Investigation of Mexiletine Hydrochloride Binding on Transition Metal Oxide Nanoparticles by Capillary Electrophoresis
Eman T. Elmorsi and Edward P.C. Lai*
Department of Chemistry, Carleton University, Ottawa, Canada
*Corresponding Author: Edward P.C. Lai, Department of Chemistry, Carleton University, Ottawa, Canada.
Received: 28 December 2023; Accepted: 26 January 2024; Published: 02 February 2024
Article Information
Citation: Eman T Elmorsi, Edward PC Lai. Investigation of Mexiletine Hydrochloride Binding on Transition Metal Oxide Nanoparticles by Capillary Electrophoresis. International Journal of Plant, Animal and Environmental Sciences. 14 (2024): 01-11.
View / Download Pdf Share at FacebookAbstract
The binding affinity of pharmaceutically active compounds (PACs) onto transition metal oxide nanoparticles (TMONPs) is a physicochemical parameter that dictates their environmental bioavailability. Capillary electrophoresis (CE) was used successfully to determine the binding affinity of mexiletine hydrochloride (MEX.HCl) onto TiO2, Co3O4, or ZnO nanoparticles individually in alkaline, neutral, and acidic aqueous suspension. The binding process was modeled using linear/nonlinear kinetics and Langmuir/Freundlich adsorption isotherms. Interestingly, TiO2 nanoparticles demonstrated the highest binding affinity of 81(±1) % at pH 9.4 for MEX.HCl in the concentration range from 15 to 75 μg/mL. The maximum binding capacity (qmax) values for TiO2, Co3O4, and ZnO at pH 9.4 were 27.0, 26.4, and 25.2(±1) mg/g, respectively. MEX.HCl molecules moved inside the porous TMONPs with a binding rate ranking of linear pseudo-second order > non-linear pseudo-second order > linear pseudo-first order > non-linear pseudo-first order. The binding isotherm favors the linear Freundlich > linear Langmuir > nonlinear Freundlich > nonlinear Langmuir models. Overall, MEX.HCl binds onto heterogeneous TMONP surfaces via electrostatic interactions and coordination bonds.
Keywords
<p>Binding affinity; Kinetics; Transition metal oxide nanoparticles; Mexiletine hydrochloride; Capillary electrophoresis</p>
Article Details
1. Introduction
The worldwide usage of pharmaceutically active compounds (PACs) has increased the release of toxic pharmaceuticals into the environment. Lately, the concentrations of PACs in water systems have been increasing to range from ng/L to mg/L levels [1]. Therefore, pharmaceutical manufacturing facilities and domestic sewage may have high PACs levels [2]. These compounds may be subject to organic and inorganic interactions, such as interactions with transition metal oxide nanoparticles (TMONPs), which can affect their bioavailability as well as their transportation into the environment [3]. PACs can be used as diagnostic agents, therapeutic treatments, and disease mitigators [4]. Salt formation of PACs is a method of choice for improving their physical properties including aqueous solubility, chemical stability, and gastrointestinal absorption [5,6]. It has been revealed that pharmaceutical salts account for about 43% of the total PACs [7]. Most of the PACs, either weakly acidic or weakly basic, contain the proper functional groups that allow for salt formation. Basic PACs often require a strong inorganic acid (HCl) that provides a counterion (Cl-) to form salts for enhanced stability in the drug formulation [8,9]. Hydrochloride salt formulation is dictated by such factors as biochemistry, pharmacokinetics, and pharmacodynamics that would enhance the overall therapeutic effects [7,10]. The stability of formed salts is mainly determined by the negative logarithm of the acid dissociation constant (pKa). A basic PAC forms a stable salt when the difference between the ionizable group pKa and the counterion pKa is greater than two or three units [11]. In the past twenty years, anionic counterions such as chloride, mesylate, bromide, acetate, and fumarate were used to form salts from basic PACs. HCl, in particular, is a safe acid and the chloride ion is abundant throughout the human body. Thus, HCl salts account for about 29% of all basic drugs. These salts are characterized by high water solubility and thermodynamic stability [8,9].
The HCl salt of mexiletine (MEX.HCl) is used as an anti-arrhythmia drug by inhibiting the sodium influx in cardiac cells which reduces the rising rate of the heart action potential [12,13]. Due to its extensive cardiac side effect profile, MEX.HCl was considered a risky drug. This led to the suspension of MEX.HCl treatment in several countries for a long period [13]. However, a recent study showed that MEX.HCl does not cause any hazardous cardiac arrhythmias and is a highly effective and inexpensive drug [13,14]. In addition, by providing real-world data on patients’ experiences through MyoPath, MEX.HCl has been used as an anti-myotonic treatment [15]. It has been reported that the annual prescription of MEX.HCl accounted for ∼ 0.1 tonnes per year in 2008 [16]. MEX.HCl is an aromatic ether and primary amine compound (pKa 9.5) known as the 2,6-dimethyl phenyl ether ofaminopropyl [17].The molecular structure of protonated mexiletine (MEX.H+) showed two binding sites the protonated nitrogen of the amine group and the lone pair of electrons on the oxygen of the phenoxy group. These binding centers would allow MEX.H+ to interact with different organic and inorganic compounds. In addition, the nitrogen of the amino group in MEX.H+ may coordinate with different cations such as Na+ and K+ ions of protein receptors [18]. MEX.HCl is extensively metabolized in men, with less than 10% of the dose being excreted unchanged in urine which ends in different aquatic environments leading to the spreading of contamination and pollution [19].
Several simple, rapid, sensitive, and selective analytical methods were reported for the determination of MEX.HCl and its synthetic impurity 2,6-dimethylphenol (DMP) [20]. A reverse-phase HPLC method was developed for the quality control analysis of MEX.HCl in various dosage forms [21]. The applicability of capillary electrophoresis (CE), capillary electro-chromatography, and micro/capillary/nano-liquid chromatography for the analysis of pharmaceutical formulations, active pharmaceutical ingredients, drug impurity testing, chiral drug separation, determination of drugs and metabolites in biological fluids was thoroughly reviewed [22].
TMONPs have gained significant attention due to their extraordinary physical, chemical, optical, and electronic properties when compared to bulk materials [23]. In addition, because of their size, shape, stability, and larger surface area, they are included in various applications. Although nanoparticles have evolved to play a prominent role in our economy, their increased use poses potential human health risks [24]. Nanoparticles may enter the environment directly or indirectly [25-27] through different sources [28] such as release during the production of raw material, during use, and after disposal of consumer products [29,30]. It has been reported that TMONPS such as TiO2 and ZnO are released into landfills, sediments, and soil because they are mostly used in cosmetics, electronics, and medicine. Therefore, TiO2 and ZnO nanoparticles are accumulated in sewage sludge during wastewater treatments [31-33]. Also, the unique properties of Co3O4 nanoparticles, are attracting enormous interest in different applications such as electrochemistry, storage of energy, sensors, and catalytic processes leading to its discharge into aquatic systems and affecting its sustainability [34]. TMONPs can be hypothetically analyzed by studying their interaction with organic molecules followed by the analysis of the molecules by capillary electrophoresis. Hence, the strength and bond type between the adsorbed compounds as MEX.HCl and TMONPs depend on the respective functional group and corresponding affinity of MEX.HCl, TMNOPs surface charge, and pH of the medium [35-37]. The objective of this study is to evaluate the binding of MEX.HCl as a model of pharmaceutical hydrochloride salt onto the surface of TMONPs such as TiO2, ZnO, and Co3O4 at different pHs to investigate its potential bioavailability in the aquatic environment.
