Effect of Ultraviolet Radiation on Human Lung Adenocarcinoma Cells in Vitro
Mengmeng Li1*, Baiqin Zhao1, Lei Han2, Hongliang Wang2, Jiaqiao Li2, Zhen Wang1, Xiao Chen3 and Xin Huang2
1Institute of Semiconductors, University of Chinese Academy of Sciences, No. 35 A, Tsinghua East Road, Haidian District College Road, Beijing,100083, China
2Department of Pathogenic Microbiology and Immunology, School of Basic Medical Sciences, Xi’an Jiaotong University Health Science Center, Xi’an, China
3Department of Neurosurgery, The First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, China
*Corresponding Author: Mengmeng Li, Institute of Semiconductors, University of Chinese Academy of Sciences, No. 35 A, Tsinghua East Road, Haidian District College Road, Beijing,100083, China
Received: 21 October 2022; Accepted: 03 November 2022; Published: 11 November 2022
Article Information
Citation:
Li M, Zhao B, Han L, Wang HL, Li J, Wang Z, Chen X, Huang X. Effect of Ultraviolet Radiation on Human Lung Adenocarcinoma Cells in Vitro. International Journal of Plant, Animal and Environmental Sciences 12 (2022): 164-174.
View / Download Pdf Share at FacebookAbstract
In this study, we compared the cellular morphology over time under different culture conditions and the effect of UV irradiation on cells elucidated the morphological characteristics of UV induced apoptosis. It was shown that UV radiation could penetrate the culture medium to reach the irradiated cells and induce DNA mutations at a small dose. Culture medium might contribute to maintain physiological responses of adherent cell. After irradiation, cells responded differently under different culture conditions. Apoptosis in the medium was dose-dependent on UV, and morphological changes began with darkening of the nucleus and then the appearance of membrane blebs at the cell edges. When there was no medium, the organelles of irradiated cells migrated from the periphery of the nucleus to the edge of the cell, and irradiation of cells containing medium did not show migration of the organelles. In temperature experiments, the cell membrane of high temperature treated cells broke, and the cells maintained the physiological activity at low temperature for a period of time.
Keywords
Cell death; Ultraviolet C; Organelle migration; Morphology
Cell death articles; Ultraviolet C articles; Organelle migration articles; Morphology articles
Article Details
In this experiment, we understood the phenomenon of UV induced on cells in different culture environments in vitro through cell proliferation and morphological characteristics. The cell damage caused by UV irradiation is accompanied by the absence or disorder of the physiological and biochemical responses of cells in response to external environment. After some time, parts of the organelles were dispersed in the medium, with the nucleus and residual cytoplasm at the edge of the cell membrane. UV acts on the nucleus to denature and condense nucleoli and chromatin, and cells with mutations in the nucleus fail to complete the physiological cycle. The organelle under suitable circumstances exited from the membrane, which expelled variant intranuclear material in the form of a membrane. More cells maintained a state of separation of cell membrane and cytoplasmic halves (Figure 1 and 2). There was a tendency for independent separation of membranous organelles, membranes, nuclear envelope in our experiments, and it may be the specific recognition of abnormal intranuclear material by the membrane during movement that expelled it automatically from nuclei that may also be variant.
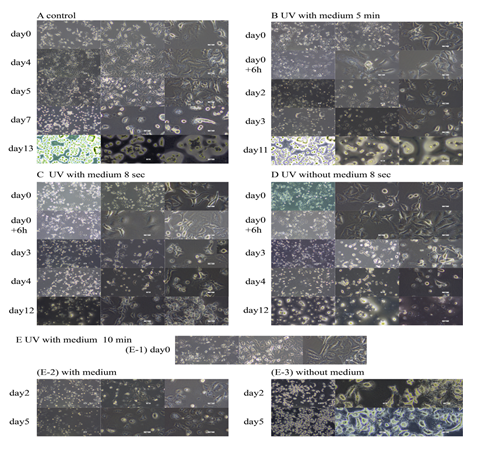
Figure 1: Images of the cells under different conditions. The pictures revealed the cells distribution and morphology. (A) Cells grew normally. (B) Cells irradiated with 5 min in culture medium. (C) Cells irradiated with 8 sec in culture medium. (D) Cells after irradiation of 8 sec. (E) cells irradiated 10 min (E-1) Cells irradiated 10 min in medium. (E-2) Containing medium. (E-3) The medium was removed after irradiation. Images from the same row are images of different sizes at the same time. Images from the same row are images of different sizes at the same time. The scale was 2 mm, 1 mm and 50 μm respectively.
