Efficacy of Biocontrol Agents, Plant Extracts and Fungicides on Fusarium Oxysporum f. sp. Ciceris
Yusuf Ali Abdulle1,5*, Abdinur Ali Osman2, Mohamed Ali Awale3, Abdihakim Osman Heile4, Muhammad Bilal5, Muhammad Nasir Subhani5
1Faculty of Agriculture, Salaam University, Mogadusho, Somalia
2Pomology Department, College of Horticulture, Jilin Agricultural University Changchun, Jilin, China
3Agronomy Department, PMAS-Arid Agriculture University Rawalpindi, Rawalpindi, Punjab, Pakistan
4Department of Environmental Science, University of Lahore, Lahore, Punjab, Pakistan
5Plant Pathology Department, Institute of Agricultural Science, University of Punjab, Lahore, Punjab, Pakistan
*Corresponding Author: Yusuf Ali Abdulle, Faculty of Agriculture, Salaam University, Mogadusho, Somalia
Received: 22 October 2021; Accepted: 01 November 2021; Published: 31 March 2022
Article Information
Citation:
Yusuf Ali Abdulle, Abdinur Ali Osman, Mohamed Ali Awale, Abdihakim Osman Heile, Muhammad Bilal, Muhammad Nasir Subhani. Efficacy of Biocontrol Agents, Plant Extracts and Fungicides on Fusarium Oxysporum f. sp. Ciceris. International Journal of Plant, Animal and Environmental Sciences 12 (2022): 034-043.
View / Download Pdf Share at FacebookAbstract
A comparative study was carried out among biological, chemical, and plant extract control against Fusarium wilt of chickpea. Chickpea is a valued crop and provides nutritious food for an expanding world population and will become progressively significant with climate change. Chickpea crop is getting an alarming threat all over the world. One of the most serious diseases regarding chickpea crop is Fusarium wilt, which is caused by Fusarium oxysporum f. sp. ciceris (Foc). In this research different experiments were conducted for the management of Fusarium wilt of chickpea in vitro; for this purpose, different fungicides, plant extract and biocontrol agents were used. All the fungicides, plant extract and bio control agents reduced the development of Foc significantly as compared to control. By all of these experiments, it is concluded that maximum control is given by Faline is the best effective against Foc and reduced the mycelial colony (80.36%) which was followed by Vampire, (69.64%), among plant extract neem leaf extract is best effective, and had given the maximum colony reduction (31.58%). While, Trichoderma harzianum showed a best biocontrol agent against Fusarium wilt of Chickpea.
Keywords
<p>Chickpea; Crop rotation; Fusarium wilt; Plant extract</p>
Article Details
1. Introduction
Being a vital crop, the Chickpea Cicer arietinum L. is considered the main pulse-crop around the world. It belongs to leguminous family. Chickpea is considered 3rd most popular crop as it contributes 15% world’s total pulse production [1]. Chickpea is primarily used for human consumption and is an important component of the Mediterranean diet and a basic food in India and Pakistan [2]. The chickpea grain is a good and cheap protein source for people in developing countries (particularly in South Asia), who are largely vegetarian, either for reasons of preference or economics [3].
