Comparative Studies on Safety of Glimepiride and Glipizide on Renal Microarchitecture and Oxidative Stress Markers of Pregnant Streptozotocin-Induced Diabetic Wistar Rats
Esubi JU1, Olojede SO1,3*, Lawal SK2,3, Medubi LJ1, Adekoya AJ1, Dauda FF1, Olusegun AP1, Osinubi AA1
1Department of Anatomy, College of Medicine of the University of Lagos, Lagos, Nigeria
2Department of Anatomy, College of Health and Allied Sciences, St. Francis University, Ifakara-Morogoro, Tanzania
3Discipline of Clinical Anatomy, Nelson Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
*Corresponding Author: Olojede Samuel Oluwaseun, Discipline of Clinical Anatomy, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, 4001, Durban, South Africa
Received: 21 March 2018; Accepted: 28 March 2018; Published: 30 March 2018
Article Information
Citation: Esubi JU, Olojede SO, Lawal SK, Medubi LJ, Adekoya AJ, Dauda FF, Olusegun AP, Osinubi AA. Comparative Studies on Safety of Glimepiride and Glipizide on Renal Microarchitecture and Oxidative Stress Markers of Pregnant Streptozotocin-Induced Diabetic Wistar Rats. J Pharm Pharmacol Res 3 (2019): 003-018.
View / Download Pdf Share at FacebookAbstract
Introduction: A relatively decreased insulin secretion and impaired response of the body to insulin are the common attributes of gestational diabetes mellitus (GDM) and Type 2 diabetes mellitus. The oral hypoglycemic agents are less invasive, improve patients’ compliance, which can be effective in maintaining the blood sugar level during pregnancy.
Aim: The overall aim of this research was to ascertain the comparative evaluation of the glimepiride and glipizide on the kidney and some maternal parameters of pregnant streptozotocin (STZ)-induced diabetic rats.
Methodology: Thirty-five (35) female Sprague-Dawley rats weighing between 120-160 g were divided into 5 groups. Groups 2-5 were induced with diabetes mellitus by intraperitoneal injection of streptozotocin (STZ). Group 1: (Control given distilled water), Group 2: (Diabetic treated with Glimepiride), Group 3: (Diabetic treated with Insulin), Group 4: (Diabetic treated with Glipizide), Group 5: (Diabetic given citrate buffer).
Results: Glimepiride and Glipizide treated groups showed statistically significant (p=0.05) improvement in oxidative stress markers, blood glucose level, body weight, hematological parameters and lipid profile when compared with diabetic and insulin groups. There was statistically significant (p=0.05) improvement on the oxidative stress marker, body weight and the restorative effect on renal histology in the group treated glimepiride when compared with glipizide and diabetic groups.
Conclusion: This work has demonstrated that the two oral hypoglycaemic agents were effective in controlling glucose intolerance during pregnancy, renal oxidative stress as well as cytoarchitectonic properties of the kidney comparable with insulin. Therefore, because of its ameliorative and restorative effects on renal oxidative stress and micro-
Keywords
<p>Kidney; Gestational Diabetes Mellitus; Oral Hypoglycaemic agents; Pancreas; Glimepiride; Glipizide</p>
Article Details
1. Introduction
Adverse maternal and prenatal outcomes have always been major complications with gestational diabetes mellitus [1]. Significant decrease in glucose intolerance has been demonstrated to possess negative effects [2]. However, the understanding of the contents of gestational glucose intolerance has been a highly controversial issue in the clinical practice and research for the past thirty years. It has become a major problem to properly diagnose pregnancy-induced diabetes mellitus because of numerous diagnostic procedures and glucose cutoffs designed for gestational glucose intolerance. The contemporary recommendations on meaning, diagnosis and grouping of gestational glucose intolerance was reviewed by WHO in the year 2010 [3, 4]. Diabetes mellitus is a set of chronic metabolic disorders of multiple causes attributed to chronic hyperglycaemia in relation to disruption of macromolecules (fat, protein and carbohydrate) metabolisms as a result of defects in either secretion or action of insulin. Manifestation of indescribable weight loss, polydipsia, polyuria which has been established by abnormal rise in blood glucose level are the indications in the detection of diabetes mellitus [3].
Relatively insufficient insulin secretion and responsiveness are the considerable similarities between gestational diabetes mellitus non-insulin-dependent diabetes mellitus. The preponderance of glucose intolerance in pregnancy account for about 2-10% in all pregnancy which may improve or disappear after childbirth [5]. Furthermore, it has been established that in about 5-10% of patients suffering from gestational diabetes mellitus was later found to suffer non-insulin dependent diabetes mellitus [5]. Thorough medical supervision during pregnancy aids better control of glucose intolerance during pregnancy. Conventionally, insulin therapy has always been the cornerstone in management of diabetes mellitus because of its efficacy in proper glucose control and because it does not cross the materno-fetal interface. Insulin is, however, an expensive and invasive treatment. Patients suffering from gestational diabetes mellitus would prefer oral hypoglycaemic agents rather than multiple injections [6]. Moreover, insulin therapy requires daily injections in which patient compliance is usually negligible. Diabetes mellitus is related to many complications such as retinopathy, neuropathy, cardiomyopathy as well as nephropathy due to increased oxidative stress and serum lipids [7]. Nevertheless, majority of the gestational diabetes cases resolve after delivery while some cases persist.
