Technologies and Challenges to Recover Energy Products from Carbohydrate Rich Effluents: A Mini Review
Dolly Kumari1, Ravi Kant Bhatia2, Radhika Singh*
1*Biohydrogen Production Lab, Department of Chemistry, Faculty of Science, Dayalbagh Educational Institute, Dayalbagh, Agra, 282005, India
2Department of Biotechnology Himachal Pradesh University, Summer Hill, Shimla-171005 (H.P.) INDIA
*Corresponding Author: Radhika Singh, Biohydrogen Production Lab, Department of Chemistry, Faculty of Science, Dayalbagh Educational Institute, Dayalbagh, Agra, 282005, India
Received: 2 July 2021; Accepted: 14 July 2021; Published: 20 July 2021
Article Information
Citation:
Dolly Kumari, Ravi Kant Bhatia, Radhika Singh. Technologies, and Challenges to Recover Energy Products from Carbohydrate Rich Effluents: A Mini Review. International Journal of Plant, Animal and Environmental Sciences 11 (2021): 423-442.
View / Download Pdf Share at FacebookAbstract
In present a large variety of effluents is available which is the result of exhaustive use of fresh water forms and human activities. The energy generation from carbohydrate rich effluents is a very fruitful approach in the present era when energy demand is enhancing day by day with population growth. Domestic as well as industrial discharge is taking place of fresh water in water resources like rivers, lakes, ponds etc. because of lack of recycling and mixing of drained water to fresh water resources. Various methods and technologies are in use for energy recovery from effluents but most of them are expensive due to high energy consumption or use of expensive chemicals and instruments in the recovery process. This review deals with methods-technologies used in energy recovery process from carbohydrate rich effluents (CREs) and the challenges faced during the energy recovery process. There are various methods including aerobic and anaerobic digestion (AD) for methane and biogas production, bio solid incineration for bioelectricity production and bio-electrochemical system (BES) for bio-hydrogen, methane and bioelectricity generation.
Keywords
<p>Energy recovery; Carbohydrate rich effluents; Bioelectricity; Biohydrogen; Anaerobic digestion; Wastewater treatment</p>
Article Details
Abbreviations:
AD - Anaerobic digestion; DF- Dark fermentation; MFCs- Microbial fuel cells BOD- Biochemical oxygen demand; WW-Wastewater; SS- Sewage sludge; CREs- Carbohydrate rich effluents; MECs- Microbial electrolysis cells; HFCs- Hydrogen fuel cells; BES- Bio-electrochemical systems; MDCs- Microbial desalination cells
1. Introduction
As we all know that water is a basic obligation of life and without water one cannot think about life. But the human deeds and advancement in industrialization ensued in enhanced amount of water along with air pollution. No one is unaffected by air and water pollution, even most of us are facing various diseases due to use of contaminated water. Water being the major constituent of our body, always accountable for our health issues. The major setback of present research is that most of the technologies are limited to lab scale and not fit to use in actual situation. Experiments, which were implemented using synthetic wastewater (WW) or single substrates, are not practically successful for treatment of real contaminated water forms [1]. The basic obligation is the treatment of WW in such a way so that the contaminants recovered, could also be utilised further.
In modern scenario WW cannot said to be a waste but it is a rich source of organic matter along with other nutrients and metal ions due to which it can be utilized for energy production [2]. There are various sources of organic rich WW like domestic WW [3], sewage sludge [4], dairy WW [5, 6], winery WW [7], petha WW [5, 8-11], phenolic WW [12], palm oil mill effluent [13], slaughter house effluent [14], medicine WW [15], glycerol/ethanol rich WW [16]. Most of these WW forms are rich in carbohydrates, nitrogen, phosphorous and other nutrients. The revival of these value-added products seems profitable due to their high cost and need of pollutant free water for drinking purposes. WW being presence of complex constituents, a single pretreatment method is not adequate for its treatment and energy recovery. A number of physical, chemical and biological methods are required separately or combinely for effective and complete WW treatment. It is obvious that the highly recovered products from WW are mainly biohydrogen and bioelectricity but now work is in progress for recovering various metals as well as chemicals using advanced technologies.
The aim of this review is to present a short and concise view on various methods/technologies used for WW treatment and energy recovery from carbohydrate rich effluents (CREs) in the form of value added products along with difficulties faced during the treatment process.
2. Carbohydrate rich effluents (CREs)
2.1 Types of CREs
Discharge from various food processing industries is rich in carbohydrates and its suitable bioprocessing has resulted in recovery of many valuable co-products. There is a large variety of CREs which is potent enough to produce energy products in the form of fuels and bioelectricity. Table 1 represents brief information about CREs, their sources, type of energy products and technology used for recovery process. It can be observed from Table 1 that AD and DF are traditional biological processes for energy recovery in the form of biohydrogen, biogas, methane, and bioethanol whereas bio-electricity generation can be attained by bio-electrochemical processes (MFCs and MECs) and nutrient recovery is accomplished by membrane ultrafiltration, reverse osmosis, forward osmosis, and electro-dialysis [16].
|
Types of effluents |
Sources |
Techniques/methods used |
Recovered Energy products |
References |
|
Cheese whey wastewater (CWW) |
Cheese processing industry |
DF (Thermophilic) |
Hydrogen |
[18] |
|
Distillery spent wash (SPW) |
Sugar industry |
DF |
Biohydrogen |
[19] |
|
Sugar mill effluent (SME) |
Sugar mill |
MFC |
Bioelectricity |
[20] |
|
Starch processing wastewater (SPW) |
Starch industry |
MFC |
Electricity |
[21] |
|
Beer brewery wastewater (BBW) |
Beer industry |
MFC |
Electricity |
[22] |
|
Olive mill wastewater (OMW) |
Olive oil industry |
DF and biological |
Biohydrogen and bioethanol |
[23] |
|
Dairy wastewater (DWW) |
Dairy or milk industry |
AD and DF |
Biohydrogen, biogas & bioethanol |
[5, 6] |
|
Food industry effluents (FIE) |
Food processing industry |
bio-processing technologies |
Biohydrogen |
[24] |
|
Poultry processing wastewater (PPW) |
Poultry processing plant |
Membrane ultrafiltration |
Protein |
[25] |
|
Sewage sludge (SS) |
Wastewater treatment plant |
Hydrothermal carbonization |
Hydrochar, bio-plastic (poly-hydroxy-alkanoate) |
[4, 26] |
|
Sugarcane bagasse hydrolysate (SBH) |
Sugarcane juice industry |
DF |
Biohydrogen |
[27] |
|
Petha wastewater (PWW) separately and with rice straw |
Petha sweet industry |
MFC and AD |
Bio-electricity, biohydrogen, methane and ethanol |
[9, 10, 11] |
|
Domestic wastewater (DW) |
Domestic use |
AD |
Biohydrogen |
[3] |
Table. 1: Carbohydrate rich effluents, their sources, techniques used and recovered energy products.