The biggest severe disease regarding chickpea crop is Fusarium wilt caused by Fusarium oxysporum f. sp. ciceris (Foc) [4, 5]. Fusarium wilt is a soil-borne pathogen well known in most of chickpea growing areas. Foc is a saprophytic fungus that may survive in soil or debris up to six years causing big yield losses in years of severe outbreaks of the disease [6]. The fungus penetrates the plant via roots; the germ tube penetrates the epidermal cells of plants, and later the hyphae extend to root cortical region and colonizes the xylem vessels, thus, preventing the upward translocation of water and essential solutes, resulting in wilt [7, 8]. It is very difficult to grip the disease throughout crop rotation due to being soil habitant and its ability to survive on for a longtime period without any host [9]. Effectiveness of wilt management can be enhanced if any type of bio-control agent used with enriched cultures like planting date [10]. Bio-control gives a choice to exploit artificial pesticides full of many benefits for better community approval and condensed ecological effect [11]. Management of fungal diseases using antagonistic microbes, known as biological control, has been the center of intense research across the globe [12]. The biocontrol fungi belonging to genus Trichoderma are ideal for infected plants of chickpea as it has capability to mingle (parasitically & symbiotically) various substrates and living organisms. It can be much effective for infected plants of chickpea with other microorganisms. It is also found that such fungi are excellent defiant microbes to innate artificial chemicals and toxins. While certain strains are difficult to control due to opportunistic invaders, quick growing and abundant spores is still strong antibiotics for every type of climatic zones [13-16]. There are different ways to manage fungal pathogens to decrease yield losses, but the most commonly used method is chemical fungicides [17]. Chemical control has been widely being used in past and present to cope with Fusarium wilt disease. Subhani et al. (2011) [18] observed the effects of 6 fungicides at four different concentrations through poisoned food technique. There was a significant decline in mycelial growth of Foc pathogen with an increase in fungicidal concentration.
Different plants have the capacity to produce secondary metabolites which are characterized by specific smell known as aromatic compounds, such as phenolic acids phenols, quinones, flavones, flavonoids, flavonols, coumarins and tannins [19]. These compounds present antimicrobial activity and help in plant resistance mechanisms against different pathogenic microorganisms including fungi and bacteria [20]. Gupta et al. (1981) [21] reported that phytinoids compound of neem, garlic, and onion inhibit the germination of spore of Foc. Grainge and Ahmed (1988) [22] found that the growth reticence of Foc caused by bulb extract of onion is attributed to existence of alkaloid compounds. Current study is designed to check in vitro the efficacy of homeo-fungicides, methanolic plant extracts at different concentration to control the causal agent of Fusarium wilt of chickpea Foc. Also to determine the effect of Trichoderma spp. against Fusarium wilt of chickpea.
2. Materials and Methods
2.1 Collection of diseased samples
Infected chickpea plants with typical symptoms of wilting were collected from Ayub Agriculture Research Institute, Faisalabad, for isolation of Foc. All the samples were transferred to M.Sc (Hons) Lab Institute of Agricultural Sciences, University of the Punjab, Lahore.
2.2 Isolation, purification and identification of fungal pathogen
The infected portions of the chickpea plant were chopped into little sections of size up to 5-10 mm. The surface of prepared samples was sterilized by a 0.1% solution of sodium hypochlorate for about one minute. The sterilized samples were washed in distilled and autoclaved water for two times and placed on the aseptic blotter paper to dry. The dried samples were transferred to petri plates containing the PDA (Potato dextrose agar) media. Five pieces of samples were placed on media plate at the same distance from the center of the plate. The plates were placed in incubator at 30±2°C for three days with alternative day and night period. Then the tips of hypha from fungal colonies were transferred to Petri plates aseptically containing PDA media. The plates were kept in incubator at 30±2°C for about a week. The fungus was identified on the basis of morphological characters with the help of relevant literature of the Wollen Webber and Reinking (1935) [23] and Synder and Hansan (1946) [24].
2.3 In-vitro management of Foc by various Trichoderma spp.
Different Biological agents were collected from First Fungal Culture Bank of Pakistan at Institute of Agriculture Sciences, University of the Punjab, Lahore, Pakistan as follows:. Trichoderma harzianum, Trichoderma viride, Trichoderma hamatum, Trichoderma atroviride, and Trichoderma longibrachiatum.
2.4 Dual culture technique
The dual culture technique was used to determine the effect of Trichoderma spp. on the pathogen. All antagonist pathogenic combinations were observed on 15 ml of PDA in 90 mm Petri dish. Nine-millimeter disc of fifteen days old fungal cultures were placed on PDA medium, one centimeter away from the edge of plate. Trichoderma spp. (9 mm disc) was placed on the opposite side of the Petri plate. In control the pathogen was alone plated on either side of the plate at the periphery. Five species of Trichoderma, T. harzianum, T. viride, T. hamatum, T. longibrachiatum, and T. atroviride were tested in vitro for their antagonistic effect on Foc. The experiment was repeated 5 times independently with 5 replicates.