2. Materials and Methods
2.1 Materials
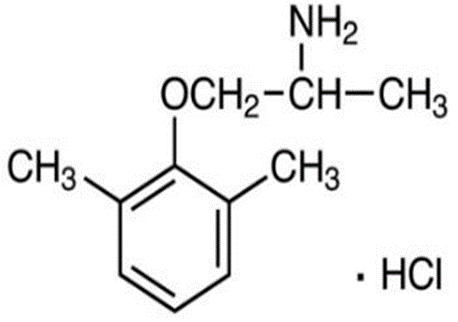
Mexiletine hydrochloride, or 1-(2,6-dimethylphenoxy)-2-propylamine hydrochloride with a molecular formula of C11H17NO·HCl and MW = 215.72 g/mol (Fig.1), Co3O4 (240.8 g/mol) nanopowder with particle size <50 nm, TiO2 (79.865 g/mol) nanopowder with particle size <50 nm and ZnO (81.406 g/mol) nanopowder with particle size <50 nm were obtained from Sigma-Aldrich (St. Louis, MO) (Figure1). Sodium phosphate dibasic (Na2HPO4) and sodium phosphate monobasic (NaH2PO4) were obtained from Fisher Scientific.
2.2 Procedures and analytical method
2.2.1 Batch adsorption procedure
Adsorption studies were conducted to study the binding affinity of MEX.HCl onto TiO2, Co3O4, and ZnO using the static technique. Experiments were conducted by simultaneously adding the required concentration of MEX.HCl onto 1 mg/mL adsorbents at pH 9.4 and at room temperature in a static technique to determine the kinetics study. Binding isotherm was obtained at five different concentrations of 15, 30, 45, 60, and 75 μg/mL at pH 5.1, 7.2, and 9.4 onto 1 mg/mL of adsorbents and at room temperature. All samples were ultrasonically homogenized for 5 minutes and allowed to undergo static adsorption for 24 hours before injecting the supernatant for CE analysis.
2.3.1 Capillary electrophoresis analysis
The binding of pharmaceutical compounds onto TMONPs was investigated using an Agilent capillary electrophoresis instrument (G1600AX CE system, Agilent Technologies, Santa Clara, USA) equipped with a capillary of 28 cm length from the inlet to the detection window. The diode array detector was set up at UV wavelengths of 200, 230, and 254 nm. Background Electrolyte (BGE) of 10 mM sodium phosphate dibasic was dissolved in deionized water and adjusted with different weights of sodium phosphate monobasic to obtain the required pH.
2.3.2 Determination of pharmaceutical compounds by CE-UV
CE was used to analyze the unknown concentrations of MEX.HCl before and after binding with TMONPs using a standard calibration curve by determining the difference in peak heights. Each binding test was repeated at least three trials, and the average result was calculated. Different solutions of MEX.HCl (100, 50, 25, 12.5,6.25,3.125, and 1.5 μg/mL) were prepared by serial dilution from the stock solution (100 μg/mL). The binding affinity (% binding) and the amount adsorbed (qe) were calculated according to Eqs. (1) and (2) respectively:
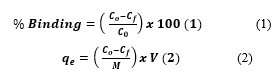
where and are the initial and final concentrations of the adsorption process, respectively. and represent the mass of nanoparticles and the volume of MEX.HCl standard solution respectively. Triplicate runs were carried out to determine the reproducibility of the analysis result and the standard deviation (SD) was calculated to verify the precision of the analysis.
2.4 Determining the point of zero charge for TMONPs
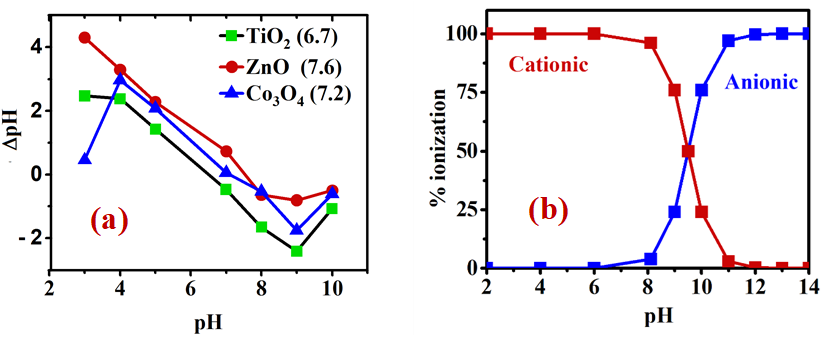
To characterize the surface charges of TiO2, ZnO, and Co3O4 nanoparticles, a titration method was used to determine their individual points of zero charge (PZC) [38,39]. The solution ionic strength was set at 1 mM KCl, followed by pH adjustment to several values between 3 and 10 using NaOH or HCl solution (0.1 M). Each solution containing 1mg/mL of nanoparticles at a different pH was homogenized using ultrasound, and then left in a static process for 24 hours at room temperature. The final pH was then measured of each solution. The point of zero charge (PZC) value could then be determined by the point of intersection on the x-axis when ΔpH was plotted versus pH.
2.5 Binding kinetics
The rate of binding of MEX.HCl onto the surface of TMONPs at given experimental conditions was investigated by linear and non-linear kinetic forms of pseudo-first order (PFO) and pseudo-second order (PSO) models along with the intraparticle diffusion model (IPD).
2.6 Binding isotherm
The relationship of adsorption isotherms at equilibrium is critical to investigate the proposed binding interaction between MEX.HCl and the surface of TMONPs. There are several equilibrium models that have been developed to describe this relationship. In this study, we used linear and non-linear forms of Freundlich and Langmuir models. OriginPro software was used for fitting the experimental data and calculating linear kinetic and isotherm parameters.
2.7 Error analysis
Error analysis functions such as the sum of the squares of the errors (SSE) was used to evaluate the linear isotherm and kinetic models (Eq. 3). For non-linear isotherm and kinetic models chi-square test (𝒳2) was used and calculated as shown in Eq. (4).
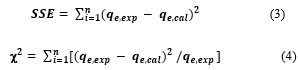
where and are the experimental and the calculated values of binding at equilibrium (Ce) of MEX.HCl,n represented the number of data points during the experiments.
It should be emphasized that the smaller the error values, the better the predictive model’s performance, indicating that there is an agreement between the experimental and calculated data, and the more the model becomes favourable.
3. Results and Discussion
3.1 Binding kinetics of MEX.HCl on TMONPs
To explain the binding mechanism, it is useful to investigate the binding rate and the rate-limiting step (mass transfer) in addition to the adsorption capacity of TMONPs. The non-linear and linear forms of pseudo-first order (PFO) and pseudo-second order (PSO) models were used to study the kinetics and to determine the rate constant of MEX.HCl binding onto TMONPs. The nonlinear and linear equations of PFO are expressed in Eqs. (5-6):
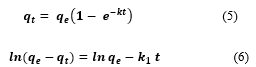
Also, the nonlinear and linear equations of PSO are represented in Eqs. (7-8):
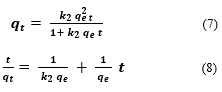
where and (mg/g) are the amount of MEX.HCl adsorbed at equilibrium and at any time (t), respectively, k1(min−1) and (g mg−1 min−1) are the PFO and the PSO rate constants respectively.