UV light penetrated the medium (approximately 1.4 mm) to act on the cells, and the irradiated cells did not proliferate compared with untreated cells. None of the cells in the culture medium immediately after being irradiated showed differences from that before irradiation [1], and the degree of damage caused by different doses of irradiation was different and exhibited different changes over time. The 8 s irradiated cells obviously changed in morphology at approximately 72 h, the cell volume was small, the nucleus and cytoplasm aggregates floated, and the morphological changes from irradiation over 5 min occurred after 6 h, and the nucleus turned dark (Figure 1). After the cells were left in the medium for a long time, a large amount was degraded. Cells irradiated without medium for 8 s lysed less than those in medium. In the irradiation experiment without culture medium, the volume of cells irradiated for 10 min had obvious shrinkage immediately after irradiation, the proportion of the nucleus increased, the nucleus lost obvious nuclear and matrix characteristics and the organelles were away from the nucleus (Figure 2). Streaking experiments of blood free media did not reveal cell migration. The lack of serum nutrients decreased the time for the cells to maintain the intact morphology, and apoptosis was faster after UV irradiation, with a large number of organelles scattered. The cells irradiated by removing the medium for 8 s partially retained the cell membrane, and UV may help keep the membrane intact.
UV induced cell damage is related to their culture environment, and the damage caused by different doses varies [2-4]. Direct irradiation of adherent cells with UV at equivalent doses resulted in faster apoptosis than those irradiated with compartment medium, and the more difficult the morphology was to maintain. With the same culture conditions, UV cell damage has a dose-dependent manner, and the larger the dose, the more serious the impairment of the physiological and biochemical reactions of the cells [4,5]. The cells showed different biochemical responses under different conditions, probably due to the difference between the presence or absence medium and the high and low doses of UV targets. UV induces DNA variations that render the cells lose their genetic replication, activate cell surface receptors, and become undirected by the transcriptional expression of irradiated cell genes [2,6-8]. Image recording, the common morphological characteristics of dead cells were reduced size, the accumulation of nuclei with matrix floating, partial detachment of the cell membrane, and scattered organelle particles. In UV irradiated cells, the obvious features started with a darkened nucleus, and the separated cell membranes are mostly transparent and did not contain organelles, in contrast to untreated cells. UV irradiation might affect the protrusion of cilia on the cell surface, the organelles of medium-free irradiated cells move from the perinuclear to the edge of the cell, the migration of organelles may result in cell death in a short time. UV irradiation did not affect cell attachment, had a significant lethal effect on cells in suspension, and the different survival states of cells have a large difference in their ability to resist UV.
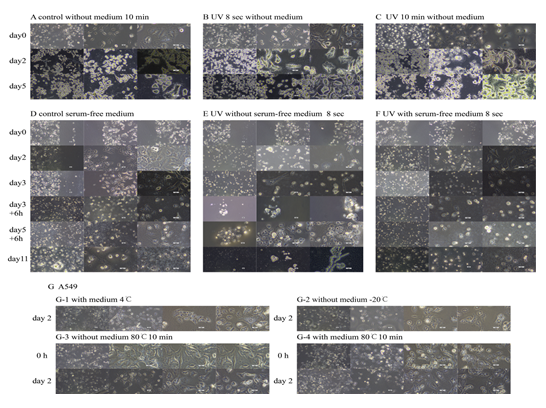
Figure 2: The cells under different culture conditions over time. (A, B and C) Images of cells morphology after removing medium. (A) Without irradiation. (B) Cells were irradiated with 8 sec without culture medium. (C) Cells irradiated with 10 min without the culture medium. (D, E and F) The cells in the serum-free medium. (D) Without irradiation. (E) Irradiated 8 sec without medium. (F) Irradiated 8 sec with medium. (G) Cells at different temperatures. (G-1) The changes in cells containing medium in 4°C; (G-2) Cells without medium in minus 20°C. (G-3) Morphological changes in cells with medium in minus 80°C for 10 min; (G-4) Cells without medium for 10 min in minus 80°C. The scale was 2 mm, 1 mm and 50 μm respectively.
In the temperature experiment, the change of cell volume may be due to different temperature effects, and the physiological activity of cells at a temperature maintained at 4 degrees was complete. At minus 20°C, there were a lot of membrane vesicles. When treated with high temperature, the cells containing medium were obviously affected, and the continuous damage caused by the heated medium to the cells was more serious than that caused by the no medium. The integrity of cell membrane and nuclear membrane in no culture medium had not been destroyed, showing a state of being separated from internal substances (Figure 2).