2.5 Preparation of plant extracts
For the preparation of plant extract, plant material was collected from the market, including bulb of onion Allium cepa, garlic Allium sativum, rhizome of ginger Zingiber officinale, and neem Azadirachta indica leaves in required quantity. Plant material was dried in the shade for some days to reduce water contents. Different concentrations viz; 5 ppm, 10 ppm, 15 ppm, and 20 ppm of plant extract were prepared by using modified Borum and Sunclair’s technique (1968) [25]. The experiment was repeated 5 times independently with 5 replicates.
2.6 Evaluation of fungicides against Foc
By poison food technique, four different fungicides were collected from First Fungal Culture Bank of Pakistan at Institute of Agriculture Sciences, University of the Punjab, Lahore, Pakistan and were evaluated against Foc. Faline, Vampire, Ventage, and Rigrous at different concentrations 5, 10, 15 and 20 ppm by using modified Borum and Sunclair’s technique, (1968) [25]. A weighed quantity of each of the fungicide was amended with sterilized chickpea seed meal agar (CSMA) to obtain required concentration of fungicides. Chickpea seed meal agar without fungicide served as control. The experiment was repeated 5 times independently with 5 replicates.
2.7 Statistical analysis
Data of fungal colony growth was recorded on 3, 5, and 7days interval and compared with control fungal growth. Data was analysed using factorial analysis of variance (ANOVA) Statistix software (Version 8.1) (Tallahassee, FL). Comparison of the treatment means were performed using Fischer’s least significant difference (LSD) test at α = 0.05). On the basis of difference in the colony growth of control and treated Petri plates the percentage inhibition of pathogen growth was calculated by using following formula given by Jagtap and Sontakke (2007) [26].
I = (C – T) / C × 100
I = Percentage inhibition.
C = Growth of pathogen in control media.
T = Growth of pathogen in treated media.
3. Results
3.1 Evaluation of Trichoderma against mycelial growth of Foc in-vitro
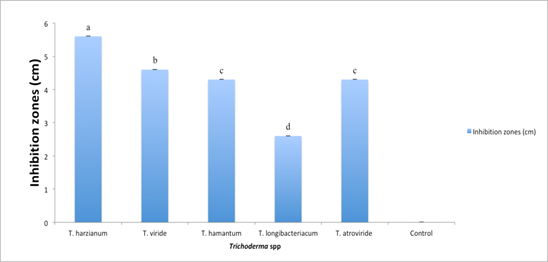
Five antagonistic species of Trichoderma viz; T. harzianum, T. viride, T. hamatum, T. longibrachiatum, and T. atroviride were tested in vitro for their antagonistic effect on F. oxysporum f. sp. ciceris. All the Trichoderma spp. reduced the development of F. oxysporum f. sp. ciceris significantly (p < 0.05) as compared to control. T. harzianum showed maximum inhibition of 5.6 mm followed by T. viride, which produced inhibition zone of 4.6 mm, T. hamatum and T. atroviride which showed the inhibition zone of 4.3 mm and T. longibrachiatum which showed inhibition zone of 2.6 mm. T. longibrachiatum proved to be least effective antagonistic Trichoderma spp. as compared to other Trichoderma spp. which showed minimum inhibition of 2.6 mm (Figure 1).