Declination in the level of glomerular filtration rate (GFR), high blood pressure and abnormal presence of albumin in the urine are the clinical manifestations of nephropathy in diabetic patients [8]. It is influenced by prolonged glomerular hyperfiltration followed by excessive protein elimination caused by diabetes. Diabetes mellitus has been reported to be the foremost cause of final stage kidney disease in many countries [9-11]. Glimepiride have been considered less likely to produce undesirable abnormally low blood sugar because it depends on extra-pancreatic effects for a larger proportion due to its hypoglycaemic effect [12]. In a study on management of Diabetes mellitus with glipizide, it was established that greater amount of reduction in A1C was observed at week 18, which remained generally steady for the period of the experiment. The steady effect with management of glipizide was likely resulted from dose up titration, which was effective throughout the study to conserve normal glycaemic control [13]. Hence this study was conceived to determine the comparative evaluation of the glimepiride and glipizide as commonly used OHAs on the kidney of pregnant streptozotocin (STZ)-induced diabetic rats and compare the outcomes with the insulin therapy which its outcome could have tendency of granting an approval to the use of glimepiride and glipizide during pregnancy, because of its easy accessibility, affordability. Moreover, acceptability of these OHAs is believed to reduce the maternal and fetal mortality and morbidity as a result of gestational diabetes mellitus.
2. Methodology
2.1 Animals
Thirty-five (35) fertile female Sprague-Dawley rats with weight ranges from 120-160 were procured from Animal Laboratory Center of College of Medicine of the University of Lagos (CMUL) and maintained in the wire-mesh cages at the animal house of the anatomy department, college of medicine of the University of Lagos. The cages were partitioned into 6 portions of equal size containing 7 female animals in each group (1-6 groups). The female rats were mated with a giant male in ratio 2:1. The base of the cage was made in such a way that allows the passage of urine and fecal pellets out of the cages. Removable trays were placed underneath each compartment to collect urine and fecal pellets released by the rats. The weights of the rats were taken and randomly divided after three weeks acclimatization into 5 main groups. It must be emphasized here that the animal research laboratory is maintained at temperature of 26-28% and 12:12 light:dark cycle. The ethical approval was given by the College of Medicine, University of Lagos Research Grants and Experimentation Ethics Committee (RGEEC).
2.2 Determination of the estrous cycle
Different phases of estrous were determined by daily routine examination of vaginal smear as described by Byers et al. [14], which are proestrous, estrous, metestrous and diestrous. Vaginal smear were taken once each day between 7 am and 10 am. During the vaginal smear procedure, each rats were held at the back. In order to obtain the vaginal smear, the tip of a 3 inch borosilicate glass medicine dropper was filled up with exactly 0.2 ml of normal saline and inserted approximately 2-3 mm inside the vagina of the rats in accordance with Ecker and Greene [15] procedure. The procedure was carefully performed in order not to injure the rat. Normal saline was released from the borosilicate glass medicine dropper into the vagina and drew with vaginal smear containing cells that were immediately placed on the histological slides and viewed under light microscope at magnification of 100 × for microscopic examination. The different phases of rat’s estrous were determined and recorded for 3 weeks with reference to Edwin et al. [16]. Animals with regular estrous cycle were used for this experiment. They were identified by different colors marking in their forehead, Tail, body, hand and leg. All the experimental rats were carefully handled with reference to the standard guide for the care and use of laboratory animals.
2.3 Induction of diabetes mellitus
Induction of diabetes mellitus was performed in overnight fasted rats by a double injection of streptozotocin (STZ) (45 mg and 35 mg/kg body weight) intraperitonealy in 0.1 M sodium citrate buffer with a pH of 4.5. The aged-matched control rats’ respectively were administered an equivalent volume of citrate buffer. The experimental rats were given rat feed, water and daily routine checks immediately after the streptozotocin (STZ) induction. Hyperglycaemia in rats were confirmed forty-eight (48) hours after induction of streptozotocin (STZ) using glucometer (Accu-check) by fasting (16 hours) blood sugar measurement of the blood drained from the tail veins of the experimental rats. The animals with fasting blood glucose level=120 mg/dl with other symptoms of diabetes mellitus such as polyphagia, polydipsia, polyuria, and weight loss were considered diabetic and included in the study.
2.4 Grouping of animals and dosage of test agents/treatment
The animals were randomly divided into 5 groups, and each group comprised of seven rats. Distilled water, insulin; glimepiride, glipizide and citrate buffer were administered once in a daily for 3 weeks by oral canular except for insulin which was intraperitoneal administered. Treatment of animals began at the onset of pregnancy after the rats have been confirmed diabetic. The animals were treated for 3 weeks as follows; Group 1: Control+distilled water (0.5 ml); Group 2: Diabetic+glimepiride (0.11 mg/kg body weight); Group 3: Diabetic+insulin (1 iu daily); Group 4: Diabetic+glipizide (0.57 mg/kg body weight); Group 5: Diabetic+citrate buffer (0.5 ml).
2.5 Determination of blood sugar using the glucometer
The most established way to test the blood sugar level is by using a blood-glucose meter (also called glucometer), a machine that analyzes the amount of glucose in one or two drops of the blood. In determining the blood sugar level using the glucose meter, a test strip was taken and placed into the test strip slot within the glucose meter, the glass pad facing upward. A small blood drop symbol would now flash. Using the scapel blade, a drop of blood was obtained from the rat-tail and placed on it. There is always some seconds delay before a value appears on the screen.
2.6 Weight assessment
Compression spring balance was used for daily assessment and monitoring of rat’s weights which serves as parameter for the physical status of the animals over the period of experiment.
2.7 Sacrifice of the rats
All the rats were sacrificed at the 19th day of gestation while still under anesthesia and all the organs were harvested.