UV radiation induces DNA damage and modulates the immune response through apoptosis [9,10], and an understanding of cell death may also help to artificially intervene in some disease states [11]. The death of the cells in this experiment underwent the process of apoptosis, and the cell death in the control group may be caused by the decrease of nutrients in the culture medium as well as the increase of metabolic wastes of the cells. UV irradiation at a low dose (3 mJ/cm2) can induce cell nucleus variation and the damage was not repaired, the metabolism of cells that had undergone radiation for a long time showed the characteristics of apoptosis, the differential effect of different doses made the physiological biochemical reactions of the cell function progress to be intervened by the degree, UV energy may have damaged the cytoplasm, organelles and other components [12]. Upon loss of the reduced physiological activity of cells following apoptosis, intracellular hydrolases were released into the solution leading to degradation of the cell remnants.
By continuously recording the morphological characteristics of cells in various conditions, it was found that the damage degree of cells to different survival conditions was different. The cells in complete medium were more metabolizing than those in blood free medium, and cell adherence was more tolerant than suspension. When higher UV energy (approximately 215 mJ/cm2) acted directly on cells, a short period (approximately 10 min) allowed organelles to migrate, away from the nucleus.
This experiment did not consider the difference of cell types, and the results also made it difficult to distinguish the difference of apoptotic process between dividing and interphase cells by UV radiation. The membrane blebs that came out at the edge of UV irradiated cells were transparent without organelles, the cells treated with high temperature and low temperature (approximately minus 20°C) in spent medium had more obvious characteristics. There may be a dynamic relationship between organelles and cell membrane in the process of apoptosis.
Declarations
Acknowledgments:
The work was supported by Institute of Semiconductors and Xi’an Jiaotong University. The work was financed by the National Natural Science Foundation of China (81702043).
Competing interest statement:
There is no competing interest.
Author contributions:
Mengmeng Li, Baiqin Zhao, Lei Han and Jiaqiao Li designed the work. The authors provided experimental materials. Mengmeng Li conducted the work, collected results and wrote this article.
Data available statement:
All data generated or analysed during this study are included in this article and its supplementary information files. All data are available.
References
- Moharikar S, D'Souza JS, Kulkarni AB, et al. Apoptotic-Like Cell Death Pathway Is Induced in Unicellular Chlorophyte Chlamydomonas Reinhardtii (Chlorophyceae) Cells Following UV Irradiation: Detection and Functional Analyses1, Journal of Phycology 42 (2006): 423-433.
- Kulms D, Poppelmann B, Schwarz T. Ultraviolet radiation-induced interleukin 6 release in HeLa cells is mediated via membrane events in a DNA damage-independent way. J Biol Chem 275 (2000): 15060-15066.
- Gentile M, Latonen L, Laiho M. Cell cycle arrest and apoptosis provoked by UV radiation-induced DNA damage are transcriptionally highly divergent responses. Nucleic Acids Res 31 (2003): 4779-4790.
- Kulms D, Poppelmann B, Yarosh D, et al. Nuclear and cell membrane effects contribute independently to the induction of apoptosis in human cells exposed to UVB radiation. Proc Natl Acad Sci USA 96 (1999): 7974-7979.
- Schwarz T, Schwarz A. Molecular mechanisms of ultraviolet radiation-induced immunosuppression. Eur J Cell Biol 90 (2011): 560-564.
- Schwarz T, Luger TA. New trends in photobiology, Journal of Photochemistry and Photobiology B: Biology 4 (1989): 1-13.
- Ravanat JL, Douki T, Cadet J. Direct and indirect effects of UV radiation on DNA and its components. Journal of Photochemistry and Photobiology B: Biology 63 (2001): 88-102.
- Gurdon JB. The Effects of Ultraviolet Irradiation on Uncleaved Eggs of Xenopus Laevis. Journal of Cell Science s3-101 (1960): 299-311.
- Lee CH, Wu SB, Hong CH, et al.Molecular Mechanisms of UV-Induced Apoptosis and Its Effects on Skin Residential Cells: The Implication in UV-Based Phototherapy. Int J Mol Sci 14 (2013): 6414-6435.
- Latonen L, Taya Y, Laiho M, et al. UV-radiation induces dose-dependent regulation of p53 response and modulates p53-HDM2 interaction in human fibroblasts. Oncogene 20 (2001): 6784-6793.