2.2 General composition of CREs
Generally, chemical composition of WW comprises of 70% organic and 30% inorganic compounds addition with various gases. The organic matter present in WW is mainly in the form of carbohydrates, proteins, and fats. Inorganic mater includes phosphorus, chlorides, heavy metals, nitrogen, sulphur, calcium carbonate alkalinity etc. Various gases commonly dissolved in WW are hydrogen sulphide (H2S), methane (CH4), ammonia (NH3), oxygen (O2), nitrogen (N2) and carbon-dioxide (CO2) [28]. Biologically, WW is encompassed of various micro-organisms from various groups and families i.e., Protista (bacteria, algae, fungi and protozoans), plants (liverworts, seedy plants, ferns and mosses) and animals [29].
2.3 Characterisation values of some CREs
General characterisation of WW can be accomplished by analyses of physical and chemical considerations. Colour, odour and turbidity are physical factors whereas pH, alkalinity, total organic carbon (TOC), biochemical oxygen demand (BOD), chemical oxygen demand (COD), total suspended solids (TSS), total dissolved solids (TDS), conductivity, nitrogen, phosphorus, heavy metals, volatile solids (VS), fats, oil and grease and gases are chemical parameters. Different types of CREs along with their typical physical and chemical properties are listed in Table 2.
|
CREsɸ |
pH |
COD (mg/L) |
BOD5 (mg/L) |
TS (g/L) |
TDS (g/L) |
TSS (g/L) |
VS (g/L) |
Alkalinity (mg/L) |
References |
|
DWW |
3.3 |
4705 |
1800 |
43.62 |
5.3 |
38.32 |
39.84 |
- |
Self |
|
PWW |
12.3 |
5882 |
580 |
5.44 |
5.22 |
0.22 |
1.64 |
2400 |
[9] |
|
DW |
- |
740 |
350 |
- |
- |
450 |
320 |
1850 |
[3] |
|
POME҂ |
4.2 |
51000 |
25000Þ |
- |
- |
18000 |
- |
- |
[30] |
|
OMW |
4.8 |
132300 |
- |
41.8 |
- |
- |
36.8 |
- |
[13] |
|
SHE* |
5.3-6.8 |
58000-201500 |
2200-9800 |
- |
- |
2.4-4.7 |
- |
- |
[14] |
|
PPW |
5.65 |
858.2 |
- |
1.17 |
- |
- |
- |
[25] |
|
|
SME |
7-7.2 |
7210 |
2850 |
- |
1.87 |
0.318 |
- |
550 |
[20] |
|
CWW |
3.3-9 |
800-102000 |
600-60000 |
- |
- |
0.1-22.0 |
- |
- |
[18] |
|
SPW |
3-4.5 |
110000-190000 |
50000-60000 |
110-190 |
25.45 |
13-15 |
80-120 |
- |
[19] |
|
BBW |
4.6-7.3 |
1096- 8926 |
1609 – 3980 |
1.29 – 12.25 |
- |
0.53 – 3.73 |
1.83 – 4.64 |
500- 10000 |
[22] |
|
OMW |
4.2-6.8 |
9080-135000 |
4750-42000 |
7.3-117 |
- |
- |
7.1-94.3 |
- |
[23] |
Table. 2: Summarising various physical and chemical parameters of different CREs.
ɸAbbreviations used and explained in table 1, ÞBOD3, ҂Palm oil mill effluent, *Slaughterhouse effluent
It can be observed from the table that most of the CREs are acidic in nature (pH ~ 3-6.8) except PWW and SME, these acidic nature effluents are not suitable for biological treatment (aerobic and anaerobic digestion), so extra efforts are required for their treatment. Most of the CREs had very high COD (4000-201500 mg/L) and BOD (500-60000 mg/L) values (except DW and PPW), which is another aspect of effluent treatment because COD removal is requisite to make the WW reusable. Other factors like TS and TDS are also included in WW treatment practice which may be improved by various techniques.
3. Methods and technologies used for energy recovery from WW
3.1 Common methods used
A number of methods have been operated for WW treatment and energy products recovery; some of them are as follows:
3.1.1 Bio-solids incineration: Prior to reuse or disposal, treatment of WW sludge is must to diminish odours and also remove disease-causing agents and resulted sludge is then referred to as bio-solids. Bio-solids are high water containing materials and are habitually dewatered prior further treatment or dumping [31]. Bio-solids immolation is an effective operation with electricity generation and has potential for significant energy recovery from CREs. Multiple hearth furnaces and fluidized bed furnaces are two equipment options, commercially available for bio-solids immolation [32]. Bio-solids are incinerated in multiple stages in hearth furnaces, to dry entering bio-solids by hot air recycle and recover the hearth generation by reduction of arriving moisture. Fluidized bed furnaces (usually more proficient, secure and at ease to operate as compared to multiple hearth furnaces) are newer technology but are applied only for continuous operations. Cleaning of exhaust gases is obligation in both technologies to avoid emissions of particulates, stink, nitrogen oxides (NOx), acid gases, hydrocarbons (methane, propane etc.) and heavy metals (Cu, Cr, Cd, Fe etc). Using both furnaces, a steam cycle power plant can be directed by bio-solids ignition. From where the high temperatura is to steam that steam then turns a turbine connected to a generator, resulted in production of electricity [33].
3.1.2 Anaerobic digestion (AD) and dark fermentation (DF): AD is an anaerobic, native microbial process, usually occurs in marshes, sedimentary lakes, municipal landfills and in ruminant abdomen. This leads the microbe assisted transformation of organic matter present in WW to valuable energy products in absence of oxygen. AD comprise a sequence of biochemical reactions namely, hydrolysis, acidogenesis, acetogenesis and methanogenesis [32]. AD can be applicable on a large variability of effluents; industrial WW, CREs, DW, sewage sludge etc. for treatment process along with energy recovery. AD is influenced by various factors (temperature, substrate loading rate, pH value, C/N ratio and concentration (conc.) of other nutrients. Temperature ranges 25-35 0C and pH 7 is appropriate for AD with varying conc. of other nutrients corresponding to the nature and composition of WW. Temperature dependant AD is categorized in three major classes: ambient (15-30 0C), mesophilic (32-39 0C) and thermophilic (50-64 0C) [1].