2.8 Hematoxylin and eosin staining
The kidney was sliced into 1 cm thick and put in the cassettes. The cassettes containing the sliced kidney were placed in the tissue processor machine where dehydration, clearing and impregnation processes were all taken place overnight for the period of 14 hours after which the cassettes were infiltrated with paraffin as an embedding agent. Each block was trimmed then sectioned about 5 µm by using a microtome [17]. H and E (hematoxylin and eosin) dye, which mounted with DPX for microscopic observations.
2.9 Blood sample collection
The rats were aneasthetized using 60 mg/kg of ketamine and 10 mg/kg of xylazine after which, five (5) mL of blood were drained from each rat, while some blood was collected in an Ethylenediaminetetraacetic acid (EDTA) bottle sample for other blood tests [18].
2.10 Hematological analysis
An automated hematology analyzer was used to analyze a well-blended blood sample collected in an EDTA bottle for complete blood count (CBC), which was later measured by a hematology analyzer made of fluorescence technology named Sysmex XS800i manufactured by Diamond Diagnostics-USA.
2.11 Hormonal profile analysis
Blood sample from the experimental rats were collected in the anticoagulants bottles and centrifuged within 2 hours. The separated plasma of blood samples that were collected were kept in a freezer at -20°C for analysis of the following hormones; progesterone, estradiol, luteinizing hormone, and follicle stimulating hormone. Hormonal assays were carried out using ELISA Kit obtained from Cayman Chemical Company, USA [19]. Laboratory Procedure Dr. Lange LP 700 equipment was used for the determination of lipid profiles [20].
2.12 Oxidative stress marker method for homogenizing sample
The kidneys of dissected rats were removed for the essence of these tests while other was used for histological tissue processing. The post mitochondria portion of the rats’ organs were prepared in the following sequences; the kidneys of the rats were cleaned in an ice cold 1.15% KCl solution, blotted and weighed. Shortly after, homogenization took place with 0.1 M of phosphate buffer (pH 7.2) whereby, the kidneys were put inside the laboratory mortar where laboratory sand was added and blended with pestle. The kidney homogenates were centrifuge at speed of 2,500 rmp for the period of 15 minutes then removed after which the supernatant was decanted and stored at degree of -20°C until analysis was carried out. Antioxidant Enzymes Assay Spectrometric analysis were used to measure the antioxidant enzymes activities of the following oxidative stress markers.
2.13 Determination of superoxide dismutase (SOD) activity
SOD activity was measured by spectrophotometric assay based on epinephrine autoxidation, described previously by Sun and Zigman. Kidney homogenates were added to cuvettes containing epinephrine (30 mM, pH 1.3) and NaHCO 3 buffer (pH 10.2) with EDTA and the change in absorbance at 320 nm was measured over 3 minutes at 25ºC. The reaction was started with the mixture of 3 mL that comprises; 2.95 mL, 0.05 M of sodium carbonate buffer (pH 10.2), 0.02 mL of homogenized kidney, 0.03 mL of epinephrine and 0.005 N HCl. The following are the content of the reference cuvette; 2.95 mL buffer, 0.02 mL of water and 0.03 mL of epinephrine. Measuring the change in the absorbance at 480 nm for the period of 5 minutes at S=4,020 M-1 cm-1 were used to estimate the enzyme activity.
2.14 Catalase (CAT) activity determination
Catalase (CAT) activities were determined as it was described by Sinha. It was assayed calorimetrically at 620 nm and expressed as µmoles of H2O2 in min/mg/kg of protein at 25°C. The composition of 1.5 mL of reaction mixture are; 1.0 mL in 0.01 M phosphate buffer with pH of 7.0, 0.1 mL of homogenized kidney and 0.4 mL of 2 M H2O2. The reaction was completed by introducing 2.0 mL of dichromate-acetic acid reagent which contained 5% potassium dichromate and glacial acetic acid blended together in the ratio 1:3 at S = 40 M-1 cm-1.
2.15 Reduced gluthathione determination
The activities of reduced GSH (glutathione) of the kidney was measured in accordance with the description stated by Sedlak and Lindsay. To the kidney homogenate, 10% of TCA was mixed and centrifuged. Furthermore, 0.5 mL of Ellman’s reagent that containing 19.8 mg of 5,5-dithiobis nitro benzoic acid (DTNB) dissolved in 100 mL of 0.1% sodium nitrate and 3.0 mL of phosphate buffer (pH 8.0, 0.2 M) were used to treat 1.0 mL of supernatant. The absorbance was later read at 412 nm with S=1.34 104 M-1 cm-1.
2.16 Lipid peroxidation malondialdehyde (MDA)
The parameter of lipid peroxidation were analyzed as it was described by Buege and Aust, where 1.0 mL of the supernatant was mixed with 2 mL of TCA-TBA-HCL reagent in the ratio (1:1:1) as follows; (thiobarbituric acid 0.37%, 0.24 N HCl and 15% TCA) tricarboxylic acid-thiobarbituric acid-hydrochloric acid reagent boiled at 100°C for 15 min, and allowed to cool. Separation of flocculent particles were carried out by centrifugation at 3,000 rpm for 10 minutes in other to remove supernatant. After which, Molar extinction coefficient for MDATBA complex of 1.56 × 105 M-1 CM1was used to estimate the MDA value.
3. Statistical Analysis
The mean blood glucose value (measured in mg/dL) and the weight (measured in grams) of the experimental rats before and after induction with diabetes were analyzed using ANOVA and were represented in the tables. All the results were presented as the mean ± standard error of the mean. Statistical analysis was ANOVA for comparisons between two groups and by analysis of variance for more than two groups and p<0.05 was considered to be statistically significant [21].