- Kanduc D, Mittelman A, Serpico R, et al. Cell death: Apoptosis versus necrosis (Review). International Journal of Oncology (2002).
- Bender K, Blattner C, Knebel A, et al. UV-induced signal transduction, Journal of Photochemistry and Photobiology B: Biology 37 (1997): 1-17.
- Kerr JF. A histochemical study of hypertrophy and ischaemic injury of rat liver with special reference to changes in lysosomes. J Pathol Bacteriol 90 (1965): 419-435.
- Pannese E. An Electron Microscopic Study of Cell Degeneration in Chick Embryo Spinal Ganglia, Neuropathology and Applied Neurobiology 2 (1976): 247-267.
- Pilar G, Landmesser L. Ultrastructural differences during embryonic cell death in normal and peripherally deprived ciliary ganglia. J Cell Biol 68 (1976): 339-356.
- Abraham R, Morris M, Hendy R. Lysosomal changes in epithelial cells of the mouse thymus after hydrocortisone treatment. Histochemie 17 (1969): 295-311.
- Kerr JF. An electron-microscope study of liver cell necrosis due to heliotrine. J Pathol 97 (1969): 557-562.
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26 (1972): 239-257.
- Popper H. Heaptocellular degeneration and death. The liver, biopsy and pathology (1988): 1087-1103.
- Kanduc D, Mittelman A, Serpico R, et al. Cell death: Apoptosis versus necrosis (Review), International Journal of Oncology (2002).
- Ellis RE, Yuan JY, Horvitz HZ. Mechanisms and functions of cell death, Annu Rev Cell Biol 7 (1991) 663-698.
- Lee CH, Wu SB, Hong CH, et al. Molecular Mechanisms of UV-Induced Apoptosis and Its Effects on Skin Residential Cells: The Implication in UV-Based Phototherapy. Int J Mol Sci 14 (2013): 6414-6435.
- Pustišek N, Šitum M. UV-radiation, apoptosis and skin, Collegium antropologicum 35 (2011): 339-341.
- Schwarz T, Schwarz A. Molecular mechanisms of ultraviolet radiation-induced immunosuppression. Eur J Cell Biol 90 (2011): 560-564.
- Schwarz T, Luger TA. New trends in photobiology. Journal of Photochemistry and Photobiology B: Biology 4 (1989): 1-13.
- Miller ML. Andringa A, Elliott J, et al. The morphological and spectral phenotype of apoptosis in HeLa cells varies following exposure to UV-C and the addition of inhibitors of ICE and CPP32, Cell Prolif 31 (1998): 17-33.
- Kulms D, Poppelmann B, Schwarz T. Ultraviolet radiation-induced interleukin 6 release in HeLa cells is mediated via membrane events in a DNA damage-independent way. J Biol Chem 275 (2000): 15060-15066.
- Kulms D, Schwarz T. Molecular mechanisms of UV-induced apoptosis, Photodermatol Photoimmunol Photomed 16 (2000): 195-201.
- Ikehata H, Ono T. The mechanisms of UV mutagenesis, J Radiat Res 52 (2011): 115-125.
- Ravanat JL, Douki T, Cadet J. Direct and indirect effects of UV radiation on DNA and its components, Journal of Photochemistry and Photobiology B: Biology 63 (2001): 88-102.
- Gentile M, Latonen L, Laiho M. Cell cycle arrest and apoptosis provoked by UV radiation-induced DNA damage are transcriptionally highly divergent responses, Nucleic Acids Res 31 (2003): 4779-4790.
- Kulms D, Poppelmann B, Yarosh D, et al. Nuclear and cell membrane effects contribute independently to the induction of apoptosis in human cells exposed to UVB radiation, Proc Natl Acad Sci USA 96 (1999): 7974-7979.
- Stanojevic-Pirkovic M, Andelkovic M, Lukovic J, et al. UV irradiation induces apoptosis in the human endometrial stromal cell line (ThESC). J BUON 25 (2020): 1541-1546.
- Moharikar S, D'Souza JS, Kulkarni AB, et al. Apoptotic-Like Cell Death Pathway Is Induced in Unicellular Chlorophyte Chlamydomonas Reinhardtii (Chlorophyceae) Cells Following UV Irradiation: Detection and Functional Analyses1. Journal of Phycology 42 (2006): 423-433.