DF is a part of AD process in which gases (biohydrogen, methane and carbon dioxide) and VFAs production from wastes takes place in lack of light and oxygen by microbial activity. It involves the obligate and facultative anaerobes (hydrogen producing bacteria) to breakdown the organic matter present in CREs. In the process, protons (H+) act as electron receiver to balance the negatively charged electrons which are generated by oxidation of organic matter and resulted in molecular hydrogen production [34]. Table 1 depicts various energy products recovered from a variety of CREs and their sources.
A number of anaerobic species are capable in energy production from WW, some of them are Saccharomyces sp., Clostridium sp., Actinomyces sp., Eubacterium sp., Lactobacillus sp., Porphyromonas sp. Methanothermobacter sp., Methanobacterium sp., Methanococcus sp., Methanomicrobium sp., Methanopyrus sp., Methanospirillium sp., etc. [35].
3.1.3 Aerobic digestion: It is rarely used method for rescue of energy products but extensively used for WW treatment and removal of pollutants. In aerobic digestion procedure, organic matter present in WW is degraded by microbes in presence of molecular oxygen. Aerobic digestion of activated sewage sludge leads to 80-85% COD removal [36].
A large variety of aerobic microbial species are utilised for degradation of different pollutants and for recovery of energy products, some of them are Pseuodomonas sp., Acinetobactor sp., Alcaligenes sp., Corynebacterium sp., Nocardia sp., Arthrobactor sp., Mycobacterium sp., Clostridium sp., Candida sp. and Gibeberella sp. etc. [35]. Various aerobic microbial species like Geotrichum candidum, Azotobacter chroococcum and Aspergillus niger were used for phenol removal from WW with 35-64% COD removal for Aspergillus niger, 12% phenol removal was obtained from OMW by a fungus Phanerochaete chrysosporium [37]. Aerobic digestion of CWW was found useful for biohydrogen production [18].
3.1.4 Bio-electrochemical systems (BES): [Microbial fuel cells (MFCs) and Microbial electrolysis cells (MECs)]: BES is a technique of electrochemical transition of organic matter present in WW to usable energy products (biohydrogen, methane, electricity etc.) by catalytic microbial activity. BES is an advanced technology over AD as it is able to treat wastes even at low COD conc. of WW and also at higher conc. of VFAs (Jadhav et al., 2017). It degrades sludge production with reduction in cost of aeration. Hence two major variants of BES (MFCs and MECs) can be used for promising and sustainable technical solutions for recovery of valuable products from CREs with high yields [39]. A number of by-products and resources (nutrients, heavy metals, minerals, and intermediate chemicals of industry) can be recaptured during redox reactions of BES. Table 3 depicts that a large variety of heavy metals can be recovered using BES.
MFCs are devices in which catalytic bacteria oxidizes organic and inorganic matter to produce biohydrogen and generate current. MECs are specific type of reactors for biohydrogen production with an external voltage source to overcome the thermodynamic barrier. MFCs are very analogous to hydrogen fuel cells (HFCs) in which protons (H+) are exchanged from an anode compartment to another cathode compartment through an electrolytic membrane [40]. Hydrogen is oxidised into electrons and protons on the anode of anodic compartment and oxygen is reduced in water on the cathode in cathode compartment in an HFC. In contrast, in MFC organic matter of WW is oxidised in the anodic chamber and electrons are transmitted to the cathode chamber. Various species of anodophilic bacteria from various bacterial families (Desulfuromonaceae, Pasteurellaceae, Clostridiaceae, Aeromonadaceae, Comamonadawereceae etc) are skilled to transfer electrons to electrodes [41]. MFCs have been used for revival of energy products from CREs (Table 1).
3.1.5 Microbial desalination cells (MDCs): MDCs are based on integrated BES which allows instantaneous WW treatment and desalination of saline water without application of any external power input or mechanical energy and pressure [42]. Use of MDCs is a sophisticated technology which plays an important role in WW treatment in addition to energy products recovery. The theory of atypical MDC is based on the fact that electron liberates from oxidation of organic matter of WW by electrogenic (catalytic) bacteria in the anode compartment which runs to the cathode compartment through an external circuit and captured by an electron acceptor. Deionisation of saline water takes place in the middle chamber through ion exchange membranes because of the potential difference in the dissolved solid conc. along with generation of immigration between anode and cathode compartments. Oxygen is commonly used and cheap electron acceptor in MDCs [43]. Use of microalgae in cathode chamber besides providing oxygen is gaining attention which also helps in CO2 absorption through photosynthesis, along with pollutant removal from agricultural WW and DW in addition to useful biomass production for biofuel generation. Recently, use of photosynthetic MDCs (PMDCs) has been reported by Kokabian et al., [44] using microalgae bio-cathode with Chlorella vulgaris sp.to study the impact of WW treatment, electricity generation, water desalination and nutrient removal capacity of MDCs. For this purpose, a mixed consortium from aerobic sludge of local WW treatment plant was utilized as microbial source in the anode of MDCs along with enrichment of the chamber with synthetic WW under anaerobic conditions. Three different process configurations were applied i.e., static fed-batch (SPMDC), continuous flow (CFPMDC) and a photo-bioreactor MDC (PBMDC). Among all three, SPMDCs were found more applicable for bioelectricity production with maximum current of 675mW/m3 (with 32.2% TDS and 64% COD removal at 35 g/L TDS conc.) due to biofilm formation while the CFPMDCs were found more appropriate for biomass production (microalgae).
3.1.6 Hydrothermal carbonisation: It is well known that the choice of WW treatment technology is influenced by the factors (type and quality of feed SS, requirement of by-products and public perceptions). So, an increase in use of hydrothermal carbonisation centred emerging technology, i.e.; polymeric carbon solid (PCS) is also pragmatic in energy recovery operations from SS in South African context. PCS technology is a thermo-chemical catalytic process used for biofuel production in an aqueous solution at elevated temperature and pressure. Optimal temperature and pressure applied are 240 0C and 3.3 MPa respectively. In PCS technology required temperature and pressure is significantly reduced by the reagents used. The reduced temperature and pressure also moderate operational costs and capital requirements. This technology is impurity tolerant, and a wide range of WW sludges can be used for energy retrieval. This is a carbon neutral process hence recycles CO2, does not contribute to global warming, and produce low toxicity products. In this process about 40-62% VS and up to 22-37% TS removal takes place on processing of sludge only. Saetea and Tippayawong [26] used hydrothermal carbonisation for hydrochar production from SS. PCS technology is more efficient as compared to AD and aerobic WW treatment and biofuel production [45].