4. Results
The results of this study was presented in Table 1-6. In Table 1, the rats in the control group show significant increase in body weights throughout the period of the experiment. The diabetic rats treated with glimepiride, insulin and glipizide show reduction in body weight at day 8 but significantly increased at the end of the experiment. The body weights of diabetic rats were significantly reduce compared with the treatment groups.
|
Groups/Days |
Day 4 |
Day 8 |
Day 12 |
Day 16 |
Day 18 |
|
Control |
156.14 ± 5.640 |
152.86 ± 3.237 |
157.71 ± 3.251 |
161.57 ± 3.359 |
165.00 ± 3.559 |
|
Glimepiride |
146.29 ± 10.111 |
145.57 ± 5.682 |
137.40 ± 4.219 |
136.00 ± 3.536 |
136.00 ± 3.536*** |
|
Insulin |
148.83 ± 2.639 |
149.20 ± 10.085 |
155.00 ± 6.028 |
155.29 ± 5.936 |
155.29 ± 5.936 |
|
Glipizide |
146.29 ± 3.988 |
142.20 ± 4.207 |
148.60 ± 9.839 |
148.20 ± 10.085 |
148.20 ± 10.085** |
|
Diabetic |
130.29 ± 5.499 |
129.57 ± 5.682* |
128.43 ± 5.473* |
128.29 ± 5.090* |
128.14 ± 5.047* |
Sig. p<0.05 *Significant when compared with Control, **Significant when compared with Diabetic ***Significant when compared with Insulin (± SEM).
Table 1: Statistics of the mean body weights of animals in (grams, g) across all groups.
|
Groups/Day |
Day 4 |
Day 8 |
Day 12 |
Day 16 |
Day 18 |
|
Control |
90.29 ± 5.499 |
89.57 ± 5.682 |
90.43 ± 5.473 |
90.29 ± 5.090 |
91.14 ± 5.047 |
|
Glimepiride |
145.29 ± 10.111 |
142.20 ± 10.085 |
139.60 ± 9.839 |
140.20 ± 10.085 |
139.20 ± 10.085** |
|
Insulin |
143.29+3.988 |
141.86 ± 3.237 |
137.40 ± 4.219 |
136.00 ± 3.536 |
136.00 ± 3.536** |
|
Glipizide |
148.83 ± 2.639 |
147.20 ± 4.207 |
144.00 ± 6.028 |
143.29 ± 5.936 |
143.29 ± 5.936** |
|
Diabetic |
156.14 ± 5.640 |
155.57 ± 6.079 |
157.71 ± 3.251 |
159.57 ± 3.359 |
158.00 ± 3.559* |
Sig. p<0.05 *Significant when compared with Control, **Significant when compared with Diabetic ***Significant when compared with Insulin (± SEM).
Table 2: Statistics of the mean blood glucose levels of animals in millimoles per litre (mmol/L) across all groups.
The glimepiride and glipizide treated groups had significant reduction in blood glucose levels, but insulin had a better blood glucose levels reduction when compared with the two oral hypoglycemic agents. The blood glucose levels of diabetic rats remain increased throughout the period of experiment. The result of this study indicates that the hematological parameters of the control rats were within normal range throughout the period of the experiment. Rats treated with insulin showed better improvement in hematological parameters than other treated groups, while diabetic rats treated with glimepiride showed improvement in hematological parameters when compared with glipizide. The significant decrease in the levels of hematological parameters were noticed in diabetic rats compared with the control and treatment groups as shown in the Table 3.
|
Groups/ Parameters |
RBC |
HCT |
HGB |
PLT |
WBC |
LYM |
GRAN |
|
Control |
7.41 ± 0.36 |
50.16 ± 3.79 |
16.58 ± 0.52 |
743.40 ± 31.09 |
6.80 ± 0.25 |
6.98 ± 0.13 |
0.54 ± 0.055 |
|
Glimepiride |
6.01 ± 0.91** |
41.70 ± 9.93** |
13.54 ± 1.92 |
641.00 ± 46.02*** |
6.46 ± 1.87*** |
4.54 ± 2.41 |
0.32 ± 0.19** |
|
Insulin |
7.07 ± 0.48** |
49.20 ± 3.92 |
14.40 ± 0.79 |
521.86 ± 291.15 |
4.58 ± 1.15 |
4.46 ± 2.25 |
0.26 ± 0.11* |
|
Glipizide |
4.58 ± 0.73 |
26.08 ± 2.89*** |
8.62 ± 0.65 |
628.60 ± 84.20 |
4.10 ± 1.12 |
3.18 ± 0.76 |
0.12 ± 0.05 |
|
Diabetic |
4.35 ± 1.566* |
31.12 ± 8.25* |
9.96 ± 1.22* |
426.00 ± 189.61* |
4.30 ± 0.29* |
4.22 ± 0.19* |
0.16 ± 0.054* |
Sig. p<0.05 *Significant when compared with Control, **Significant when compared with Diabetic, ***Significant when compared with Insulin (± SEM). Key: RBC-red blood cell; HGB-heamoglobin; PLT-platelet; PCT-plateletcrit; MPV-mean platelet volume; WBC-white blood cell; LYM-lymphocyte; GRAN-granulocyte.