Supplementary Information
Earlier studies judged the cellular necrosis condition of injured tissues by electron microscopy observation [13-15], and the cells were subjected to electron microscopy observation to judge the evolution of death and explore the effects of drugs on cells [16,17]. Apoptosis refers to cell death that is controlled by genes and can be a metabolic response caused by a variety of factors, and the typical process of apoptosis is divided into two parts, firstly manifesting in cell shrinkage, separation of protrusions formed on the cell surface, chromatin pyknosis, nuclear pyknosis, and subsequently shedding of membrane vesicles from the cell membrane surface to form spherical or ovoid debris apoptotic bodies. Apoptotic bodies contain a pyknotic nucleus and cytoplasm, organelles that are intact in composition and their size depends on the cellular components present in the cytoplasmic protrusions that generate the apoptotic bodies [18]. Unlike apoptosis, cell necrosis is mainly characterized by the rupture of the cell membrane, organelle swelling, and cell volume enlargement, and is not genetically regulated [19,20]. There is also a more nuanced distinction between cell death, caused by a variety of environmental factors that can be interpreted in terms of their morphological characteristics and their death pathways, which are more studied at the genetic level [21].
UV can induce skin cancer, suppress the immune system, and cause chronic skin damage and aging [22-24]. UV irradiated corneocytes increase the release of cytokines such as interleukins, which participate in the proliferation and differentiation of cells and regulate immune and inflammatory responses [22,25-27]. Many studies have shown that UV radiation has a killing effect on cells. The cytoplasm of apoptotic HeLa cells after UV (254nm) irradiation showed dark spherical shape in membrane vesicles, the number of cytoplasmic ribosomes was greatly increased, the nucleolar material was abundant containing a large number of individual granule components, the chromatin was concentrated on the nuclear membrane, the number of apoptotic cells was dose dependently increased, and the apoptosis of cells with the addition of caspase inhibitors was reduced [26]. UV light is first absorbed by intracellular chromophores, which then convert energy into biochemical signals to exert biological effects, such as altering mammalian gene expression, and common chromophores include nucleic acids, amino acids, etc. [28]. UV induces DNA damage in cells and may also activate cell surface receptors, triggering cellular responses [29,30]. Ultraviolet (UVB) irradiation can activate the cellular mitochondrial apoptotic pathway [31]. When cells were exposed to UVB at 4°C and DNA damage was removed by photolysis enzymes, UV induced apoptosis was almost completely inhibited [32]. Apoptotic morphology and drug treatment, inactivation of UV irradiated cells can be explained by images [33,34].
In this experiment, we compared the changes of cell state under different culture conditions with UV irradiation over time and the effects of high and low temperature on cells, observed the cell morphological changes and damage under different experimental conditions, recorded the physiological and biochemical responses of cells to environmental changes, elaborated the morphological characteristics of apoptosis caused by UV, and discussed the continuous responses that may be caused by UV.
By observing and recording the changes of UV irradiated cells with time, the effects of UV on different states of cells were compared, and the cell damage caused by UV was evaluated.
1.1 As shown in Figure 1A, A549 adhered to the wall and grew in an irregular polygon shape. The nucleus is mirror like and translucent, and the number of nucleoli was inconsistent. The cells in interphase were larger, and organelles were scattered near the nucleus, with a small amount distributed in the far away area from the nucleus. During division, the cells were small and mostly triangular. There were ciliary connections between cells and small villi on the membrane surface. After 4 days, the number of cells increased significantly. After 5 days, there were a large number of cells, some cells were still in the division stage, and there was a small amount of dead cell debris in the culture medium. On the 7th day, the number of cells decreased, the medium turned yellow, and the overall cell volume became smaller, showing the characteristics of dividing cells. There were a large number of aggregated clusters of round dead cells, and long villi stretched out on the cell surface. There was gray translucent cell membrane prolapse at the edge of the cell. When the cell membrane of some cells was separated from the polymer of nucleus and cytoplasm, the membrane contained a large number of ribosome particles. There were opaque gray membrane vesicles floating alone in the medium. For smaller cells, the cell membrane was detached from one or both sides, and multiple intact membrane vesicles appeared on the cilia stretched out on the cell surface of larger interphase cells. On the 13th day, after the medium split, the dried medium contained a large number of dehydrated cytoplasmic polymers of dead cells, the cell volume was very small, the morphology of nucleus and perinuclear aggregates and cell membrane detached from both sides was maintained, and there were crystalline crystals.