3.2 Energy products recovered from CREs
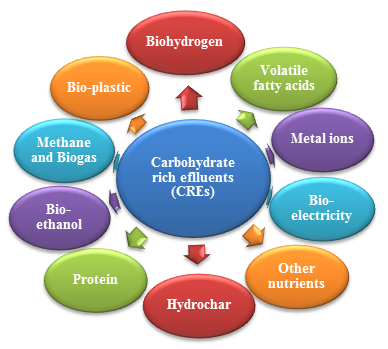
A great variety of value-added products can be recovered from CREs (fig. 1) using different methods. Biological treatment of effluents is a very convenient method which helps in pollutant removal along with production of valuable products. Some of them are as follows:
3.2.1 Biohydrogen: Biohydrogen being carbon neutral, gaining attention as future fuel to reduce the greenhouse gas emission because methane and carbon dioxide are the major products of fossil fuel combustion [46]. Biohydrogen can be produced either in presence of light (biophotolysis and photofermentation) or in absence of light (DF, MFC and MEC) [47]. DF and MFC are more suitable for biohydrogen production from CREs as compared to light process. Optimum biohydrogen production can be achieved either by heat pretreatment of inoculum [48] or by high dilution rate [49] to suppress the activity of inhibitory microbes and by lowering the pH of reactors [11]. Mixed microbial culture is more reliable over pure microbial source for maximum biohydrogen production because more care is required for maintaining the pure culture and mixed culture have a wide variety of microbes for biological conversion of organic matter [50] into valuable products. A large variety of CREs have been used for biohydrogen production, some of those are depicted in Table 1.
3.2.2 Bio-methane and Biogas: Methane is the major constituent of biogas and a co-product of hydrogen in AD of CREs. It can also be produced abiotically (hydrogenophilic-methanogenation) or biologically by fermentation of organic matter [51]. AD is more efficient over aerobic digestion process for methane production due to low energy consumption and high energy production in the form of methane. Various WW forms have been used for methane production, i.e., petha WW [11]; acidic discharged affluent of sugarcane juice after hydrogen fermentation [28] etc. Co-treatment of WW with carbon rich food waste can result in maximized methane yield and improved energy balance of WW treatment plants [52].
3.2.3 Bio-ethanol: The economic importance of bioethanol is well known as it has been used in gasoline blends with the name E10, E85 etc. to reduce carbon monoxide emission and improve the efficiency of petrol. So, there was a hack in ethanol demand and prices in last decades which inspired researchers for ethanol production from CREs. Before it, ethanol was produced only from biomass. Various WWs have been used for bio-ethanol production with the help of mixed microbial culture or through bio-electrochemical conversion of CREs [53]. Federico et al. [23] used oil mill WW and olive pomace, Ezgi and Canan [54] used apple pomace hydrolysate and rice straw with petha WW and dairy WW was used by Kumari et al. [9] for bioethanol and methane production. Steinbusch et al. [55] recovered ethanol and butanol from WW in the cathodic chamber. Bioethanol was also produced by acidification of gelatin rich WW using an up flow anaerobic reactor at different temperatures [56].
3.2.4 Recovery of valuable metals: Heavy metals present in WW effluents are poisonous to human beings, animals, and environment, even present in small amounts. There are various technologies for heavy metal removal from WW like physical, chemical, physico-chemical and biochemical but bio-electrochemical system (BES) is more proficient among all these practises for removal of even small concentration of heavy metals present in the discharge. Various metals i.e., Cr, Cu, Fe, Cd, Zn, Ni, Pb, Au, iron oxide, elemental selenium, uranium etc. have been picked up in satisfactory amount (90-99.9%) in last decades [38].
|
Metals recovered |
Removal % |
Anode/Cathode reaction |
Redox potential |
Reference |
|
Copper (Cu) |
97.8 |
Cu2+ + 2e − → Cu |
E0 = 0.337 V |
[57] |
|
Zinc (Zn) |
97 |
Zn2+ +2e− → Zn |
E0 = −0.762 V |
[58] |
|
Lead (Pb) |
47.5 |
Pb2+ + 2e− → Pb |
E0 = −0.130 V |
[59] |
|
Nickel (Ni) |
99 |
Ni2+ + 2e–→ Ni |
E0 = 0.250 V |
[60] |
|
Cadmium (Cd) |
90 |
Cd2+ + 2e–→ Cd |
E0 = 0.400 V |
[61] |
|
Mercury (Hg) |
90-99.3 |
Hg2+ + 2e–→ Hg |
E0 = 0.911 V |
[62] |
|
Vanadium (V) |
26.1 |
V5+ + 5e–→ V |
E0 = 0.991 V |
[40] |
|
Chromium (Cr) |
99.5 |
Cr4+ + 4e–→ Cr |
E0 = 1.330 V |
[63] |
|
Silver (Ag) |
99.91 |
Ag+ + e–→ Ag |
E0 = 0.799 V |
[64] |
|
Gold (Au) |
- |
Au3+ + 3e–→ Au |
E0 = 1.001 V |
[65] |
|
Iron (Fe3+) |
99 |
Fe2+ + 3H2O → Fe (OH)3↓+ 3H+ +e– |
E0 cell= 0.28 V |
[66] |
|
Selenium (Se) |
98 |
Se (aq.) + 4e– → Se |
E0 = 0.41V |
[67] |
|
Uranium (U) |
87 |
U6+ + 2e– → U4+ |
E0Cathode= −0.042 V |
[41] |
Table. 3: Reactions involved in the recovery of different metals from wastewater.
3.2.5 Synthesis of biopolymer and bioplastic: Poly-hydroxy-alkanoates (PHAs) are biologically degradable bio-plastics which are obtained from bacterial cells, but its production is expensive as compared to low cost petro-chemically derived plastics which limits the production of PHAs [4, 68]. Alcohols, carboxylic acids (VFAs), diols and biopolymers (poly−β−hydroxybutyrate) are also obtained during the PHA synthesis pathway, utilising CO2 as the carbon source in BES by an external power supply [68]. Municipal sewage sludge was used for the production of bioplastic (poly-hydroxy-alkanoate) in Netherland through rich culture route (using selected bacterial species) and mixed culture route [4].
3.2.6 Volatile fatty acids (VFAs): AD of organic matter, present in CREs resulted in production of various VFAs (acetic acid, propionic acid, butyric acid, lactic acid, caproic acid etc.) along with alcohols. Yu et al. [56] used gelatin-rich WW for VFAs and alcohol production by acidification of WW using an up flow anaerobic reactor at different pH and temperatures. pH affects metabolic pathways, in most of the studies acetate and butyrate were major products while low pH favours formation of butyrate. Propionate production is dominant over butyrate at pH 7 and above [1]. Results showed that VFAs and alcohol production quotient was enhanced with increasing temperature. Gelatin degradation efficiency was also enhanced from 60% to 97.5% when pH was changed from 4.0 to 7.0. It shows that pH has more effect on AD as compared to temperature.