3.2 Evaluation of fungicides against mycelia growth of Foc in vitro
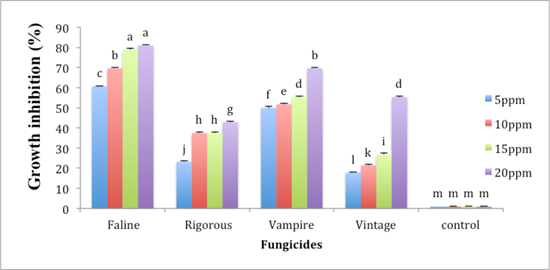
Fungi toxicity of fungicides varied greatly among each other and their concentration. Analysis of variance showed highly significant results fungicides, their concentration and interaction between fungicides and fungicide concentrations (p < 0.05). The development of fungus was tested for a period of 7 days against different concentrations of fungicides, maximum mycelia growth inhibition was given by Faline at 20 ppm concentration, which was 80.36% followed by Vampire which gave 69.64%, Ventage gave 55.36% and Rigrouse showed 42.86% inhibition (Figure 2).
None of the fungicides completely checked the mycelia growth of Foc. But there was a continuous trend in reduction in mycelia growth of Foc with the boost in application of fungicides.
3.3 Evaluation of methanolic plant extract against mycelia growth of Foc in vitro
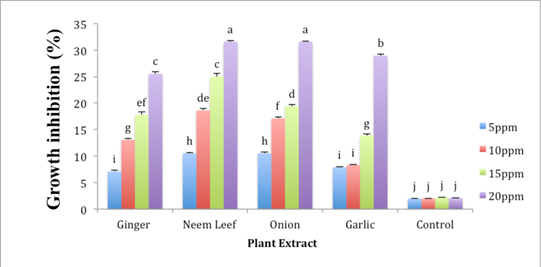
Fungitoxic effects of four methanolic plant extracts viz; garlic extract, ginger extract, neem leaf extract, and onion at four different concentrations viz; 5, 10, 15, and 20 μg/ml were tested in vitro by applying poisoned food technique. Analysis of variance showed highly significant results of plant extracts, their concentration and interaction between plant extracts and their concentrations (p < 0.05). Mycelial growth of Foc was decreased with the increase in the concentration of plant extracts. The growth of fungus was tested for period 7 days against different concentration of plant extract, maximum mycelia growth inhibition was given by neem leaf extract and Onion bulb extract at 20 ppm concentration which was 31.58 and 31% respectively, followed by Garlic bulb extract with 28.3% and Ginger rhizome extract with 25.53% inhibition (Figure 3). None of the plant extracts completely checked the mycelia growth of Foc. But there was a continuous trend in reduction in mycelia increase of Foc with the boost in absorption of plant extract.
4. Discussion
Fusarium wilt caused by Foc is a major constraint to chickpea C. arietinum L. production around the world (Khan et al., 2002) [27]. This study determined the effect of different Trichoderma spp., fungicides and methanolic plant extracts against mycelia growth of the causal agent of Fusarium wilt of chickpea. According to some research evidence, it is proved that there are continuous verified-diverse microorganisms that can be helpful for infected plants of chickpea as natural antagonists [28]. Biological control mechanisms are contemplated as significant measures for disease management because chemical fungicides adversely affect other non-target [29]. There are several bodies of evidence which support the fact that some microorganisms cause growth inhibition of pathogens by impairing their metabolisms and/or establishing a parasitic relationship [30]. Mostly in greenhouse and adverse field conditions some fungilike Trichoderma spp. are found valuable as bio-control agents [31].
In our study we tested different Trichoderma spp. against Fusarium wilt. All the Trichoderma spp. reduced the growth of Foc significantly but T. harzianum produced larger inhibition zone as compared to other antagonistic organisms. Our results are in line with previous studies (Subhani et al., 2020) who reported that T. harzianum reduced number of wilted chickpea plants with 67.93% reduction in disease incidence.
In fungicides, four fungicides were used viz; Ventage, Vampire, Rigorous, and Faline. All of these fungicides showed the inhibition effect on mycelia growth of Foc. By analysis of data, it was concluded that different fungicides suppressed the growth of mycelium at different levels as Faline at 20 ppm concentration, which was 80.36% followed by Vampire, which gave 69.64%, Ventage gave 55.36% and Rigrouse 42.86% inhibition, when the data was recorded at different concentrations similar trend was shown. By these results we observed that newly developed fungicides have a positive impact on mycelia growth inhibition of causal agent of Fusarium wilt of chickpea. Some chemicals were used by (Khaleel et al., 2014 [32]) and found that vampire showed best results against Fusarium wilt of Pea at different concentrations as compared to rigorous and Ventage at different concentrations.