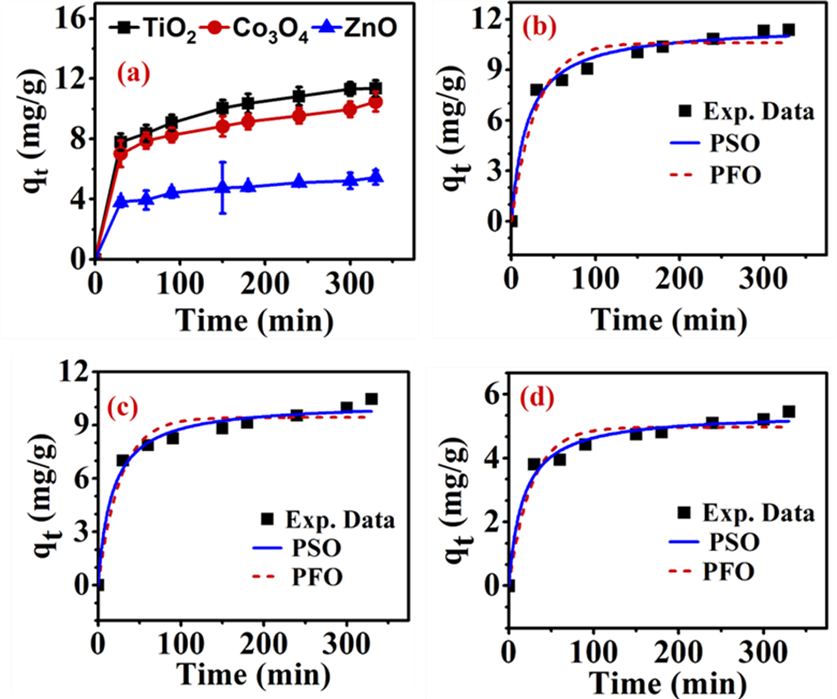
The non-linear forms of PFO and PSO for the binding of MEX.HCl onto the surface of TMONPs (TiO2, ZnO and Co3O4) are represented by plotting the values of qt versus the bindingtime (t) and shown in Figure 2(a-d). The results displayed in Figure 2(a) show an initial rapid stage followed by a slower stage, which may be suggestive of various interactions between MEX.HCl and the surface of TMONPs. In the rapid stage the binding quantity (qt) of MEX.HCl started to increase with the increase of the contact time. Then the adsorption rate decreases with increasing the time where most binding took place at about 240 minutes for TiO2, Co3O4, and ZnO with an experimental value (qe,exp) being equal to 11.37 ± 0.53, 10.54 ± 0.52 and 5.45 ± 0.47 mg/g respectively. The binding process then reached equilibrium at 300 minutes. In the rapid stage, the surface of TMONPs provides a large number of binding sites for MEX.HCl resulting in a high rate to occur. The rate slows down with increasing time due to the decrease in the number of vacant sites. In addition, the repulsive forces of the bonded MEX.HCl molecules tend to prevent more MEX.HCl molecules to bind at other sites and lead to reaching equilibrium. Figure 2 (b-d) shows fitting the binding data to the nonlinear plots of PFO
and PSO kinetic models.
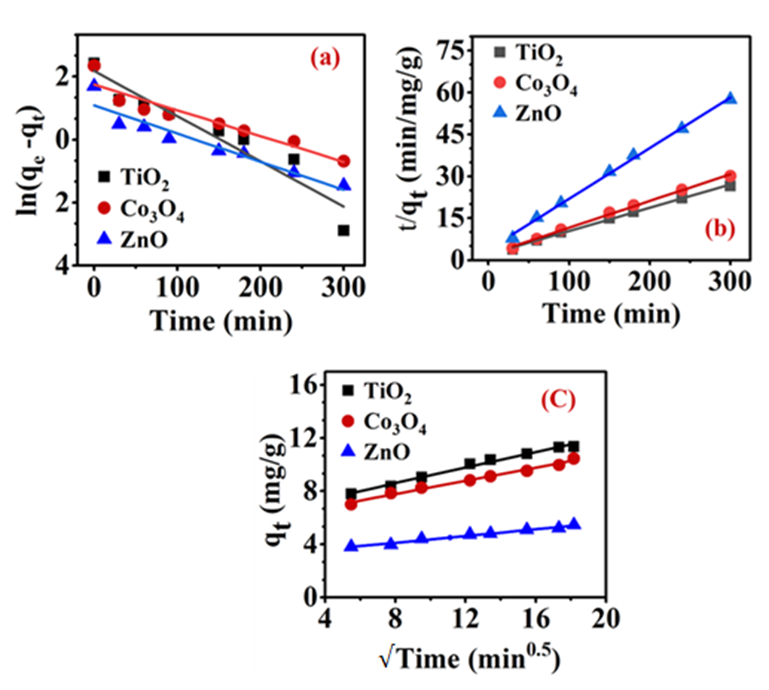
Also, Figure 3 (a-b) represented fitting the linear plots of PFO and PSO kinetic models for TiO2, Co3O4, and ZnO respectively. To compare the application of the proper model displaying the goodness of fit for the experimental data, the error analysis function of sum of square errors (SSE) Eq. (3), chi-square test (𝒳2) Eq. (4) and the coefficient of determination (R2) were used. The determined parameters for non-linear and linear kinetic models along with the values of error analysis functions are presented in Table 1.
The results of binding MEX.HCl onto the surface of TiO2 at pH 9.4 indicated that values of R2 from linear and non-linear PSO model in the range of 0.987 to 0.998 are higher than the values for linear and non-linear PFO (0.882 to 0.957). Also, the values of calculated adsorbed quantity at equilibrium (qe, Cal) for linear and non-linear PSO (12.15 ± 0.002 and 11.61 ± 0.309) are very similar to the experimental adsorbed quantity (qe, exp) (11.37 ± 0.53). Furthermore, the values of SSE (1.33) for linear PSO and (1.24) for non-linear PSO respectively, are lower than the obtained values of (4.26) for linear PFO and (1.45) for non-linear PFO. Similarly, binding of MEX.HCl onto the surface of Co3O4 showed that the values of R2 were 0.987 to 0.991 for non-linear and linear forms of PSO which are higher than 0.898 to 0.959 for linear and non-linear PFO model respectively. The values of R2 along with the values of SSE for the binding of MEX.HCl onto the surface of ZnO resulted in a similar trend as shown in Table 1. This indicated that the non-linear and linear forms of pseudo-second order fit well with the data and are appropriate to represent the binding of MEX.HCl onto the surface of TiO2, Co3O4, and ZnO nanoparticles. However, the linear form of PSO has a goodness of fit better than its non-linear form. Therefore, representing the kinetic binding of MEX.HCl onto the surface of TMONPs is in the order of linear PSO > non-linear PSO > linear PFO > non-linear PFO.