3.2.7 Bioelectricity: BES is a convenient technology for bio-electricity generation using catalytic microbial community for conversion of organic matter present in CREs [38]. MFCs are generally operated for bio-electricity generation. Similar to hydrogen fuel cells, in MFCs protons are exchanged from an anode to cathode compartment through an electrolytic membrane [40]. Single chambered microbial fuel cells (SCMFC) were applied for bioelectricity generation from PWW using graphite electrodes [48]. Some CREs used for bio-electricity generation through MFCs, are depicted in Table 1.
3.2.8 Hydrochar and other biochemicals: As compared to bio-energy production from WW, biochemical production is more feasible [69]. Sewage sludge was used for hydrochar production by hydrothermal carbonization [26]. Industrial wastes can be utilised as inexpensive raw sources of integrated fermentation processes [70]. CREs and WW which are not suitable for human and animal use can be converted into high-value products using pure culture or co-culture processes. Various chemicals like lipase, protease, glycerol, acetic acid, lactic acid, biomass protein can be manufactured through anaerobic fermentation and aerobic treatment of WW [71]. CWW was used by [18] for biohydrogen production along with co-production of VFAs (acetate, butyrate, iso-butyrate, propionate, lactate, and formate) with a yield of 118 to 27,012 mg/L.
3.2.9 Recovery of Protein and other nutrients:
Various nutrients like phosphate, sulphate, orthophosphate, and ammonia can also be regained by treatment of WW. Swine WW being rich in phosphate, its treatment is a critical concern. By its treatment, 27% phosphate recovery and 70-82% phosphate removal efficiency were achieved in BES [72, 73]. Orthophosphate was recaptured from digested sewage sludge (600 mg/L) by the metabolic activity of Escherichia coli [74]. Blázquez et al. [75] reported a novel bio-electrochemical method for regaining of sulphate from sulphate rich WW, where elemental sulphur recovery was done by autotrophic sulphate reducing bacteria and sulphide oxidising bacteria. Membrane ultrafiltration was used for protein synthesis from poultry industry WW [25].
3.2.10 Microalgal growth followed by biodiesel production: Presence of microalgal biomass in water bodies is an indication that the water of the source is polluted but this algal biomass plays dual role; first in treating WW and second biomass production followed by biofuel (biodiesel and bio-electricity) production [73]. This algal biomass can also be used as animal fodder as well as bio-fertilizers. A few algal species are capable in producing up to 80% of oil as its storage product and can also be capable to produce about 23 times more oil than the best oil-seed plant [29]. Oil trans-esterification is a technique, used to produce biodiesel and glycerol from algal biomass. Biodiesel can be used to produce energy products and glycerol may be burn directly as a fuel or can be further converted to other biofuels (bioethanol and biohydrogen) by fermentation process. Microalgae were also used in phycoremediation of various WW sources [3, 29]. Chlorella vulgaris sp. was utilised by Kokabian and Gude [43] in MDCs for nutrient removal and bio-electricity generation from WW.
4. Challenges to recover energy products from effluents
The cross relationship among power and water and the organic content of WW can inspire energy restoration operations from many potential sources, comprising municipal WW and other CRE treatment facilities [32]. Through incorporation of AD with biogas utilization and bio-solids incineration with electrical energy production, WW efficiencies can diminish electricity intake by 4.7 to 83% [31]. There are many other challenges which must be considered for efficient utilisation of WW to transform it into energy related and other valuable products. Various physical and chemical factors (pH, substrate loading rate, COD, BOD, and different temperature) affect the biohydrogen production rate [76]. Altering organic matter of WW whichever increasing with poorer WW flows that deliberate wastes or decreasing with enriched waste management- leads further uncertainty into these energy rescue assessments [77].
The operation of anaerobic treatment plants at low temperature is also a challenge which not only downhearted the kinetics of all living process but also proliferations the dispersed methane in the discharge as methane is nearly 1.5 times additionally solvable at 15°C competed to 35°C [57]. This makes performance of AD difficult to produce the valuable energy products from the WW. This problem may be short out by the use of hydrothermal carbonisation in which reagents used are capable to moderate the temperature and pressure of the reaction.
It is evident from Table 3 that many industrial effluents have small or large quantity of heavy metal ions which are not appropriate for fermentative bacteria accountable for biohydrogen and VFAs production. These metals may cause inhibition of biological process resulted in low amount of energy products regain [1]. Some of the microbes present in WW forms can also inhibit another microbe’s activity. Study revealed that Lactobacillus paracasei and Enterococcus durans inhibited the production of hydrogen when co-cultured with Clostridium sp. [1].
WW is mainly constituted of various organic materials and nitrogenous wastes. Most of the organic matter is transformed to methane but there is the probability of forming nitrous gas (laughing gas) during the partial nitration procedure from the nitrogenous wastes and this nitrous gas is a very potent greenhouse gas. Nitrous gas is produced at stress atmospheres for nitrification or denitrification bacteria for instance at low oxygen and pH-values. Sometime phenolic substances are inhibitory to microbes [78, 79] so much care should be taken in assortment of microbes for production of energy commodities. Production of PHAs from SS is technically an interesting approach but its high cost makes the process less feasible at practical level and its cost can be reduced by maximizing the VFAs production along with PHA storage capacity [4].
Moreover, day by day the complexity of WW also a rising concern due to addition of new chemical from all walks of life which not only makes it more difficult to recover energy from WW but also makes the WW treatment practice more energy-intensive in the future. Hence in order to manage the WW more efficiently and to recover the important energy related products, these all challenges have to address in a holistic way to overcome all the issues related to the WW treatment.
5. Future perspectives to control water pollution and enhance energy recovery
Our present environmental conditions are getting very worse in a speedy way and most of the water bodies are now contaminated, and addition of more and more pollutants is in use. So, for our environmental concern, the future plan should be to focus on production of biodegradable fertilizers or bio fertilizers, because a large amount of contaminants are produced through our unhealthy agronomic practices due to the extreme use of synthetic fertilizers and pesticides. Presents unhealthy way of medicinal waste discarding is another major problem because a high quantity of antibiotics and other medicines are found in water bodies which make water unfit to use further. These chemicals finally get intensified to the water resources and contaminate them. Some of the steps which could help to solve the present problem can be listed as:
- Presently, most of the MFCs utilised are designed for lab scale purposes, the utility of MFC should be scale up to use for energy retrieval from large amount of WW at practical level.