As shown in Figure 1B, there was no significant difference in cell morphology after 5 min of UV irradiation. After 6 hours, the nucleus darkened from mirror shape. After 2 days, the number of cells decreased slightly, the cell debris adhered to the wall, the nucleus suspended, the nucleus pyknosis into a mass, and membrane vesicles appeared at the edge of cell adhesion. On the third day, most of the cells appeared as the remains of membrane vesicles, and the cell membrane was detached from both sides. After 12 days, the medium dried, the cells adhered to the wall and died, and the nuclear morphology of contraction and pyknosis was maintained, and the organelle particles were obvious.
When the irradiation time was reduced to 8 s, as shown in Figure 1C, apoptosis occurred on the third day after irradiation, the total number of cells decreased, there were various cell debris in the culture medium, and the more complete cells were in the division stage. On day 4, the number of debris increased. After the culture medium was dried, the cells aggregated and died, and the pyknotic cells mainly existed in the form of nucleus. Some cells adhere to the wall and have complete morphology, with obvious nucleoli, nuclear organelles and other structures.
The apoptosis of cells irradiated with the culture medium for 8 s was faster, and the number of debris was more at the same time. As shown in Figure 1D, there were opaque membrane vesicles in the culture medium, fewer adherent cells, and most cells were in the division stage. On the 4th day, the number of cells continued to decrease, some organelle particles adhered to the wall, cell debris gathered, and some cells extended long villi. After the medium was dried, a large number of scattered membranous organelles and a small number of membranous organelles solidified, and some maintained the form of membrane prolapse.
As shown in Figure 1E-1, when the irradiation time in the culture medium was 10 min, there was no immediately significantly different from the control group. On the second day, the death phenomenon was that the nucleus became dark and the cell membrane was separated from the nucleus and matrix. Some cells had small membrane vesicles at the edge, and the nuclear membrane was separated. As shown in Figure 1E-2, there were transparent and gray individual membrane bubbles floating in the culture medium. There were no organelles and colloids in hyaline membrane cells. Gray membrane cells contained cytoplasmic matrix. The contents of membrane cells wrapped with cell residues were on one side and edge of the membrane, showing a trend of efflux. On the 5th day, some cells remained attached to the wall, and the whole nucleus solidified and gathered into a mass.
After 10 minutes of irradiation, the cell volume decreased on the second day after removing the culture medium. As shown in Figure 1E-3, the colloidal substances in the cell concentrated and gathered around the nucleus, the contraction edge of the cell membrane was dispersed, there were large organelle particles in the cell, the membrane was agglomerated and solidified, fine cilia were stretched on the membrane, the fibrin and ribosome constituting the cell skeleton were solidified on the culture dish, and the nuclear glia and chromatin were agglomerated and solidified as a whole. On the 5th day, there was no cytoskeleton and other structures did not change significantly.
1.2 As shown in Figure 2A, there was no significant change in the cell state after 10 minutes without irradiation. The cell volume in the division stage was small, the brightness was high, the interphase cell was dark, the volume was large, and the interphase cell had better adhesion ability. There was a small amount of dead cell debris. The cell density was low, there were few connections, the nucleoli of the cells were condensed, some gathered into balls, some nuclei were colloidal and transparent, and the nucleoli were obvious. Organelle particles such as ribosomes were mainly concentrated and distributed around the nucleus. On the second day, the cells at different stages remained in their state of coagulation. The cytoplasmic matrix, fibrin and ribosomes constituting the cytoskeleton were solidified on the culture dish, and the scattered membrane organelle particles were randomly distributed. The cell volume became smaller, the cytoplasmic cell membrane condensed and shrank, there were fine cilia on the membrane, and the nuclear glia agglutinate and solidify. The nucleosome particles of some interphase cells agglutinated. On the 5th day, the crystalline skeleton disappeared, the cell membrane solidified on the Petri dish did not change significantly, the cytoplasmic color deepened, the nucleoplasm did not shrink, and it was always in a translucent mirror shape. There were large organelle particles in the cell membrane.
As shown in Figure 2B, the cells irradiated with the culture medium for 8 s had no obvious changes. On the second day, there was no obvious cytoskeleton. The cells in the interphase had obvious nucleosome particle aggregation, the nucleus remained colloidal translucency, the cytoplasm contracted, the cytoplasmic matrix and a large number of ribosome particles scattered outside the membrane and solidified in the culture dish. There were short cilia on the cell surface, and some cells had no obvious contraction. On the fifth day, the cells did not change significantly, and the nucleus remained gel.