Table 3: Statistics of the mean hematological parameters of animals in across all groups.
|
Groups/ Parameters |
CHOL |
TG |
HDL |
LDL |
AST |
ALT |
ALP |
|
Control |
1.34 ± 0.05 |
0.68 ± 0.25 |
1.04 ± 0.25 |
0.58 ± 0.08 |
41.60 ± 11.28 |
15.00 ± 1.87 |
20.60 ± 29.67 |
|
Glimepiride |
2.08 ± 0.16*** |
0.88 ± 0.08 |
0.96 ± 0.18*** |
0.94 ± 0.74 |
65.60 ± 1.52*** |
22.80 ± 10.13*** |
22.80 ± 10.69 |
|
Insulin |
1.94 ± 0.46 |
0.76 ± 0.11 |
1.08 ± 0.18 |
0.76 ± 0.47 |
41.60 ± 12.30 |
17.60 ± 1.52 |
21.40 ± 3.44 |
|
Glipizide |
2.22 ± 0.16 |
0.98 ± 0.40 |
0.86 ± 0.18** |
0.90 ± 0.14 |
49.60 ± 14.22** |
27.80 ± 6.14* |
101.00 ± 13.27*** |
|
Diabetic |
2.46 ± 0.26* |
1.18 ± 0.16* |
0.54 ± 0.05* |
1.00 ± 0.16 |
75.00 ± 10.79* |
30.00 ± 11.85* |
109.80 ± 1.30* |
Sig. p<0.05 *Significant when compared with Control, **Significant when compared with Diabetic, ***Significant when compared with Insulin (± SEM).
Key: CHOL-cholesterol; TG-triglycerides; HDL-high density lipoprotein; LDL-low density lipoprotein; AST-aspartate aminotransferase; ALT-alanine aminotransferase; ALP-alkaline phosphatase.
Table 4: Statistics of the mean lipid profile of animals across all groups.
The levels of lipid profiles were within the normal limit in the control group. Table 4 clearly shows that levels of HDL were normal in rats treated with insulin but decreased in glipizide and glimepiride and significantly reduced in diabetic rats. Diabetic rats treated with insulin showed some level of increase in cholesterol, triglyceride and low density lipoprotein compared with control but decreased when compared with other treatment groups and diabetic group. On the other hand, glimepiride worked better on the cholesterol, triglyceride and low density lipoprotein compared with glipizide and diabetic groups. The levels of cholesterol (chol), triglyceride and low density lipoprotein were significantly high in diabetic rats when compared with control and treatment groups. It was clearly shown in the Table 4 that the levels of AST, ASL and ALP were within the normal range in control rats and rats treated with insulin, while the levels of AST and ALT were increased to some extent in rats treated with glimepiride and glipizide. Moreover, the levels of ALP were significantly low in the rats treated with glimepiride but higher in rats treated with glipizide. The levels of AST, ALP and ASL were significantly increased in diabetic rats while the levels of ASL were also increased in diabetic rats.
|
Group/ Parameters |
PROG |
E2 |
LH |
FSH |
PRL |
|
Control |
21.75 ± 3.23 |
46.10 ± 11.02 |
0.42 ± 0.16 |
1.00 ± 0.16 |
1.00 ± 0.14 |
|
Glimepiride |
15.71 ± 1.01** |
21.92 ± 2.06** |
0.22 ± 0.11*** |
1.08 ± 0.65*** |
0.38 ± 0.18*** |
|
Insulin |
13.88 ± 1.77** |
21.16 ± 0.78 ** |
0.56 ± 0.09** |
0.72 ± 0.42** |
1.18 ± 0.08** |
|
Glipizide |
15.24 ± 2.02*** |
21.95 ± 1.45 |
0.16 ± 0.09 |
1.90 ± 1.14*** |
0.28 ± 0.08 |
|
Diabetic |
12.90 ± 0.44* |
14.52 ± 0.81* |
0.14 ± 0.05* |
0.20 ± 0.07* |
0.42 ± 0.15* |
Sig. p<0.05 *Significant when compared with Control, **Significant when compared with Diabetic ***Significant when compared with Insulin, (± SEM). Key: LH-luteinizing hormone; FSH-Follicle stimulating hormone; PRL-prolactin; PROG-Progesterone; E2-estradiol.
Table 5: Statistics of the mean hormonal profile of animals across all groups.
Rats in control group show normal hormonal profile values. In the same way, decreased in the hormonal profile values except FSH values, were observed in the rats treated with glimepiride and glipizide when compared with control group. The hormonal profile levels of the diabetic rats were significantly decreased compared with the control and treatment groups as it was revealed in the Table 5.
|
Groups/Parameters |
GSH |
SOD |
CAT |
MDA |
|
Control |
8.71 ± 1.51 |
94.23 ± 3.74 |
428.42 ± 91.89 |
0.65 ± 0.32 |
|
Glimepiride |
2.91 ± 0.36** |
83.99 ± 5.47** |
562.37 ± 24.97** |
1.82 ± 0.25** |
|
Insulin |
3.00 ± 1.54** |
94.23 ± 3.74** |
678.45 ± 52.71** |
1.67 ± 0.25** |
|
Glipizide |
2.25 ± 0.62** |
77.64 ± 11.49 |
625.64 ± 103.00** |
1.86 ± 0.66** |
|
Diabetic |
0.72 ± 0.58* |
77.20 ± 9.58* |
816.46 ± 42.12* |
4.95 ± 0.52* |
Sig. p<0.05 *Significant when compared with Control, **Significant when compared with Diabetic ***Significant when compared with Insulin (± SEM). Key: GSH-glutathione; SOD-superoxide dismutase; CAT-catalase; MDA-Malondialdehyde.
Table 6: Statistics of the mean oxidative stress markers of animals across all groups.
Table 6 shows the result of the oxidative stress markers of the experimental rats. The levels of glutathione reductase and super oxide dismutase (SOD) were significantly reduced in diabetic rats when compared with rats treated with insulin and rats in control group while levels of CAT and MDA were significantly reduced in control group and rats treated with insulin but significantly increased in diabetic rats. Rats treated with glimepiride and glipizide shows significant increase in the levels of glutathione reductase and SOD compared with diabetic rats but not as higher as control group and rats treated with insulin, also noticed were significant reduction in the levels of CAT and MDA in the rats treated with glimepiride and glipizide but glimepiride shows the best result compared with glipizide group.
