide area, presumably in all prefectures in Japan. The data here suggest that examining a large cohort of songbird species will confirm that the suspicion of dialects leads to a profound understanding of dialects in songbirds and other species, including humans, if possible.
5. Conclusion
Male wrens can be expected to vocalize in the context of different dialects of their species. Wrens are sedentary songbirds with one of the most complex songs; thus, it can be suggested that dialects in songbirds are acquired through vocalization learning during the juvenile phase.
Acknowledgements
We thank K. Hamaguchi, K. Abe, M. Fujishiro, and R. Matsui for discussions, advice, and criticism, which greatly benefited this project. This study was supported in part by JSPS KAKENHI grants 25861630 and 16K18378 (to H.F.).
Funding statement
This study was supported in part by JSPS KAKENHI grants 25861630 and 16K18378 (to H.F.).
Conflicts of interest
The authors declare no conflicts of interest regarding the publication of this article.
Ethics approval statement
All procedures were approved (approval number: 16-080) by the Kawasaki Medical School Animal Care and Use Committee, in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals for experiments.
Data availability statement
The data supporting this study's findings are available from the corresponding author, HF, upon reasonable request.
References
- Narler P, Tamura M. Song "dialects" in three populations of White-crowned Sparrows. The Condor 64 (1962): 368-377.
- Petrinovich L, Patterson The responses of white?crowned sparrows to songs of different dialects and subspecies. Zeitschrift für Tierpsychologie 57 (1981) 1-14.
- Konishi M. The role of auditory feedback in the control of vocalization in the white-crowned sparrow. Zeitschrift fur Tierpsychologie 22 (1965): 770-783.
- Abe K, Matsui S, Watanabe D. Transgenic songbirds with suppressed or enhanced activity of CREB transcription factor. Proceedings of the National Academy of Sciences of the United States of America 112 (2015): 7599-7604.
- Aronov D, Andalman AS, Fee MS. A specialized forebrain circuit for vocal babbling in the juvenile songbird. Science (New York, N.Y.) 320 (2008): 630-634.
- Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. The Journal of comparative neurology 165 (1976): 457-486.
- Brainard MS, Doupe AJ. Translating birdsong: songbirds as a model for basic and applied medical research. Annual review of neuroscience 36 (2013): 489-517.
- Pfenning AR, Hara E, Whitney O, et al. Convergent transcriptional specializations in the brains of humans and song-learning birds. Science (New York, N.Y.) 346 (2014): 1256846.
- Reiner A, Perkel DJ, Bruce LL, et al. Revised nomenclature for avian telencephalon and some related brainstem nuclei. The Journal of comparative neurology 473 (2004): 377-414.
- James LS, Davies R, Mori C, et al. Manipulations of sensory experiences during development reveal mechanisms underlying vocal learning biases in zebra finches. Developmental neurobiology 80 (2020): 132-146.
- Nottebohm F, Kelley DB, Paton JA. Connections of vocal control nuclei in the canary telencephalon. The Journal of comparative neurology 207 (1982): 344-357.
- Fujimoto H, Hasegawa T, Watanabe D. Neural coding of syntactic structure in learned vocalizations in the songbird. The Journal of neuroscience 31 (2011): 10023-10033.
- Fujimoto H, Ohgomori T, Abe K, et al. Time-dependent localization of high- and low-sulfated keratan sulfates in the song nuclei of developing zebra finches. The European journal of neuroscience 42 (2015): 2716-2725.
- Marler P, Tamura M. culturally transmitted patterns of vocal behavior in sparrows. Science (New York, N.Y.) 146 (1964): 1483-1486.
- Liu WC, Kohn J, Szwed SK, et al. Human mutant huntingtin disrupts vocal learning in transgenic songbirds. Nature neuroscience 18 (2015): 1617-1622.
- Marler P. Birdsong: The acquisition of a learned motor skill. Trends in Neurosciences 4 (1981) 88-94.
- Tian LY, Brainard MS. Discrete Circuits Support Generalized versus Context-Specific Vocal Learning in the Songbird. Neuron 96 (2017): 1168-1177.
- MM Milligan, J Verner. Inter-populational song dialect discrimination in the white-crowned sparrow. The Condor 73 (1971): 208-213.
- Prather JF, Peters S, Nowicki S, Mooney R. Precise auditory-vocal mirroring in neurons for learned vocal communication. Nature 451 (2008): 305-310.
- Tachibana RO, Oosugi N, Okanoya K. Semi-automatic classification of birdsong elements using a linear support vector machine. PloS One 9 (2014): e92584.
- James LS, Sakata JT. Vocal motor changes beyond the sensitive period for song plasticity. Journal of neurophysiology 112 (2014): 2040-2052.
- Okanoya K, Yamaguchi A. Adult Bengalese finches (Lonchura striata var. domestica) require real-time auditory feedback to produce normal song syntax. Journal of neurobiology 33 (1997): 343-356.
- Sakata JT, Brainard MS. Online contributions of auditory feedback to neural activity in avian song control circuitry. The Journal of neuroscience 28 (2008): 11378-11390.
- Warren TL, Tumer EC, Charlesworth JD, et al. Mechanisms and time course of vocal learning and consolidation in the adult songbird. Journal of neurophysiology 106 (2011): 1806-1821.
- Piristine HC, Choetso T, Gobes SM. A sensorimotor area in the songbird brain is required for production of vocalizations in the song learning period of development. Developmental neurobiology 76 (2016): 1213-1225.
- Sakata JT, Brainard MS. Real-time contributions of auditory feedback to avian vocal motor control. The Journal of neuroscience : the official journal of the Society for Neuroscience 26 (2006): 9619-9628.
- Wohlgemuth MJ, Sober SJ, Brainard MS. Linked control of syllable sequence and phonology in birdsong. The Journal of neuroscience : the official journal of the Society for Neuroscience 30 (2010): 12936-12949.
- Jin X, Costa RM. Start/stop signals emerge in nigrostriatal circuits during sequence learning. Nature 466 (2010): 457-462.
- Martiros N, Burgess AA, Graybiel AM. Inversely Active Striatal Projection Neurons and Interneurons Selectively Delimit Useful Behavioral Sequences. Current biology 28 (2018): 560-573.e5.
- Bocanegra Y, García AM, Pineda D, et al. Syntax, action verbs, action semantics, and object semantics in Parkinson's disease: Dissociability, progression, and executive influences. Cortex 69 (2015): 237-254.
- Bronfeld M, Yael D, Belelovsky K, et al. Motor tics evoked by striatal disinhibition in the rat. Frontiers in systems neuroscience 7 (2013): 50.
- Milligan M. Vocal responses of white-crowned sparrows to recorded songs of their own and another species. Animal Behaviour 14 (1966): 356-361.


 Impact Factor: * 4.1
Impact Factor: * 4.1 Acceptance Rate: 75.32%
Acceptance Rate: 75.32%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 