Current and future directions in the prevention and treatment of Malaria
Nardeen Perko, Tewodros Kebede, Shaker A. Mousa*
The Pharmaceutical Research Institute, Albany College of Pharmacy and Health Sciences, 1 Discovery Drive, Rensselaer, NY, 12144 USA
*Corresponding author: Shaker A. Mousa, The Pharmaceutical Research Institute, Albany College of Pharmacy and Health Sciences, 1 Discovery Drive, Rensselaer, NY, 12144 USA.
Received: 01 July 2022; Accepted: 07 July 2022; Published: 02 September 2022
Article Information
Citation: Nardeen Perko, Tewodros Kebede, Shaker A. Mousa. Current and future directions in the prevention and treatment of Malaria. Journal of Pharmacy and Pharmacology Research 6 (2022): 131-138
View / Download Pdf Share at FacebookAbstract
Malaria is an infectious disease caused by the Plasmodium parasite, which is carried by the Anopheles mosquito. Among the four Plasmodium species that can infect humans, Plasmodium vivax and Plasmodium falciparum are the most common. A person can contract the disease after being bitten by an infected insect and can invade and destroy human cells on a deadly rampage through a development cycle. Millions of people die every year from malaria, most of whom live in undeveloped countries in Africa and Asia, where the disease is endemic. Preventative efforts such as vector control, insecticide-treated mosquito nets, seasonal malaria chemoprevention, and intermittent preventive treatment for infants and pregnant women are an important place to start reducing malaria transmission. Through the World Health Organization and many researchers’ efforts, vaccines such as RTS,S/AS01 and PfSPZ exist among many others currently in clinical trials. Also, treatment guidelines have not changed for a long time, and many of the anti-malarial drugs currently have had cases of resistance. Malaria and COVID-19 can have similar presentation and common symptoms including fever, breathing difficulties and acute onset headache, which may lead to misdiagnosis of malaria for COVID-19 and vice versa. This review highlights the development of prevention and treatment strategies that aim to reduce malaria cases worldwide and put into perspective future clinical trials.
Keywords
<p>malaria, prevention, treatment, vaccines, plasmodium, resistance</p>
Article Details
1. Introduction
Malaria is one of the deadliest infectious diseases in the world. In 2018, there were approximately 228 million cases worldwide, 85% of which occurred in 19 countries. In the last decade, cases declined from 585,000 to 405,000, with the highest decline in the WHO African region. Approximately 67% of deaths that occur worldwide are of children that are five years old or younger. The countries with the highest burden include Africa, Ghana, and Nigeria, which experienced an increased number of cases in 2018 compared with 2017. On the other hand, over the same period, countries as India and Uganda experienced a decrease in the number of cases. Table 1 shows the countries that have been free of indigenous cases for three consecutive years [1].
Table 1: List of countries that have eliminated malaria from 2000 and onward.
These countries have had zero indigenous malaria cases for three years consecutively.
|
Year |
Countries |
|
2000 |
Egypt |
|
United Arab Emirates |
|
|
2004 |
Kazakhstan |
|
2007 |
Morocco |
|
Syrian Arab Republic |
|
|
Turkmenistan |
|
|
2008 |
Armenia |
|
2011 |
Iraq |
|
2012 |
Georgia |
|
Turkey |
|
|
2013 |
Argentina |
|
Kyrgyzstan |
|
|
Oman |
|
|
Uzbekistan |
|
|
2014 |
Paraguay |
|
2015 |
Azerbaijan |
|
Sri Lanka |
|
|
2016 |
Algeria |
|
2017 |
Tajikistan |
WHO awards a certification of elimination for countries that have achieved complete malaria transmission cessation and prevention.
Malaria has a significantly negative impact on pregnant women as it poses consequences of anemia and results in newborns delivered with low birth-weight [1]. If the WHO does not keep pushing efforts forward and motivate through its mission, global incidences could make their way to 1 billion cases based on the current trajectory. Therefore, malaria is a challenging infectious disease, particularly in Africa, primarily due to its transmission mode and the challenges faced with its prevention and treatment.
Almost all malaria cases are transmitted exclusively by mosquitoes of the genus Anopheles. It is found and reproduces in warm, humid climates where water pools provide a perfect breeding ground [2]. The disease is caused by a small parasite called Plasmodium that lives in the mosquito and infects and destroys human red blood cells [2, 3]. Four parasite species in the genus Plasmodium can infect human beings: Plasmodium vivax, Plasmodium malaria, Plasmodium ovale, and Plasmodium falciparum [3]. While P. vivax and P. falciparum are each responsible for about 40% of malaria infections, P. falciparum is far more deadly and causes the vast majority of malaria-related deaths [2, 3]. Plasmodium falciparum is prevalent in nations in Sub-Saharan Africa, where active cases exist. Thus, the genus Anopheles is known for its aid in malaria transmission fueled by the plasmodium parasite, which has a rapid infection onset.