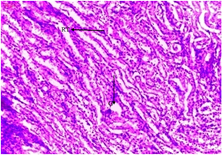
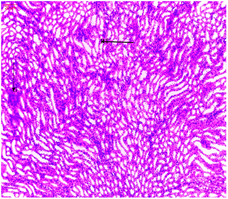
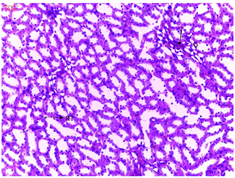
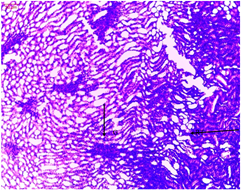
4.1 Histological analysis
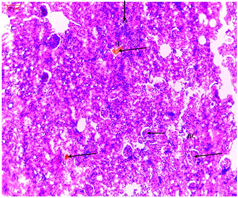
The histological analyses shows normal glomeruli and tubules of the rats in control group without odema, congestion or hemorrhage.
The histological sections of the diabetic rats treated with glimepiride shows mild cortical congestion and degeneration of glomerulus but better improvement in renal histology were observed in diabetic rats treated with glimepiride compared with glipizide.
Mild cortical congestion and degeneration were noticed in the glomeruli and tubules of diabetic rats treated with insulin.
The histological sections of the diabetic rats treated with glipizide shows severe cortical congestion and degeneration of glomeruli.
There were severe congestions and hemorrhage in the glomeruli and tubules of the diabetic rats.
Discussion
There have been great attainments regarding prevention and management but the prevalence of gestational diabetes mellitus in Africa is still wide-ranging since few decades [22]. Over the years, insulin injection, food and life style adjustment has been known as the only treatment modalities for gestational diabetes mellitus as physicians believed that oral hypoglycemic agents (OHAs) had the tendency to cause congenital abnormalities and other complications both to the mothers and the developing children but it now generally accepted that the information available on the safety of these OHAs are not substantial enough for this conclusion [23]. Hence this research was designed to assess the comparative effects of glimepiride, glipizide and insulin on the kidney of STZ-induced diabetic pregnant wistar rats. The mean body weight across the experimental groups shows statistical differences within, and between the groups. The decrease in body weight in the diabetic rats observed in this study was also reported in the previous study [24], which revealed that decrease in body weight in diabetic rats may indicate loss or degradation of structural proteins that can contribute to decrease in body weight [24].
Likewise, the result of this research is in accordance with recent report [25] that reveals weight of rats on insulin therapy increased throughout the study, whereas that of untreated diabetic rats remain relatively unchanged. However, glimepiride group shows statistical significant reduction in body weights compared with control, insulin and diabetic groups (Table 1), therefore, the result of this finding confirmed the benefit of glimepiride in gestational diabetes as earlier reported [26]. The result of blood glucose levels reveal that the two (2) oral hypoglyceamic agents show better glycemic control. More so, insulin had the best result in controlling blood glucose levels when compared with the two oral hypoglyceamic agents. Previous study also revealed that controlling blood glucose with insulin has the potential to be the most effective blood glucose-lowering therapy [27]. Nevertheless, the glyceamic control of rats treated with glimepiride showed better result when compared with glipizide, similar findings were reported in the previous studies [28, 29]. The blood glucose levels of diabetic rats remain increased throughout the period of experiment. The previous study also reported that the blood glucose levels of STZ-induced diabetic rats were significantly higher [30], this homogeneously corroborates with the result of this study.
The reasons for the increase in blood glucose level in diabetic rats were reported [31], where it was revealed that effect of streptozotocin on ß-cells leads to development of insufficient production of insulin and consequently, the elevation of blood glucose level occur. There was no significant difference in the pack cell volume (PCV) in all the treated groups, but significantly higher than the diabetic untreated group. This indicates that all the three treatments significantly improved the level of PCV in a diabetic condition. However, insulin proved to be more effective on MPV and other haematological parameters according to the results of this study which corroborates with previous study that reported improvement in the heamatological parameters of the diabetic rats treated by insulin [32].
Consistent with the literature, the results of this study reveals that the oral hypoglycemic agents have positive impact on blood cells, though conflicting results have been reported for major heamatological parameters especially WBC and PLR counts in diabetes as it was also reported by previous study [33]. It was observed from the result of this study that the levels of Cholesterol, TG and VLDL-C levels were significantly decreased while HDL-C levels were significantly increased in the rats treated with insulin when compared with diabetic rats. Similar results were found in another study [34]. Meanwhile, both glimepiride and glipizide could restore cholesterol levels which were comparable to the control. This finding is in conformity with previous study that reveals oral antidiabetic drugs are not only affordable and effective hypoglycemic agents especially in combination therapy but can also decrease serum lipids and thereby aids in the prevention and management of atherosclerosis and its complications in T2DM [35], but contrary to the report that [36] insulin controls blood glucose and lipid profile while glimepiride had no amelioration. Previous study also reported lipid changes as a result of improved glycaemic control in diabetic rats treated with oral hypoglycaemic agents [37]. The activities of cholesterol, LDL, TG, serum aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase were significantly increased in diabetic rats when compared with control group and other treatment groups. Similar results were reported [38].