|
Model/ TMONPs |
TiO2 |
||||
|
qe, exp (mg/g) |
qe, calc (mg/g) |
k1(min-1), K2 (g mg-1 min-1) |
R2 |
SSE (mg2/g2) |
|
|
PFO Linear |
11.37 ± 0.53 |
8.87 ± 0.39 |
0.014 |
0.882 |
4.26 |
|
PFO Non-linear |
11.37 ± 0.53 |
10.60 ± 0.34 |
0.034 |
0.957 |
1.45 |
|
PSO Linear |
11.37 ± 0.53 |
12.15± 0.002 |
0.003 |
0.998 |
1.33 |
|
PSO Non-linear |
11.37 ± 0.53 |
11.61 ± 0.309 |
0.0046 |
0.987 |
1.24 |
|
Co3O4 |
|||||
|
PFO Linear |
10.54 ± 0.52 |
5.75 ± 0.18 |
0.008 |
0.898 |
3.26 |
|
PFO Non-linear |
10.54 ± 0.52 |
9.42 ± 0.293 |
0.037 |
0.985 |
2.6 |
|
PSO Linear |
10.54 ± 0.52 |
10.54 ± 0.003 |
0.005 |
0.991 |
1.16 |
|
PSO Non-linear |
10.54 ± 0.52 |
10.28 ± 0.28 |
0.0058 |
0.959 |
0.23 |
|
ZnO |
|||||
|
PFO Linear |
5.45 ± 0.47 |
2.97 ± 0.19 |
0.009 |
0.912 |
0.97 |
|
PFO Non-linear |
5.45 ± 0.47 |
4.97 ± 0.16 |
0.038 |
0.956 |
6.54 |
|
PSO Linear |
5.45 ± 0.47 |
5.69 ± 0.004 |
0.007 |
0.996 |
0.35 |
|
PSO Non-linear |
5.45 ± 0.47 |
5.40± 0.152 |
0.011 |
0.984 |
1.57 |
Table 1: Linear and non-linear kinetic parameters and error analysis functions for binding of 20 µg/mL of MEX.HCl onto TMONPs (TiO2, Co3O4, and ZnO) at pH 9.4 and at room temperature. SD of the slope and y-intercept were used to determine uncertainties.
Furthermore, the intra-particle diffusion (IPD) model (Weber-Morris) was used to determine the rate-limiting step and the diffusion mechanism as shown in Eq. (9)
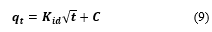
where is the rate constant of intraparticle diffusion (mg/g.min1/2) and provides information that is directly proportional to the boundary layer thickness (mg/g) on the diffusion [40].
An increase in the value of C indicates an increase in the boundary layer thickness and a decrease in the external mass transfer, which in turn leads to an increase in internal mass transfer. The values of kid and C can be obtained from the slope and intercept, respectively.
Figure 3 (c) shows the linear relationship between qt and square root of time, for the IPD model. If the straight line passes through the origin, the rate limiting step of the binding process can be attributed only to the intraparticle diffusion process [41]. Table 2 indicates that the IPD model is suitable for the depiction of experimental data because the R2 values range from 0.980 to 0.988.
|
TMONPs |
Intra-particle diffusion |
|||
|
kid (mg/g.min1/2) |
C (mg/g) |
R2 |
SSE (mg2/g2) |
|
|
TiO2 |
0.29 ± 0.01 |
6.26 ± 0.17 |
0.988 |
0.146 |
|
Co3O4 |
0.25 ± 0.01 |
5.78 ± 0.16 |
0.985 |
0.109 |
|
ZnO |
0.13 ± 0.01 |
3.10 ± 0.01 |
0.98 |
0.048 |
Table 2: Intra-particle diffusion kinetic parameters for binding of 20 µg/mL MEX.HCl to TiO2, Co3O4, and ZnO nanoparticles at room temperature at pH 9.4.
However, none of the straight lines pass through the origin. Significant intercept (C) values of 6.26 ± 0.17, 5.78 ± 0.16, and 3.10 ± 0.098 (mg/g) were obtained in the order TiO2 > Co3O4 > ZnO. It is noted that these results agree with their corresponding intraparticle diffusion rates ( ) of 0.29 ± 0.01 > 0.25 ± 0.01 > 0.13 ± 0.01 respectively. According to these trends, MEX.HCl is bound to the internal surfaces of TMONPs, and the rate-limiting step consisted of intra-particle diffusion [42]. The IPD model assumes that mass transfer occurs as a result of the diffusion of MEX.HCl molecules within the pores of TMONPs [41].
2.1 Binding affinity of MEX.HCl onto TMONPs
Binding affinity measures the strength of binding between the adsorbent and the adsorbate. Depending on the binding affinity values at equilibrium, more insight into the adsorption process can be gained for potential improvements [43]. To investigate the adsorption isotherm and evaluate the affinity of MEX.HCl onto TMONPs, the linear and non-linear forms of Freundlich and Langmuir isotherm models were used. To compare the application of the proper model displaying the goodness of fit for the experimental data, two error analysis functions as the sum of square errors (SSE) Eq. (3), and the chi-square (R2) Eq. (4) test along with the coefficient of determination (R2) were used.
The Freundlich isotherm assumes that different sites with several adsorption energies are involved on a heterogeneous surface. The non-linear form Eq. (10) can be transformed into the linear form Eq. (11) and expressed as follow:
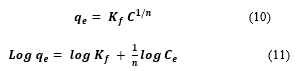
Where, is the measure of the capacity of adsorbent (TMONPs) and is the measure of how an affinity for the adsorbate (MEX.HCl) varies with changes in adsorption density. Higher values indicate stronger adsorbate/adsorbent affinities and better distribution of adsorbate towards the surface of the adsorbent [44]. Also, an increase in values indicate high capacity of adsorbent to adsorbate.
On the other hand, Langmuir adsorption isotherm proposed a monolayer binding onto a homogeneous adsorbent surface that possesses identical and energetically equivalent sites. The non-linear form Eq. (12) and the transformed linear form Eq. (13) can be written as:
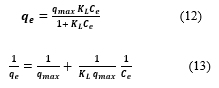
Where (L/g) is the Langmuir isotherm constant, (mg/g) and (mg/L) is the amount adsorbed and the liquid concentration of the adsorbate at equilibrium respectively, and (mg/g) is the maximum amount of MEX.HCl adsorbed per (g) of adsorbent.
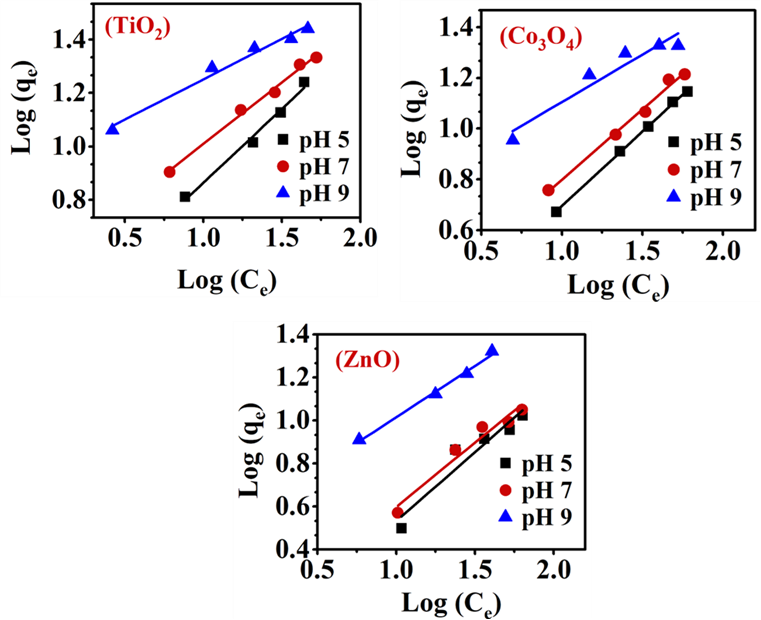
The linear plots of Freundlich for TiO2, Co3O4, and ZnO nanoparticles at pH 5.1, pH 7.2, and pH 9.4 are presented in Figure 4. The data of log Ce versus log qe fitted well with the Freundlich equation based on both the good R2 values (in the range of 0.981- 0.993) and the low SSE values (in the range of 2x10-5- 5x10-5) in Table 3. The results, which represent the adsorption capacities for MEX.HCl, increased with higher pH levels in the order of pH 5.1 < pH 7.2 < pH 9.4 for TMONPs. At pH 9.4, the value reached 8.9 L/g for TiO2 > 5.38 L/g for Co3O4 > 3.47 L/g for ZnO. Also, values, which represent the affinity of TMONPs for MEX.HCl, also increased with higher pH levels in the order of pH 5.1 < pH 7.2 < pH 9.4. At pH 9.4, the value reached 3.3 for TiO2 > 2.7 for Co3O4 > 2.1 for ZnO. All these results
indicate binding of MEX.HCl molecules with a distribution of heterogenous sites on the surface of each TMONP as controlled by a physisorption mechanism.