- Use of electrochemical, bio-electrochemical, and biological expertise for WW treatment can generate increased amount of electricity in a WW treatment plant and can also help in reduction of greenhouse gas emissions [80].
- Advancement in the optimisation of the present technologies may be obtained by using two directional mathematical models.
- Application of hybrid approach (production of more than one useful product in a single process) i.e., integrated hydrogen and methane production using CREs.
- Selection of best microbial species, responsible for specific biofuel production and pollutant elimination along with energy generation by WW treatment. This would consequence in reduction of the operation cost and incinerated energy for the process [74].
- Addition of a very small quantity of catalytic substance (mostly metal ions) can build up the microbial process, implicated in the by-product’s recovery from WW [81].
- Use of various CREs along with lignocellulosic wastes (rice straw, wheat straw etc.) for pretreatment and biofuel production process using anaerobic co-digestion and fermentation [5, 8, 9 and 82].
- Use of different nanomaterial to ease the recovery of value-added products and heavy metals from waste effluents [83, 84, 85].
- Application of conventional and novel materials for heavy metal adsorption from wastewater treatment process [86].
- Implementation of bentonite clay with magnetic nanoparticles for the treatment of food industry wastewater to make it reusable [87].
Conclusions
Among all the technologies which are in use, BES and hydrothermal carbonization seems more promising to fulfill present demand of WW treatment plants. These technologies are applicable on a large variety of WW effluents and recover more than one energy product in a single step, hence save operational costs of the treatment process. Integrated processes (MDCs) can be also fruitful to reduce treatment expenses with low energy input or energy utilized can be recovered from the electricity generated during the process. So our aim should have to develop hybrid and integrated approach which leads the maximum energy recovery from the WW with zero waste product generation. Thus zero waste management is the healthy practice for future to reduce the risk of upcoming hazards of the polluted water.
Acknowledgement
Authors would like to thank the Head of the Chemistry Department and the Director of Dayalbagh Educational Institute, Agra, India for providing all basic requirements for the completion of this research. Authors would also like to acknowledge University Grant Commission (UGC) India for providing UGC-BSR Fellowship in Sciences to assist the project.
Funding
This work was supported by University Grant Commission for providing ‘UGC Research Fellowship in Sciences’ [F.25-1/2014-15(BSR)/7-191/2007(BSR) dated 7.10.2015].
References:
- Li C, Fang HHP. Fermentative Hydrogen Production from Wastewater and Solid Wastes by Mixed Cultures. Crit Rev Env Sci Tec 37 (2007): 1-39.
- Kumari D, Singh R. Recent Advances in Bioremediation of Wastewater for Sustainable Energy Products. In: Rathoure AK editor. Zero Waste: Management Practices for sustainability, CRC Press, Taylor & Francis Group, Boca Raton (2019): 247-276.
- Rawat I, Ranjith KR, Mutanda T, et al. Dual role of microalgae: Phycoremediation of domestic wastewater and biomass production for sustainable biofuels production. Appl Energy 88 (2011): 3411-3424.
- Bluemink ED, Nieuwenhuijzen AFV, Wypkema E, et al. Bio-plastic (poly-hydroxy-alkanoate) production from municipal sewage sludge in the Netherlands: a technology push or a demand driven process? Water Sci Technol 74 (2016): 353-358.
- Kumari D, Chahar P, Singh R. Effect of ultrasonication on biogas and ethanol production from rice straw pretreated with petha wastewater and dairy wastewater. International Journal of Current Engineering and Scientific Research (IJCESR) 5 (2018): 65-73.
- Luo J, Ding L, Benkun Q, et al. A two-stage ultrafiltration and nanofiltration process for recycling dairy wastewater. Bioresour Technol 102 (2011): 7437-7442.
- Li YC, Liu YF, Chu CY et al. Techno-economic evaluation of biohydrogen production from wastewater and agricultural waste. Int J Hydrog Energy 37 (2012): 15704-15710.
- Kumari D, Singh R. Coupled green pretreatment of petha wastewater and rice straw. Environmental and Sustainability Indicators 5 (2020a): 100013.
- Kumari D, Singh R. Ultrasonic Assisted Petha Wastewater Pretreatment of Rice Straw for Optimum Production of Methane and Ethanol using Mixed Microbial Culture. Renew Energy 145 (2020b): 682-690.
- Singhal Y, Singh R. Comparison of Graphite Electrode Types on Generation of Bioelectricity from Petha Industry Wastewater Using Single Chamber Microbial Fuel Cells (SCMFC). Adv Sci 20 (2014b): 1578-1581.
- Singhal Y, Singh R. Energy Recovery from Petha Industrial Wastewater by Anaerobic Digestion. International Journal of Science and Engineering 3 (2015): 146-151.
- Satsangee R, Ghose P. Anaerobic degradation of phenol using a cultivated mixed culture. Appl Microbiol biotechnol 34 (1990): 127-130.
- Maragkaki AE, Fountoulakis M, Gypakis A, et al. Pilot-scale anaerobic co-digestion of sewage sludge with agro-industrial by-products for increased biogas production of existing digesters at wastewater treatment plants. Waste Manage 59 (2017): 362-370.
- Fuchs W, Binder H, Mavrias G, et al. Anaerobic treatment of wastewater with high organic content using a stirred tank reactor coupled with a membrane filtration unit. Water Res 37 (2003): 902-908.
- Sivaramakrishna D, Sreekanth D, Sivaramakrishnan M, et al. Effect of system optimizing conditions on biohydrogen production from herbal wastewater by slaughterhouse sludge. Int J Hydrog Energy 39 (2014): 7526-7533.
- Ajao V, Millah S, Gagliano MC, et al. Valorization of glycerol/ethanol-rich wastewater to bioflocculants: recovery, properties, and performance. J Hazard Mater 375 (2019): 273-280.
- Xie M, Shon HK, Gray SR, et al. Membrane-based processes for wastewater nutrient recovery: Technology, challenges, and future direction. Water Res 89 (2016): 210-221.
- Azbar N, Dokgöz FTÇ, Keskin T, et al. Continuous fermentative hydrogen production from cheese whey wastewater under thermophilic anaerobic conditions 34 (2009): 7441-7447.
- Eniyon UK, Ananthara Raj C, Atun RC, et al. Synthesis of Bio-Hydrogen Renovated with Carbohydrate Rich Wastewater Utilizing Monitoring Based Agitable UASB Reactor. International Journal of Advance Engineering and Research Development (IJAERD) 5 (2018): 781-788.