Plants have the ability to manufacture aromatic derived metabolites, including phenols, phenolic acids, quinones, flavones, flavonoids, flavonols, tannins and coumarins [19]. In our experiment we used methanolic plant extract of onion, ginger, neem leaf and garlic and checked their efficacy against Foc. By analysis of data we recorded that maximum mycelia growth inhibition was given by neem leaf extract and Onion bulb extract at 20 ppm concentration which was 31.58 and 31 % respectively, followed by Garlic bulb extract which gave 28.3% and Ginger rhizome extract which gave 25.53% inhibition. Our results are in line with previous findings of (Khaleel et al., 2014 [32]; Shukla and Dwivedi, 2012 [33]) who agree to the studies and use of plant extracts instead of pesticides.
By all of these experiments, it is concluded that maximum control was given by Faline which is the most effective against F. oxysporum, and is followed by Vampire, among plant extract neem leaf extract is best effective, and T. harzianum is best biocontrol agent in lab conditions. More studies are required under field conditions to standardize the concentrations of homeo-fungicides and methanolic plant extracts against Fusarium wilt of chickpea.
Acknowledgements
This research was financially supported by the Institute of Agricultural Science, University of the Punjab, Lahore, Pakistan.
Conflict of Interest
The authors declare no conflict of interest.
References
- Agricultural Production Year Book. Food and Agriculture Organization of the United Nations (FAO) (2019).
- Millan T, Winter P, Jüngling R, et al. A consensus genetic map of chickpea (Cicer arietinum) based on 10 mapping populations. Euphytica 175 (2010): 175-118.
- Lichtenzveig J, Scheuring C, Dodge J, et al. Construction of BAC and BIBAC libraries and their applications for generation of SSR markers for genome analysis of chickpea, Cicer arietinum Theoretical and Applied Genetics 110 (2005): 492-510.
- Dubey SC, Suresh M. Evaluation of Trichoderma species against Fusarium oxysporum sp. ciceris for integrated management of chickpea wilt. Biological Control 40 (2007): 118-127.
- Halila MH, Strange RN. Screening of Kabuli chickpea germplasm for resistance to Fusarium wilt. Euphytica 96 (1997): 273-279.
- Chen W, Sharma H C, Muehlbauer FJ. Compendium of chickpea and lentil diseases and pests. American Phytopathological Society (APS) Press (2011).
- Recorbet G, Steinberg C, Olivain C, et al. pathogenesis-related marker molecules for Fusarium oxysporum. New Phytologist 159 (2003): 73-92.
- Jiménez-Díaz RM, Castillo P, Jiménez-Gasco M, et al. Fusarium wilt of chickpeas: Biology, ecology and management. Crop Protection 73 (2015): 16-27.
- Haware MP, Nene YL. Races of Fusarium oxysporum sp. ciceri. Plant Diseases 66 (1982): 809-810.
- Landa BB, Navas-Cortes IA, Jiménez-Diaz RM. Integrated Management of Fusarium wilt of Chickpea with Sowing Date, Host Resistance, and Biological Control. Phytopathology 94 (2004): 946-960.
- Reino JL, Guerrero RF, Hernandz-Galan R, et al. Secondary metabolites from species of the biocontrol agent Trichoderam. Phytochemistry Reviews 7 (2008): 89-123.
- Kilani-Jaziri S, Bhouri W, Skandrani I, et al. Phytochemical, antimicrobial, antioxidant and antigenotoxicpotentials of Cyperus rotundus extracts. South African Journal of Botany 77 (2011): 767-776.
- Monte E. Understanding Trichoderma, between biotechnology and microbial ecology. International Microbiology 4 (2001): 1-4.