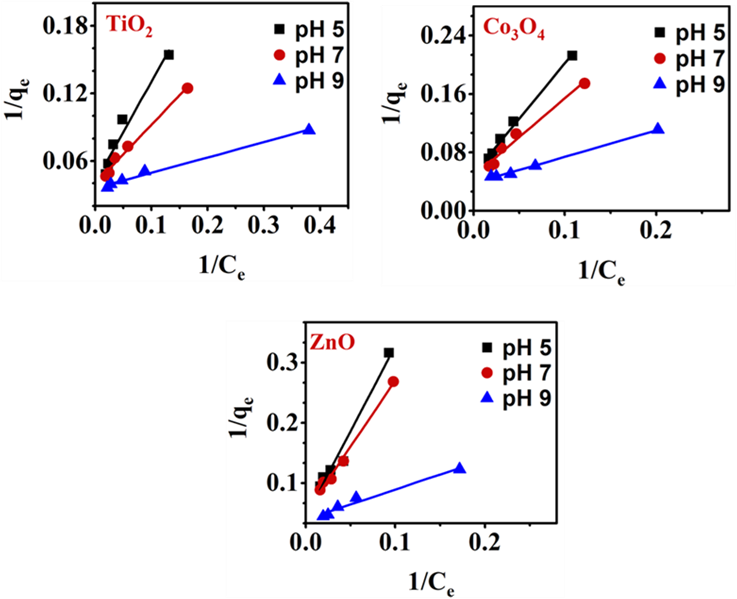
The Langmuir isotherm model was next tested by plotting 1/qe versus i/Cein Figure 5. Data fitting in Table 3 for TiO2 nanoparticles produced reasonable R2 values (in the range of 0.958 to 0.991) but high SSE values (in the range of 9×10-4 to 3×10-3). The maximum capacity (qmax) of TiO2 predicted from Langmuir model was the highest and increased from 24.28 mg/g at pH 5.1 to 27.85 mg/g at pH 9.4. By comparison, the results for Co3O4, and ZnO nanoparticles increased from 19.8 to 26.4 mg/g and from 19.5 to 25.2 mg/g respectively. The best binding capacity for MEX.HCl is again provided by TiO2 nanoparticles. Taking both isotherm models together, our interpretation of all results is that MEX.HCl binding forms a monolayer of adsorbate molecules on the TMONPs surface that possesses identical or energetically equivalent sites, followed by accumulation of additional molecules on the heterogeneous surface with different adsorption energies. This proposed mechanism is similar to the adsorption of Cu (II) onto the surface of Biochar composites [45].
The experimental data were further subjected to the non-linear forms of Freundlich and Langmuir and the results are shown in Figure 6 and Table 3 and 4 respectively. The results of non-linear Freundlich indicated that the values of R2 were in the range from 0.985 to 0.987, 0.984 to 0.999 and 0.954 to 0.970 for TiO2, Co3O4, and ZnO at pH 9.4 to pH 5.1 respectively. Also, the values of Chi-Sqr test were very small as 0.249 to 1.15, 0.023 to 0.484 and 0.24 to 0.470 for TiO2, Co3O4, and ZnO at pH 9.4 to pH 5.1 respectively. This results showed that the non-linear form of Freundlich is also sutitable to fit the experimental data well for TiO2 and Co3O4. While the linear form is more suitable than the non-linear form for ZnO. In addition, the R2 values for the non-linear form of Langmuir were in the range of 0.960 to 0.978, 0.904 to 0.992 and 0.924 to 0.965 for TiO2, Co3O4, and ZnO at pH 9.4 to pH 5.1 respectively. The values of Chi-Sqr test were higher than the values of non-linear Freundlich as 1.10 to 1.67, 0.143 to 0.904, and 0.578 to 1.60 for TiO2, Co3O4, and ZnO at pH 9.4 to pH 5.1 respectively. Therefore, the linear form of Langmuir is better than the non-linear form of Langmuir isotherm to express the binding process. In general the binding process is in the order of linear Freundlich > linear Langmuir > nonlinear Freundlich > non-linear Langmuir.
Table 3: Parameters of Linear and non-linear Freundlich model for the adsorption of MEX.HCl onto TiO2, Co3O4, and ZnO at different pHs (pH 5.1, pH 7.2, and pH 9.4), [MEX.HCl]0 = 15, 30,45,60, and 75 µg/mL at room temperature.
Table 4: Parameters of Linear and non-linear Langmuir model for the adsorption of MEX.HCl onto TiO2, Co3O4, and ZnO at different pHs (pH 5.1, pH 7.2, and pH 9.4), [MEX.HCl]0 = 15, 30,45,60, and 75 µg/mL at room temperature.
2.2 Favorability of the Binding Process
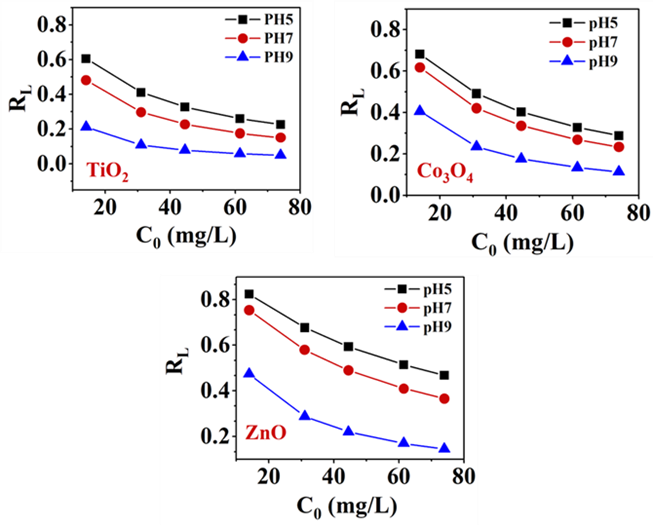
The indication of the favorability of the binding of MEX.HCl onto the surface of TMONPs can be investigated from both the value obtained from Freundlich and the Langmuir separation factor (RL) value [45]. The binding process is favorable when 0 < RL < 1, and unfavorable when RL > 1. RL is a dimensionless parameter that can be calculated:
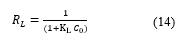
where is Langmuir constant (mg/g) and is the initial concentration of MEX.HCl (mg/L).