- Kumar R, Singh L, Zularisam AW. Bioelectricity Generation and Treatment of Sugar Mill Effluent Using a Microbial Fuel Cell. J Clean Energy Technol 4 (2016): 249-252.
- Lu N, Zhou SG, Zhuang L, et al. Electricity generation from starch processing wastewater using microbial fuel cell technology. Biochem Eng 43 (2009): 246-251.
- Wang X, Feng YJ, Lee H. Electricity production from beer brewery wastewater using single chamber microbial fuel cell. Wat Sci Tech 57 (2008):1117-1121.
- Federico B, Giuseppe M, Bernardo R, et al. Selection of the best pretreatment for hydrogen and bioethanol production from olive oil waste products. Renew Energy 88 (2016): 401-407.
- Kapdan IK, Kargi F. Bio-hydrogen production from waste materials. Enzyme Microb Technol 38 (2006): 569-582.
- Lo YM, Cao D, Argin S, et al. Recovery of protein from poultry processing wastewater using membrane ultrafiltration. Bioresour Technol96 (2005): 687-698.
- Saetea P, Tippayawong N. Recovery of Value-Added Products from Hydrothermal Carbonization of Sewage Sludge. Hindawi Publishing Corporation (2013): 1-6.
- Reddy K, Nasr M, Kumari S, et al. Biohydrogen production from sugarcane bagasse hydrolysate: effects of pH, S/X, Fe2+, and magnetite nanoparticles. Environ Sci Pollut R 24 (2017): 8790-8804.
- Alissara R, Sureewan S, Chakkrit S. Methane production from acidic effluent discharged after the hydrogen fermentation of sugarcane juice using batch fermentation and UASB reactor. Renew Energy 86 (2016): 1224-1231.
- Kong QX, Li L, Martinez B, et al. Culture of microalgae Chlamydomonas reinhardtii in wastewater for biomass feedstock production. Appl Biochem Biotechnol 160 (2010): 9-18.
- Deshon SP, Wei Tan, Kaman IA, et al. Palm oil mill effluent treatment using coconut shell – based activated carbon: Adsorption equilibrium and isotherm. MATEC Web of Conferences 87 (2017): 03009, ENCON 2016.
- Turovskiy IS. Biosolids or Sludge? The Semantics of Terminology. Water & Wastes Digest Retrieved (2015).
- Agdag ON, Sponza DT. Co-digestion of mixed industrial sludge with municipal solid wastes in anaerobic simulated landfilling bioreactors. J Hazard Mater 140 (2007): 75-85.
- Roy MM, Dutta A, Corscadden K, et al. Review of biosolids management options and co-incineration of a biosolid-derived fuel. Waste Manage 31 (2011): 2228-2235.
- Wang J, Wan W. Factors influencing fermentative hydrogen production: a review. Int J Hydrog Energy 34 (2009): 799-811.
- Christy PM, Gopinath LR, Divya D. Microbial Dynamics During Anaerobic Digestion of Cow Dung. International Journal of Plant, Animal and Environmental Science (IJPAES) 4 (2014): 86-94.
- Rozzi A, Malpei F. Treatment, and disposal of olive mill effluents. Int Biodeter Biodegr 47 (1996): 135–144.
- Dhouib A, Aloui F, Hamad N, et al. Pilot-plant treatment of olive mill wastewaters by Phanerochaete chrysosporium coupled to aerobic digestion and ultrafiltration. Process Biochem 41 (2006): 159–167.
- Jadhav DA, Ray SG, Ghangrekar MM. Third generation in bio-electrochemical system research – A systematic review on mechanisms for recovery of valuable by-products from wastewater. Renew Sustain Energy Rev 76 (2017): 1022-1031.
- Pham TH, Rabaey K, Aelterman P, et al. Microbial fuel cells in relation to conventional anaerobic digestion technology. Eng Life Sci 6 (2006): 285-292.
- Zhang BG, Zhou SG, Zhao HZ, et al. Factors affecting the performance of microbial fuel cells for sulfide and vanadium (V) treatment. Bioproc Biosyst Eng 33 (2010): 187-194.
- Lovley DR. The microbe electric: conversion of organic matter to electricity. Curr Opin Biotech 19 (2008): 564-571.
- Cao X, Huang X, Liang Xiao PK, et al. A new method for water desalination using microbial desalination cells. Environ Sci Technol 43 (2009): 7148-7152.
- Kokabian B, Gude VG. Sustainable photosynthetic biocathode in microbial desalination cells. Chem Eng J 262 (2015): 958-965.
- Kokabian B, Ghimire U, Gude VG. Water deionization with renewable energy production in microalgae-microbial desalination process. Renew Energy 122 (2018): 354-361.
- Zvimba J, Musvoto E. Watewater treatment, from waste to worth – converting wastewater sludge into high-value products. The Water Wheel January/February (2018): 31-33.
- Ballesteros I, Negro MJ, Oliva JM, et al. Ethanol production from steam-explosion pretreated wheat straw. Appl Biochem Biotechnol 130 (2006): 496-508.
- Singh R. Fermentative biohydrogen production using microbial consortia. In Gupta V, Tuohy M, editors. Biofuel Technologies, Springer, Berlin, Heidelberg (2013): p. 273-300.
- Singhal Y, Singh R. Effect of microwave pretreatment of mixed culture on biohydrogen production from waste of sweet produced from Benincasa hispida. Int J Hydrog Energy 39 (2014a): 7534-7540.
- Chen CC, Lin CY, Chang JS. Kinetics of hydrogen production with continuous anaerobic cultures utilizing sucrose as the limiting substrate. Appl Microbiol Biotechnol 57 (2001): 56-64.
- Pachapur VL, Kutty P, Pachapur P et al. Seed Pretreatment for Increased Hydrogen Production Using Mixed-Culture Systems with Advantages over Pure-Culture Systems. Energies 12 (2019): 530.
- Villano M, Aulenta F, Ciucci C, et al. Bio-electrochemical reduction of CO2 to CH4 via direct and indirect extracellular electron transfer by a hydrogenophilic methanogenic culture. Bioresour Technol 101 (2010): 3085-3090.
- Guven H, Ersahin ME, Dereli KK, et al. Energy recovery potential of anaerobic digestion of excess sludge from high-rate activated sludge systems co-treating municipal wastewater and food waste. Energy 172 (2019): 1027-1036.
- Rosenbaum M, Aulenta F, Villano M, et al. Cathodes as electron donors for microbial metabolism: which extracellular electron transfer mechanisms are involved? Bioresour Technol 102 (2011): 324-333.
- Ezgi E and Canan T. Production of bioethanol from apple pomace by using co-cultures: Conversion of agro-industrial waste to value added product. Energy 88 (2015): 775-782.