- Harman GE, Howell CR, Viterbo A, et al. Trichoderma species - opportunistic, virulent plant symbionts. Nature Reviews Microbiology 2 (2004): 43-56.
- Woo SL, Lorito M. Exploiting the interactions between fungal antagonists, pathogens and the plant for biocontrol. In: Vurro, M. and Gressel, J. (Eds.), Novel Biotechnologies for Biocontrol Agent Enhancement and Management. Springer, Dordrecht (2007): 107-130.
- Druzhinina IS, Seidl-Seiboth V, Herrera-Estrella A, et al. Trichoderma: the genomics of opportunistic success. Nature Reviews Microbiology 9 (2011): 749-759.
- Abawi GS, Widmer TL. Impact of soil health management practices on Soil Borne pathogens, nematodes and root diseases of vegetable crops. Applied Soil Ecology 15 (2000): 37-47.
- Subhani MN, Sahi ST, Hussain S, et al. Evaluation of various fungicides for the control of gram wilt caused by Fusarium oxysporum sp. ciceris. African Journal of agricultural research 6 (2011): 4555-4559.
- Cowan MM. Plant products as antimicrobial agents. Clinical Microbiology Reviews 112 (1999): 564-582.
- Das K, Tiwari RKS, Shrivastava DK. Techniques for evaluation of Medicinal plant products as antimicrobial agent: Current methods and future trends. Journal of Medicinal Plants Research 4 (2010): 104-111.
- Gupta JS, Agarwal MB, Dixit RB, et al. Effect of metabolites from different host plants on conidial germination of Colletotrichum graminicola and C. capsici. Geobios 8 (1981): 226-228.
- Grainge M, Ahmed S. Poisonous plants and plants controlling human and animal pest. Hand book of plants with pest control properties. John Wiley and Sons Limited (1988): 309.
- Wollen Webber HW, Reinking OA. Die verbreitung Fusaritm in der Hature. (The Distribution of Fusarium in nature), Berlin, R. Fried Land Und, Sohm (1935): 80.
- Synder WC, Hausen HN. The species concept in Fusarium. American Journal of Botany 27 (1940): 64-67.
- Borum DF, Sinclair JB. Evidence for systemic protection against Rhizoctonia solani with Vitavax in cotton seedlings. Phytopathology Journal 58 (1968): 976-980.
- Jagtap GP, Sontakke PL. Management of chickpea wilt caused by Fusarium oxysporum sp. Cicero. African journal of Agricultural Research 2 (2007): 692- 697.
- Khan IA, Alam SS, Haq A, et al. Selection for resistant to wilt in relation with phenols in Chickpea. International chickpea and Pigeon pea Newsletter 9 (2002): 19-20.
- Cook RJ. Advances in plant health management in the 20th century. Annual Review of Phytopathology 28 (2000): 95-116.
- Köhl J, Kolnaar R, Ravensberg WJ. Mode of action of microbial biological control agents against plant diseases: Relevance beyond efficacy. Frontiers in Plant Science 10 (2019): 845.
- Panth M, Hassler SC, Baysal-Gurel F. Methods for Management of Soil borne Diseases in Crop Production. Agriculture 10 (2020): 16.
- Hermosa R, Viterbo A, Chet I, et al. Plant-beneficial effects of Trichoderma and of its genes. Microbiology 158 (2012): 17-25.
- Khaleel M, Subhani MN, Ali A, et al. In vitro evaluation of homeo-fungicdes and methanolic plant extracts against mycelial growth of Fusarium oxysporum sp. pisi causing wilt disease in pea. Pakistan Journal of Phytopathology 26 (2014): 247-251.
- Shukla A, Dwivedi SK. Bioefficacy of plant extracts against Fusarium species causing wilt in pulses. International Organization of Scientific Research 2 (2012): 136-44.


 Impact Factor: * 4.1
Impact Factor: * 4.1 Acceptance Rate: 75.32%
Acceptance Rate: 75.32%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 