In this present research, insulin shows significant effect on the progesterone, oestrogen and prolactin as obtainable in the rats in control group except E2. This is in agreement with study carried out by Pournaghi et al. (2012). Furthermore, the mean levels of PROG, E2, LH, FSH and PRL were found to be lower in diabetic rats compare with control and treatment groups. Similar results have been reported in the previous study [39], but contrary findings were reported in another study [40]. The result of the oxidative stress markers shows that all the treatment groups had renal MDA which were comparable to the control and significantly lower than the diabetic untreated group, analogous results have been reported by different authors. Recent study [41] have reported that conditions such as; ß-cell dysfunction, dyslipidemia, insulin resistance and impaired glucose tolerance which would later lead to Type 2 Diabetes mellitus are majorly caused by an increased oxidative stress. Similarly, another study [42] have attributed reduction in the antioxidant enzymes levels (superoxide dismutase and glutathione peroxidase) of ß-Cells and higher sensitive to oxidative stress [42] to the major cause of the above effect.
Oxidative stress exposure to ß-cells initiated the increment in the level of cyclin-dependent kinase inhibitor 1 secretion, reduced level of insulin mRNA, as well as ATP and Calcium flux reductions in the organs like; mitochondria and cytosol which lead to apoptosis [43]. Hence, glimepiride and glipizide have shown to be effective in reducing hyperglycaemic induce renal oxidative stress which agrees with a finding that reveals glimepiride promotes anti-oxidant status in diabetic animals and reduce nuclei damage and sperm abnormalities [44]. Moreover, glimepiride and glipizide groups showed normal glomeruli on renal histology which were similar to the control. They also showed mild cortical congestion and haemorrhage compared to insulin. This infers that both glimepiride and glipizide were not toxic to the organ rather ameliorated the effect of diabetes on renal tissue, the result agree with a report which shows that STZ induced diabetes caused degenerative changes in renal histology of rabbit following the treatment of glimepiride the renal morphology was recovered [45] as well as report that reveals mild and moderate renal impairment in rats treated with glimepiride [46].
Conclusion
This work has demonstrated that the two oral hypoglycaemic agents were effective in management of gestational diabetes mellitus, renal oxidative stress as well as cytoarchitectonic properties of the kidney comparable with insulin. Therefore because of its ameliorative and restorative effects on renal oxidative stress and micro-architectonic properties of the kidney, glimepiride could be tempting alternative drug of choice for the management of gestational diabetes mellitus.
Disclosure
The authors report no conflict of interest.
References
- Ali S, Dornhorst A. Diabetes in pregnancy: health risks and management. Postgraduate Medical Journal 87 (2011): 417-427.
- World Health Organization. Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy. 2013 report of a WHO consultation (2013).
- Yogev, Chen, Hod, et al. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: preeclampsia. American Journal of Obstetrics and Gynecology 202 (2010): 255-257.
- World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications: 1999 report of a WHO consultation (1999).
- National Diabetes Clearinghouse (NDIC): National Diabetes Statistics 2011. U.S. Department of Health and Human Services (2011).
- Langer O. When diet fails: insulin and oral hypoglycaemic agents as alternatives for management of gestational diabetes mellitus. Journal of Maternal and Fetal Medicine 11 (2002): 218-225.
- Rondi S, Peddolla R, Venisetty RK. Neuro, cardio, and reno protective activities of rosuvastatin in streptozotocin-induced type 2 diabetic rats undergoing treatment with metformin and glimepiride. J Adv Pharm Technol Res 5 (2014): 78-83.
- Sun YM, Su Y, Li J, et al. Recent advances in understanding the biochemical and molecular mechanism of diabetic nephropathy. BiochemBiophys Res Commun 433 (2013): 359-361.
- He F, Xia X, Wu XF, et al. Diabetic retinopathy in predicting diabetic nephropathy in patients with type 2 diabetes and renal disease: a meta-analysis. Diabetologia 56 (2013): 457-466.
- Mohamad SM, Haidari F, Shiri MR. Comparison of effect of resveratrol and vanadium on diabetes related dyslipidemia and hyperglycemia in streptozotocin induced diabetic rats. Adv Pharm Bull 1 (2011): 81-86.
- Jonnalagadda VG, Ram-Raju AV, Pittala S, et al. The prelude on novel receptor and ligand targets involved in the treatment of diabetes mellitus.Adv Pharm Bull 4 (2014): 209-217.
- Dills DG and Schneider J. Clinical evaluation of glimepiride versus glyburide in NIDDM in a double-blind comparative study. HormMetab Res 28 (1996): 426-429.
- Camilo J, Ferreira A, Marre M, et al. Efficacy and Safety of sitagliptin versus glipizide in patients with type 2 Diabetes and moderate-to-severe chronic renal insufficiency. Diabetes Care 36 (2013): 1067-1073
- Byers SL, Wiles MV, Dunn SL, et al. Mouse estrous cycle Identification Tool and Images. PLos ONE 7 (2012): e35538.
- Ecker JL, Greene MF. Gestational diabetes mellitus. New England Journal of medicine 358 (2001): 2061-2063.
- Edwin S, Jarald EE, Deb L, et al. Wound Healing and Antioxidant Activity of Achyranthesaspera. Pharmaceut Biol 46 (2008): 824-828.
- Jemai H, Sayadi S. Heart Histopathology and Oxidative Features in Diabetic Rats and Protective Effects of Oleuropein. Advances inBioscience and Biotechnology 6 (2015): 383-389.
- Vague P, Raccah D, Juhan-Vague I. Hemobiology, Vascular Disease, and Diabetes with Special Reference to Impaired Fibrinolysis. Metabolism Suppl 1 (1992): 2-6.
- Pushpa and Kalavathy. Effect Of Mehani on Hormonal Profile in Wistar Rats Induced with Polycystic Ovary Syndrome. Biology 1 (2013): 21-24.