The results in Figure 7, represented the plot of separation factor (RL) versus the initial concentrations of MEX.HCl at different pHs and at room temperature. It is indicated that all the values of RL for TiO2, Co3O4, and ZnO at different pHs lie between 0 and 1, which indicated the favorability of binding of MEX.HCl onto the surface of TMONPs.
2.3 Effect of pH on the binding affinity of MEX.HCl onto TMONPs
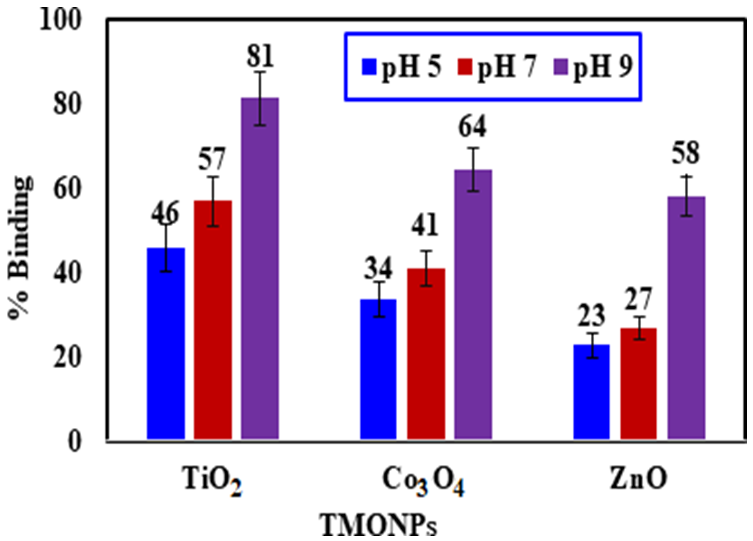
To investigate the effect of pH on the binding of MEX.HCl onto TMONPs, experiments were performed on TiO2, Co3O4, and ZnO at pH 5.1, 7.2 and 9.4 and at room temperature. The % bindings of MEX.HCl onto TiO2, Co3O4 and ZnO are presented in Figure 8. The results indicated that MEX.HCl can bind onto TiO2, Co3O4, and ZnO nanoparticles in alkaline, neutral, and acidic solutions. TiO2 attained the highest % binding compared to Co3O4, and ZnO at all three solution pH levels. At pH 5.1, mainly MEX.H+ (20 µg/mL) bound to TiO2 up to 46 ± 1.5 %, which is higher than 34 ± 1.2 % for Co3O4 and 23 ± 1 % for ZnO. According to the pKa and PZC values, MEX.H+ binds to TiO2 nanoparticles due to coordination bonding. Importantly, the binding of MEX.H+ to TiO2 increased to 57 ± 1.2 % at pH 7.2 and reached 81 ± 1 % at pH 9.4. At pH 7.2 the surface charge of TiO2 becomes slightly negative which may enhance the electrostatic interaction with the positively charged MEX.H+. At pH 9.4, the surface of TiO2 becomes more negatively charged, thus enhancing the electrostatic attraction to MEX.H+ and resulting in the highest %binding. Also, Co3O4 binds to MEX.H+ with 34 ± 1.2 % at pH 5.1 and increases in an alkaline medium to reach 64 ± 2.1 % at pH 9.4. The empirical PZC value of Co3O4 is 7.2, which is higher than the PZC value of TiO2, leading to an increase in the amount of positive charge at pH 5.1 on the Co3O4 surface. Thus, the electrostatic repulsion between the positively charged conjugate acid ions and the positive H+ ions on the surface of Co3O4 in an acidic medium lead to a decrease in the binding %. This may be attributed to the synergistic effect of the redox cycle of Co2+/Co+3 on the surface of Co3O4 which contributes to an increase in the positive charge and lead to a decrease in the binding % of (MEX.H+) [46]. Similar results were shown for the adsorption of pharmaceuticals such as tetracycline.HCl (TEC) [47]. It was noted that the adsorption process at different pH levels was related to the molecular structure of TEC and the surface charges on the adsorbent surface. Furthermore, the opposite trend was reported for the adsorption of different negatively charged ions of emerging contaminants (atenolol, carbamazepine, ciprofloxacin, diclofenac, gemfibrozil, and ibuprofen) on porous graphene (PG) [47]. The adsorption was drastically reduced above pH 9.4 due to an increase in the electrostatic repulsion between those negatively charged ions and the PG nano-sheet covered with negative hydroxide ions, as confirmed by both the pKa value of TEC and the PZC value for PG [48]. Furthermore, the binding of MEX.HCl onto ZnO was lower in acidic and neutral mediums with 23 ± 1 % and 27 ± 1.6 % respectively. However, the % binding increased to reach 58 ± 1.7 % at pH 9.4 due to increasing the surface negative charge. Similarly, it was reported that the adsorption of asphaltene was lower on the surface of ZnO compared to Co3O4 [49].
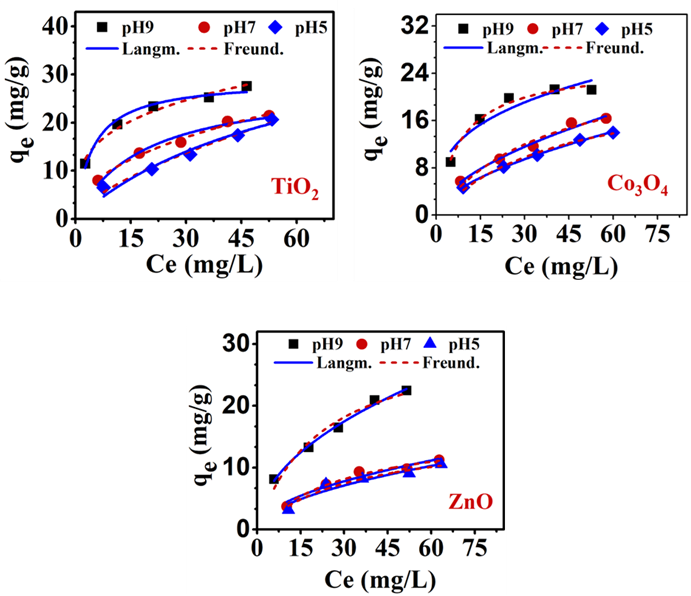
2.4 Effect of initial [MEX.HCl] on the binding to TMONPs surfaces
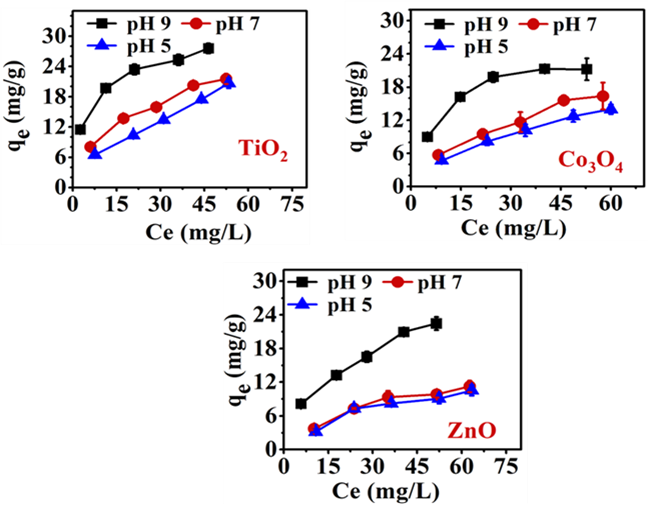
The relationship between the qe (equilibrium adsorption capacity) values of TMONPs and Ce (the equilibrium concentration of MEX.HCl) in an aqueous solution is presented in Figure 9. Increasing the initial concentrations of MEX.HCl in the solution from 15 to 75 µg/mL increased the driving force which may enhance the growth of its binding to TMONPs surfaces. The binding of MEX.H+ onto the surface of TiO2 starts to grow from 21 ± 1 mg/g at pH 5.1 and increases to 28 ±1 mg/g at pH 9.4. Also, the binding of MEX.H+ grows on the surface of Co3O4 from 14 ± 1 mg/g at pH 5.1
to 21 ± 2 mg/g at pH 9.4, while ZnO exhibited low growth of binding from 11 ± 1 mg/g at pH 5 to 22 ± 1 mg/g at pH 9.4 after 24 hr.