After 10 minutes of irradiation with the culture medium removed, as shown in Figure 2C, the cells immediately showed obvious differences. All cells showed the characteristics of cells in the division stage, with reduced volume, disappearance of nucleoli, irregular aggregation of nuclear core corpuscles and obvious nuclear membrane. The granular membrane of interphase organelles was obvious and moved from the vicinity of nucleus to the edge of cytoplasm. There were filament connections between cells. On day 2, the cytoskeleton coagulated on the Petri dish. The boundary of cell membrane was not clear, the nuclear membrane was obvious, the cytoplasmic area was significantly reduced, the cell volume shrank, some organelles were separated from cells, and organelle particles such as ribosomes were distributed on the culture dish. The granules in the nucleus increased, and the nucleosome granules were irregularly condensed into nucleolar morphology. No villi appeared on the cell surface. On the 5th day, the color of the inner membrane of the nucleus deepened, some membrane containing structures solidified into crystals, the cell edges dispersed, and the morphology of some cells was normal.
As shown in Figure 2D, the adherent cells of normal growth were cultured in serum-free medium for 2 days. The cell morphology was complete and the volume was smaller than that of ordinary cells. Some cell nucleoli contracted and there were fine hair connections between cells. On the 4th day, a large number of cells died, and there were many cells with small volume and cell membrane in the division stage. The cell membrane of interphase cells was not obvious, and the nuclei agglomerate into semilunar balls. Some cell contents were semi separated from the cell membrane. Then a large number of cell debris and fragments appeared. The debris was the surrounding aggregate of nucleus and perinucleus, with or without membrane structure (no membrane state or semi separated from the membrane). The cell fragments were closed. A large number of cell fragments were distributed on the dry medium, and the nucleoli of some cells were prominent. The scribed area did not increase throughout the process.
There was no significant change when cells were irradiated with serum-free medium for 8 s. As shown in Figure 2F, on the second day, the number of cells decreased, a large number of cells died and lysed, some cells adhered to the wall, and the cells basically maintained their morphology. The cell debris was suspended in the culture medium, the debris was the nucleus and the aggregates around the nucleus, and the debris was in a membranous state or the cell membrane was detached. On the 4th day, the cell debris increased, the cell fragments on the culture medium were obvious, and the cells had no complete cell structure. After that, when the culture medium was not solidified, there were suspended spherical cell clusters containing semi separated cell membranes. On the 6th day, the culture medium was dry, the cells clustered, some intracellular organelles were lost, and the perinuclear aggregates were at the edge of the cell membrane. On the 11th day, the cell morphology completely disappeared, leaving a large number of cell debris particles. The remnants of some cells on the culture medium retained the cell contour, and some cell debris were crystalline.
The cells were placed in the culture medium for a long time at 4 °C, as shown in Figure 2G-1. The cell volume decreased, the basic shape was complete, there were villi on the surface, the nucleus was gray and spherical, and the volume increased. The cells were at minus 20°C without the culture medium, when taken out, the cells were in granular fixed arrangement. As shown in Figure 2G-2, the basic morphology of the cells was complete, the volume was reduced, the nucleus was mirror shaped and the volume increased, many transparent membrane vesicles were detached from the edge of the cells, and there were no organelles in the membrane vesicles. As shown in Figure 2G-3, the basic morphology of cells after high temperature treatment without culture medium was complete, ribosomes and other organelles were evenly dispersed in the cells, the nuclear boundary was not obvious, the nucleoli were clustered, the nuclear membrane was out of the nucleus, and the transparent membrane vesicles at the cell edge were out. After being put back into the incubator for 2 days, the cells adhered to the wall, the volume decreased, the organelles were scattered, and there was a suspended transparent nuclear membrane in the culture medium. The cells containing the medium were severely damaged after high temperature, as shown in Figure 2G-4. The cell morphology was incomplete, the volume was reduced, and the medium had gray membrane bubbles floating. The nucleoli condensed into crescent or mass, the organelles were distributed disorderly, and there were short villi on the cell surface. After being placed in the incubator for 2 days, the cell state did not change.
Methods
Experimental conditions
The cells A549 (human lung adenocarcinoma cells) used in the experiment were donated by Xi'an Jiaotong University.
The complete medium group of cells was: DMEM (basic 1× Dulbecco's modified Eagle medium GIBCO / Hylone), 10% serum (Biological industries), 1% streptomycin (100× Genview). Culture environment: cell constant temperature incubator (air, 95%, CO2, 5%, temperature 37°C).