- Jain HR, Shetty V, Singh GS, et al. A Study of Lipid Profile in Diabetes Mellitus. International Journal of Scientific Study 4 (2016): 56-61.
- Kim HY. Analysis of variance (ANOVA) comparing means of more than two groups. Restor Dent Endod 39 (2014): 74-77
- World Health Organization. Global Health Estimates. Deaths by Cause, Age, Sex and Country, 2000-2012. Geneva (2014).
- Osinubi AA, Adesiyun AE, Ajayi GO. Comparative effects of three herbs and standard hypoglycaemic agents on blood glucose in normoglycaemic, hyperglycaemic and alloxan-induced diabetic male rats. Afr J Endocrinol Metab 7 (2008): 6-11.
- Portha B, Levacher C, Picon L, et al. Diabetogenic effect of streptozotocin in the rat during the perinatal period. Diabetes 23 (1974): 889-895.
- Schaschkow A, Mura C, Dal S, et al. Impact of the Type of Continuous Insulin Administration on Metabolism in a Diabetic Rat Model. J Diabetes Res (2016).
- Ferrannini E, Fonseca V, Zinman B, et al. Fifty-two-week efficacy and safety of vildagliptinvs.glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin monotherapy. Diabetes Obesity and Metabolism 11 (2000): 1463-1326.
- Hermansen K, Mortensen LS, Hermansen ML. Combining insulins with oral antidiabetic agents: effect on hyperglycemic control, markers of cardiovascular risk and disease. Vasc Health Risk Manag 4 (2008): 561-574.
- Cheta DM, Lim J, Chan EK, et al. Glimepiride-induced prevention of diabetes and autoimmune events in the BB rat: Revised. Life Sci 57 (1995): 2281-2290.
- Krauss H, Kozlik J, Gryzymislawski M, et al. The influence of glimepiride on the oxidative state of rats with streptozotocin-induced hyperglycemia. Med Sci Monit 10 (2003): BR389-BR393.
- Pournaghi P, Sadrkhanlou RA, Hasanzadeh K, et al. An investigation on body weights, blood glucose levels and pituitary-gonadal axis hormones in diabetic and metformin-treated diabetic female rats. Vet Res Forum 3 (2012): 79-84
- Rossetti L, De Fronzo RA, Gharezi R, et al. Effect of metformin treatment on insulin action in diabetic rats: in vivo and in vitro correlations. Metabol 39 (1990): 425-435.
- Olojede SO, Lawal SK, Medubi LJ, et al. Studies on Cardiac Cytoarchitectonic and Biochemical Indices in Pregnant Streptozotocin-Induced Diabetic Rats Treated with Gliclazide and Insulin. Journal of Pharmacy and Pharmacology 6 (2018): 38-51.
- Demirtas L, Degirmenci H, Akbas EM, et al. Association of hematological indices with diabetes, impaired glucose regulation and microvascular complications of diabetes.Int J ClinExp Med 8 (2015): 11420-11427
- Aslan I, Kucuksayan E, Aslan M. Effect of insulin analog initiation therapy on LDL/HDL subfraction profile and HDL associated enzymes in type 2 diabetic patients. Lipids in Health and Disease 12 (2013): 54.
- Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabet care 29 (2006): 1963-1972.
- Vlassara H, Torreggiani M, Post J, et al. Role of oxidants/inflammation in declining renal function in chronic kidney disease and normal aging; Kidney international. Supplement 76 (2009): 3-11.
- Buse JB, Tan MH, Prince MJ, et al. The effects of oral anti-hyperglycaemic medications on serum lipid profiles in patients with type 2 diabetes. Diabetes ObesMetab 6 (2004): 133-156
- Mwafy SN and Yassin MM. Antidiabetic activity evaluation of glimepiride and Nerium oleander extract on insulin, glucose levels and some liver enzymes activities in experimental diabetic rat model. Pak J Biol Sci 14 (2011): 984-990.
- Ebrahimi-Mamaghani M, Saghafi-Asl M, Pirouzpanah S, et al. Association of Insulin Resistance with Lipid Profile, Metabolic Syndrome, and Hormonal Aberrations in Overweight or Obese Women with Polycystic Ovary Syndrome. J Health PopulNutr 33 (2015): 157-167.
- Mor E, Zograbyan A, Saadat P, et al. The insulin resistant subphenotype of polycystic ovary syndrome: clinical parameters and pathogenesis. Am J Obstet Gynecol 190 (2004): 1654-1660.
- Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes 6 (2015): 456-480.
- Tiedge M, Lortz S, Drinkgern J, et al. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes 46 (1997): 1733-1742.
- Maechler P, Jornot L, Wollheim CB. Hydrogen peroxide alters mitochondrial activation and insulin secretion in pancreatic beta cells. J Biol Chem 274 (1999): 27905-27913.
- Rabbani SI, Devi K, Khanam S. Role of Pioglitazone with Metformin or Glimepiride on Oxidative Stress-induced Nuclear Damage and Reproductive Toxicity in Diabetic Rats. Malays J Med Sci 17 (2010): 3-11.
- Mir S, Fayaz A, Darzi M, et al. The Influence of Glimepiride on the Biochemical and Histomorphological Features of Streptozotocin - Induced Diabetic Rabbits Pakistan Journal of Nutrition 7 (2008): 404-407.
- Cefalu WT, Leiter LA, Yoon K-H, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet 382 (2013): 941-950.


 Impact Factor: * 3.3
Impact Factor: * 3.3 Acceptance Rate: 74.39%
Acceptance Rate: 74.39%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 