2.5 Binding Mechanism of MEX.HCl
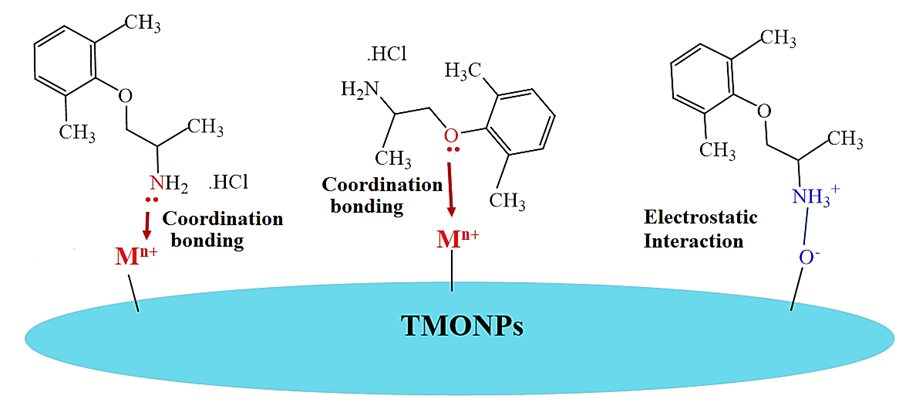
As suggested above, the mechanism of MEX.HCl binding to the TMONPs was based on the pKa value and the molecular structure of MEX.HCl versus the surface charge of TMONPs at the three pH levels studied. MEX.HCl (with pKa 9.5) and the TMONPs (TiO2, Co3O4, and ZnO) with PZC of 6.7, 7.2 and 7.6 respectively would carry different charges in the solution due to protonation-deprotonation processes (Figure 10). Optimal binding occurred at pH 9.4 where MEX.H+ acts as the conjugate acid with a slight positive charge and the surface of each TMNOPs bears a negative charge. This strongly suggests that the adsorption mechanism may involve electrostatic interaction between the negative OH- groups on the TMONPs surface and the positive charge of MEX.H+. Furthermore, coordination bonding between the amino/phenoxy group of MEX.HCl and the Zn2+, Co2+/Co3+, and Ti4+on the surface of TMONPs may also contribute to the overall binding mechanism as shown by the schematic diagram Figure 11.

4. Conclusion
Capillary electrophoresis was used to investigate the binding of MEX.HCl onto the surface of TiO2, Co3O4, and ZnO at different pHs and at room temperature. MEX.HCl binds onto TMONPs through electrostatic interaction and coordinated bonding. The kinetics and adsorption isotherms of the adsorption process, as well as the influence of pH on the adsorption process, were investigated. Alkaline water at pH 9.4 led to efficient binding of MEX.HCl molecules, at 81 ± 1 %, 64 ± 2 % and 58 ± 2 % for TiO2, Co3O4, and ZnO respectively. Investigation of rate-limiting steps by the IPD model revealed that MEX.HCl binding occurred also within the internal surface of porous TMONPs, in the order of TiO2 > Co3O4 > ZnO. Although both Freundlich and Langmuir models contribute to the binding affinity of MEX.HCl on TMONP surfaces, the former model showed better fitting with higher R2 values and lower SSE uncertainties. The binding isotherm fits well with the Freundlich model, and the binding kinetics followed the PSO model involving IPD. It can be concluded that MEX.HCl molecules bind onto heterogenous sites on TMONPs mainly under the control of physisorption. The binding capacity (qmax) was maximal at pH 9.4, following the order of 27.0 > 26.4 > 25.2 mg/g for TiO2, Co3O4, and ZnO respectively. The small values, at different pH levels, confirmed favorable binding of MEX.HCl onto the studied TMONPs, mainly accomplished via electrostatic interaction and coordination bonding.
Credit statement
Edward Lai: Conceptualisation, resources, review & editing, supervision, project administration, funding acquisition. Eman Elmorsi: investigation, visualisation, methodology, formal analysis, writing original draft, review & editing.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors acknowledge support from the Natural Sciences and Engineering Research Council of Canada (NSERC) [RGPIN-2018- 05320] for the financial support of this research.
References
- Alnajjar M, Hethnawi A, Nafie G, et al. Silica-alumina composite as an effective adsorbent for the removal of metformin from water. J Environ Chem Eng 7 (2019): 102994.
- Mutiyar PK, Mittal AK. Occurrences and fate of selected human antibiotics in influents and effluents of sewage treatment plant and effluent-receiving river Yamuna in Delhi (India). Environ Monit Assess 186 (2014): 541-557.
- Committee for medicinal products for human use assessment report (2018).
- Kumar V, Bansal V, Madhavan A, et al. Active pharmaceutical ingredient (API) chemicals: a critical review of current biotechnological approaches. Bioengineered 13 (2022): 4309-4327.
- Elder DP, Holm R, Diego HL de. Use of pharmaceutical salts and cocrystals to address the issue of poor solubility. Int J Pharm 453 (2013): 88-100.
- Tran TTD, Tran PHL. Nanoconjugation and encapsulation strategies for improving drug delivery and therapeutic efficacy of poorly water-soluble drugs. Pharmaceutics 11 (2019): 325.
- Wiedmann TS, Naqwi A. Pharmaceutical salts: theory, use in solid dosage forms and in situ preparation in an aerosol. Asian J Pharm Sci 11 (2016): 722-734.
- Bharate SS. Recent developments in pharmaceutical salts: FDA approvals from 2015 to 2019. Drug Discov Today 26 (2021a): 384-398.
- Bharate SS. Carboxylic acid counterions in FDA-approved pharmaceutical salts. Pharm Res 38 (2021b): 1307-1326.
- Gupta D, Bhatia D, Dave V, et al. Salts of therapeutic agents: chemical, physicochemical, and biological considerations. Molecules 23 (2018): 1719.
- Lee H. Salt formation of pharmaceutical compounds and associated genotoxic risks. In: Pharmaceutical Industry Practices on Genotoxic Impurities. CRC Press (2014): 404-445.
- Nakagawa H, Munakata T, Sunami A. Mexiletine block of voltage-gated sodium channels: isoform- and state-dependent drug–pore interactions. Mol Pharmacol 95 (2019): 236-244.
- Modoni A, D’Amico A, Primiano G, et al. Long-term safety and usefulness of mexiletine in a large cohort of patients affected by non-dystrophic myotonias. Front Neurol 11 (2020): 300.