- Steinbusch KJ, Hamelers HV, Plugge CM, et al. biological formation of caproate and caprylate from acetate: fuel and chemical production from low grade biomass. Energy Environ Sci 4 (2011): 216-224.
- Yu HQ, Fang HHP. Acidogenesis of gelatin-rich wastewater in an up flow anaerobic reactor: influence of pH and temperature. Water Res 37 (2003): 55-66.
- Liang M, Tao HC, Li SF, et al. Treatment of Cu2+−containing wastewater by microbial fuel cell with excess sludge as anodic substrate. Environ Sci Technol 32 (2011): 179-185.
- Tao HC, Lei T, Shi G, et al. Removal of heavy metals from fly ash leachate using combined bio-electrochemical systems and electrolysis. J Hazard Mater 264 (2014): 1-7.
- Modin O, Wang X, Wu X, et al. Bio-electrochemical recovery of Cu, Pb, Cd, and Zn from dilute solutions. J Hazard Mater 235-236 (2012): 291-297.
- Qin B, Luo H, Liu G, et al. Nickel ion removal from wastewater using the microbial electrolysis cell. Bioresour Technol 121 (2012): 458-461.
- Abourached C, Catal T, Liu H. Efficacy of single−chamber microbial fuel cells for removal of cadmium and zinc with simultaneous electricity production. Water Res 51 (2014): 228-233.
- Wang Z, Lim B, Choi C. Removal of Hg2+ as an electron acceptor coupled with power generation using a microbial fuel cell. Bioresour Technol 102 (2011): 6304-6307.
- Li Z, Zhang X, Lei L. Electricity production during the treatment of real electroplating wastewater containing Cr6+ using microbial fuel cell. Process Biochem 43 (2008):1352-1358.
- Choi C, Cui Y. Recovery of silver from wastewater coupled with power generation using a microbial fuel cell. Bioresour Technol 107 (2012): 522-525.
- Varia JC, Martinez SS, Velasquez−Orta S, et al. Microbiological influence of metal ion electrodeposition: studies using graphite electrodes, [AuCl4] - and Shewanella putrefaciens. Electrochim Acta 115 (2014): 344-351.
- Lefebvre O, Neculita CM, Yue X, et al. Bio-electrochemical treatment of acid mine drainage dominated with iron. J Hazard Mater 241-242 (2012): 411-417.
- Yang L, Wu Z, Wu J, et al. Simultaneous removal of selenite and electricity production from Se−laden wastewater by constructed wetland coupled with microbial fuel cells. In: Banuelos GS, Lin ZQ, Yin X, editors. Selenium in the environment and human health, London: Taylor & Francis (2014): p. 212–214.
- Chen BY, Liu SQ, Hung JY, et al. Reduction of carbon dioxide emission by using microbial fuel cells during wastewater treatment. Aerosol Air Qual Res 13 (2013): 266-274.
- Bungay HR. Confessions of a bioenergy advocate. Trends Biotechnol 22 (2004): 67-71.
- Montgomery R. Development of biobased products. Bioresour Technol 91 (2004): 1-29.
- Largus TA, Khursheed K, Muthanna HAl-D, et al. Production of bioenergy and biochemicals from industrial and agricultural wastewater. Trends Biotechnol 22 (2004).
- Ichihashi O, Hirooka K. Removal, and recovery of phosphorus as struvite from swine wastewater using microbial fuel cell. Bioresour Technol 114 (2012): 303-307.
- Xiao L, Young EB, Berges JA, et al. Integrated photo−bio-electrochemical system for contaminants removal and bioenergy production. Environ Sci Technol 46 (2012): 114590-11466.
- Fischer F, Bastian C, Happe M, et al. Microbial fuel cell enables phosphate recovery from digested sewage sludge as struvite. Bioresour Technol 102 (2011): 5824-5830.
- Blázquez E, Gabriel D, Baeza JA, et al. Treatment of high−strength sulphate wastewater using an autotrophic biocathode in view of elemental sulfur recovery. Water Res 105 (2016): 395-405.
- Parihar RK, Upadhyay K. Production of Bio-Hydrogen Gas from Wastewater by Anaerobic Fermentation Process: A Review. Int J Chem Stud 3 (2015): 07-14.
- Jun WL. Anaerobic Co-digestion of Brown Water and Food Waste for Energy Recovery. Daniel Thevenot, 11th edition of the Worldwide Workshop for Young Environmental Scientists (WWW-YES-2011) - Urban Waters: resource or risks? Arcueil, France, June 6-10 (2011).
- Parag RG. Treatment of wastewater streams containing phenolic compounds using hybrid techniques based on cavitation: A review of the current status and the way forward. Ultrason Sonochem 15 (2008): 1-15.
- Tontti T, Poutiainen H, Heinonen-Tanski H. Efficiently treated sewage sludge supplemented with nitrogen and potassium is a good fertilizer for cereals. Land Degrad Dev 28 (2016).
- Tang J, Zhang C, Shi X, et al. Municipal wastewater treatment plants coupled with electrochemical, biological and bio-electrochemical technologies: Opportunities and challenge toward energy self-sufficiency. J Environ Manage 234 (2019): 396-403.
- Banu JR, Eswari AP, Kavitha S, et al. Energetically efficient microwave disintegration of waste activated sludge for biofuel production by zeolite: Quantification of energy and biodegradability modelling. Int J Hydrog Energy 44 (2019): 2274-2288.
- Kumari D, Singh R. Pretreatment of lignocellulosic wastes for biofuel production: A critical review. Renew Sustain Energy Rev 90 (2018): 877-891.
- Srivastava N, Srivastava M, Mishra PK, et al. Advances in nanomaterials induced biohydrogen production using waste biomass. Bioresour Technol 307 (2020): 123094.
- Naseem T , Durrani The role of some important metal oxide nanoparticles for wastewater and antibacterial applications: A review. Environmental Chemistry and Ecotoxicology3 (2021): 59-75.
- Yaqoob AA, Parveen T, Umar K, et al. Role of Nanomaterials in the Treatment of Wastewater: A Review. Water 12 (2020): 495.
- Chai WS, Jie YC, Kumar PS, et al. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J Clean Prod 296 (2021): 126589.
- Mateus A, Torres J, Bolivar WM, et al. Implementation of magnetic bentonite in food industry wastewater treatment for reuse in agricultural irrigation. Water Resour Ind 26 (2021): 100154.


 Impact Factor: * 4.1
Impact Factor: * 4.1 Acceptance Rate: 75.32%
Acceptance Rate: 75.32%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 