The cells were cultured in 100 mm cell culture dish (Corning) with 10 mL complete medium. After a large number of cells grew, the cells were washed with phosphate buffer for three times and digested with 2 mL trypsin (EDTA 0.25% trypsin EDTA 1×) for 3-5 minutes. The medium (2 mL) was added to terminate and disperse the cells. The cells were collected in a 15 mL centrifuge tube for 1000 rpm and 5 min. The cells were resuspended and placed in a 60 mm Petri dish (SORFA) with 4 mL medium. The adherent cells with appropriate density were used for the irradiation experiment. The culture medium used in the scribing experiment did not contain serum. The medium was not changed after the irradiation experiment. There was no statistical significance in the experiment, and the results did not show cell viability.
Experimental instruments and the UV energy
The wavelength of the ultraviolet light-emitting diode used in the ultraviolet radiation experiment was 265-278 nm (BRT-B35CD7A1CSD, Taiwan High Lighting Power). The irradiance was measured by UVC-LED Probe (LS125, Shenzhen Linshang). During irradiation, the Petri dish was placed directly below the lamp bead, and the distance between the lamp bead and the target surface was about 2.7 cm where the irradiance was measured in different time. The irradiance measured at the target surface and the average value was about 0.3591 ± 0.05 mW/cm2 (N=576, P<0.0001). All data was available. The data was analyzed by Normality and Lognormality Tests, One sample t and Wilcoxon test and Descriptive statistics. The statistical tests were performed by Prism 8 Version 8.2.1 (279).The energy was calculated by the following formula:
E=I×t
Where Ε is UV dosage (mJ/cm²), Ι is the irradiance detected on the surface of targets (mW/cm²), and t is the irradiation time (s). And But if all data were used for the UV energy for 5 min and 10 min, the energy was about 2.9‵ 107.7‵ 215.5 mJ/cm2.
In this experiment, when the lamp bead of the instrument was lit, the heat dissipation in the driving system was poor, and there was the statistical error of irradiation dose. It was only used as a reference for the actual ultraviolet energy. The effect of lamp bead heat on cells was ignored. An inverted microscope (Optikam PRO8 Digital Camera) was used for image recording. Due to the different density of each component of the cell, the various structures of light passing through the cell showed dark contrast under the inverted microscope.
Irradiation experiment
The cells for experimental groups were human lung adenocarcinoma cells. The image was not statistically significant.
For the normally cultured cells, the relative time interval of continuous image recording was 0 h, day 3 + 9 h, day 4 + 13 h, day 7, day 12 + 6 h. The irradiation time was 8 s, 5 min and 10 min respectively. The cells for irradiation were with culture medium and without the culture medium. The latter was added with culture medium after irradiation and cultured in constant temperature incubator. After irradiation, the liquid was not changed in the whole experimental process. The corresponding recording time of cells irradiated for 5 minutes was 0 h, 6 h, day 1 + 9 h, day 2 + 14 h, day 10 + 14 h. The corresponding recording time of the two groups irradiated for 8 s was 0 h, 6 h, day 2 + 11 h, day 3 + 15 h and day 11 + 11 H. The image recording time of 5 groups irradiated for 10 min and 8 s without medium was 0 h, day 2 + 4 h and day 5 + 4 h.
For blood free medium irradiation, the cells were divided into two parts along the diameter of the Petri dish with a 200 μL cube which were not in contact with each other. The irradiation group was placed at 2.7 cm to irradiate the cells containing culture medium and de culture medium respectively. After irradiation, the blood-free medium was added. The cells were put into the incubator after taking photos. The image recording interval was 0 h, day 2, day 3, day 3 + 8 h, day 5 + 6 h, day 11.
The cells were treated at different temperatures. The cells containing the culture medium were placed at 4 °C and were placed at minus 20 °C after removing the culture medium. The bottom of the culture dish containing the culture medium and the cells without the culture medium was placed in a constant temperature water bath at 80 °C for 10 minutes and at room temperature for 5 minutes. After the heat dissipated, the cells were observed under the microscope. The culture medium was removed and placed in the incubator. The image recording interval was about 53 hours.
Supplementary Table 1: Characteristics of A549 in petri dish with the medium.
Supplementary Table 2: Characteristics of A549 in petri dish without the medium.


 Impact Factor: * 4.1
Impact Factor: * 4.1 Acceptance Rate: 75.32%
Acceptance Rate: 75.32%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 