- Statland JM. Mexiletine for symptoms and signs of myotonia in nondystrophic myotonia. JAMA 308 (2012): 1357.
- Díaz-Manera J, Urtizberea JA, Schey C, et al. Impact of restricted access to, and low awareness of, mexiletine on people with myotonia: a real-world European survey. Neuromuscular Disorders 33 (2023): 208-217.
- Kasprzyk-Hordern B. Pharmacologically active compounds in the environment and their chirality. Chem Soc Rev 39 (2010): 4466-4503.
- Andrews JL, Nilsson Lill SO, Freitag-Pohl S, et al. Derisking the polymorph landscape: the complex polymorphism of mexiletine hydrochloride. Cryst Growth Des 21 (2021): 7150-7167.
- Remko M, Smieško M, Benová A. Theoretical study of mexiletine and its interaction with cationic and anionic receptor sites. Il Farmaco 54 (1999): 653-659.
- Akıncı E, Yüzbasıoglu Y, Coskun F. Hemodialysis as an alternative treatment of mexiletine intoxication. Am J Emerg Med 29 (2011):1235.e5-1235.e6.
- Belal TS, Haggag RS, Shaalan RA. Selective and stability-indicating methods for the simultaneous determination of mexiletine hydrochloride and/or its related substance: 2,6-dimethylphenol. J AOAC Int 91 (2008): 720-730.
- Kaushik S, Alexander KS. A modified reverse-phase HPLC method for the analysis of mexiletine hydrochloride. J Liq Chromatogr Relat Technol 26 (2003): 1287-1296.
- Aturki Z, Rocco A, Rocchi S, et al. Current applications of miniaturized chromatographic and electrophoretic techniques in drug analysis. J Pharm Biomed Anal 101 (2014): 194-220.
- Agnihotri AS, Varghese A, M N. Transition metal oxides in electrochemical and bio sensing: a state-of-art review. Appl Surf Sci Adv 4 (2021): 100072.
- Huang Y-W, Wu C, Aronstam RS. Toxicity of transition metal oxide nanoparticles: recent insights from in vitro studies. Materials 3 (2010): 4842-4859.
- Kaegi R, Voegelin A, Sinnet B, et al. Behavior of metallic silver nanoparticles in a pilot wastewater treatment plant. Environ Sci Technol 45 (2011): 3902-3908.
- Al-Kattan A, Wichser A, Vonbank R, et al. Characterization of materials released into water from paint containing nano-SiO2. Chemosphere 119 (2015): 1314-1321.
- Meier C, Voegelin A, Pradas del Real A, et al. Transformation of silver nanoparticles in sewage sludge during incineration. Environ Sci Technol 50 (2016): 3503-3510.
- Bundschuh M, Filser J, Lüderwald S, et al. Nanoparticles in the environment: where do we come from, where do we go to? Environ Sci Eur 30 (2018): 6.
- Gottschalk F, Sun T, Nowack B. Environmental concentrations of engineered nanomaterials: review of modeling and analytical studies. Environ Pollut 181 (2013): 287-300.
- Tolaymat T, el Badawy A, Genaidy A, et al. Analysis of metallic and metal oxide nanomaterial environmental emissions. J Clean Prod 143 (2017): 401-412.
- Mueller NC, Nowack B. Exposure modeling of engineered nanoparticles in the environment. Environ Sci Technol 42 (2008): 4447-4453.
- Keller AA, McFerran S, Lazareva A, et al. Global life cycle releases of engineered nanomaterials. J Nanopart Res 15 (2013): 1692.
- Sun TY, Bornhöft NA, Hungerbühler K, et al. Dynamic probabilistic modeling of environmental emissions of engineered nanomaterials. Environ Sci Technol 50 (2016): 4701-4711.
- Ates M, Demir V, Arslan Z, et al. Toxicity of engineered nickel oxide and cobalt oxide nanoparticles to artemia salina in seawater. Water Air Soil Pollut 227 (2016): 70.
- McNamara WR, Milot RL, Song H, et al. Water-stable, hydroxamate anchors for functionalization of TiO2 surfaces with ultrafast interfacial electron transfer. Energy Environ Sci 3 (2010): 917.
- Gulley-Stahl H, Hogan PA, Schmidt WL, et al. Surface complexation of catechol to metal oxides: an ATR-FTIR, adsorption, and dissolution study. Environ Sci Technol 44 (2010): 4116-4121.
- Brennan BJ, Llansola Portolés MJ, Liddell PA, et al. Comparison of silatrane, phosphonic acid, and carboxylic acid functional groups for attachment of porphyrin sensitizers to TiO2 in photoelectrochemical cells. Phys Chem Chem Phys 15 (2013): 16605.
- Ibanez JG, Hernandez-Esparza M, Doria-Serrano C, et al. The Point of Zero Charge of Oxides. In: Environmental Chemistry. Springer New York, New York, NY (2008): 70-78.
- Taoufik N, Elmchaouri A, Anouar F, et al. Improvement of the adsorption properties of an activated carbon coated by titanium dioxide for the removal of emerging contaminants. J Water Process Eng 31 (2019): 100876.
- Uma Maheswari B, Sivakumar VM, Thirumarimurugan M. Synthesis of novel nanobioadsorbent for the effective removal of Pb2+ and Zn2+ ions—adsorption, equilibrium, modeling, and optimization studies. In: Nano-Biosorbents for Decontamination of Water, Air, and Soil Pollution. Elsevier (2022): 503-528.
- Campos NF, Barbosa CM, Rodríguez-Díaz JM, et al. Removal of naphthenic acids using activated charcoal: kinetic and equilibrium studies. Adsorp Sci Technol 36 (2018): 1405-1421.
- Pholosi A, Naidoo EB, Ofomaja AE. Intraparticle diffusion of Cr(VI) through biomass and magnetite coated biomass: a comparative kinetic and diffusion study. S Afr J Chem Eng 32 (2020): 39-55.
- Ayawei N, Ebelegi AN, Wankasi D. Modelling and interpretation of adsorption isotherms. J Chem 2017 (2017): 1-11.
- Liu S. Cooperative adsorption on solid surfaces. J Colloid Interface Sci 450 (2015): 224-238.
- Hussain A, Yousaf U, Rahman Ch U, et al. Synthesis and application of modified orchard waste biochar for efficient scavenging of copper from aqueous solutions. Arab J Sci Eng 47 (2022): 333-345.
- Fu W, Du X, Su P, et al. Synergistic effect of Co(III) and Co(II) in a 3D Structured Co3O4/Carbon felt electrode for enhanced electrochemical nitrate reduction reaction. ACS Appl Mater Interfaces 13 (2021): 28348-28358.
- Khalil AME, Memon FA, Tabish TA, et al. Nanostructured porous graphene for efficient removal of emerging contaminants (pharmaceuticals) from water. Chemical Engineering Journal 398 (2020): 125440.
- Tabish TA, Memon FA, Gomez DE, et al. A facile synthesis of porous graphene for efficient water and wastewater treatment. Sci Rep 8 (2018): 1817.
- Hosseini SA, Hagjoo R, Baninaam M. Adsorption of asphaltenes onto CaO, Co3O4, Fe3O4 and ZnO supported on 13X zeolite - an isothermal study. Pet Sci Technol 37 (2019): 2330-2337.


 Impact Factor: * 4.1
Impact Factor: * 4.1 Acceptance Rate: 75.32%
Acceptance Rate: 75.32%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 