The parasite goes through an intricate development cycle within the mosquito. Depending on the parasite species and environmental conditions, the cycle may last anywhere from eight days to over two weeks [2]. While an infected mosquito is feeding, parasite sporozoites residing in its salivary glands release onto the human dermis, where they may remain for several hours before migrating into the bloodstream [2-4]. Once it enters, the sporozoites travel to the liver, invade hepatocytes and begin the liver-stage of development [2-4]. Over 5 to 16 days, a single parasite replicates into 10,000 to 40,000 merozoites [2, 3]. Once this stage is complete, the infected hepatocytes rupture and the merozoites release into the bloodstream where they infect red blood cells and begin the asexual erythrocyte development stage [2, 3]. (Some dormant parasites, called hypnozoites, can remain in the hepatocytes where they can potentially cause a relapse in the future [3].) The parasites then multiply as they infect more and more red blood cells [2, 3]. The infected erythrocytes then rupture, releasing pathogenic merozoites, which trigger the onset of fever and chills, the most common clinical symptoms associated with malaria [2]. In this stage, P. falciparum becomes more virulent than other species of the parasite, as red blood cells infected with P. falciparum tend to congregate in the capillaries of many organs, including the brain [2, 3]. During this stage of asexual replication, a given number of parasites will then begin sexual reproduction by differentiating into female and male gametocytes, which are non-pathogenic [2, 3]. If a mosquito bites a person during this stage, the gametocytes can enter the uninfected insect where they reproduce and spawn a new generation of sporozoites [2, 3]. Therefore, the parasites grow from sporozoites to gametocytes in approximately a couple of weeks, which warrant prevention and treatment modalities.
According to the 2019 malaria world report, the WHO has a strategic plan to address and combat malaria’s growing concern. It has set an ambitious target backed with a strategic and thorough plan to reduce 90% of malaria mortality rates and case incidences globally compared to 2015 by 2030. Additionally, in the same period, the WHO aims to eliminate malaria from 35 countries to which it was transmitted. The last fourth core target is to prevent the re-establishment of malaria that is disease-free. These four core global strategies set the tone for the efforts to push forward preventative measures and treatments. There was an increase in funding from the US private sector in 2018 to aim the drug research and development movement in developing a single-exposure radical cure [1]. This review highlights prevention and treatment strategies and the advancements that have happened over the past ten years and what future directions await.
2. Prevention
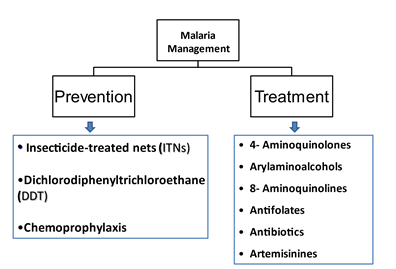
When combating infectious disease, an essential initial aspect that lays the foundation for disease control is prevention. There are a few preventative strategies in place that aim at lowering the incidences of malaria. Earlier prevention strategies, such as vector control, are still recommended but cannot be the primary prevention method. Table 2 compiles the prevention strategies outlined by WHO. See figure 1 for current prevention and treatment strategies.
Table 2: Summary of the World Health Organization’s prevention strategies in its 2019 world malaria report
|
Prevention Strategy |
Description/Standard of Use |
|
Vector Control |
Reducing the odds of mosquitoes biting humans |
|
ITNs |
Bed nets treated with insecticides to kill mosquitos as they serve as a form of personal protection. |
|
Indoor Residual Sprays (IRS) |
Residual insecticide sprayed inside housing structures |
|
Seasonal Malaria Chemoprevention (SMC) |
Administered to children 5 to 59 months of age in areas whose malaria transmission rates are seasonally high in the Sahel subregion of Africa. |
|
Intermittent Preventative Treatment in Infants (IPTi) |
In areas of moderate to high malaria transmission, WHO recommends IPTi to infants which often corresponds to their routine vaccination schedule. |
|
Intermittent Preventative Treatment in Pregnancy (IPTp) |
In areas of moderate to high malaria transmission, WHO recommends IPTp to pregnant women |
Commodities to help with prevention are being delivered globally and have been for some time. For example, between 2016 and 2018, over 600 million insecticide-treated mosquito nets (ITNs) reached sub-Saharan Africa, India, Uganda, Ethiopia, and the United Republic of Tanzania. In terms of residual spraying, it used to be that indoor residual spraying showed some protection when used to spray inside homes with insecticides; but their protection declined to only about 2% in 2018. However, some studies have found ways to combine their use with other preventative strategies such as ITNs to maximize protection [1, 5]{World Health Organization, #172}. Also, in areas where transmission is categorized to be moderate or high risk, the WHO recommends that they receive, mostly women and children, intermittent preventative therapy (IPTp/IPTi). Furthermore, one of the most significant advances in malaria prevention is vaccinations such as RTS, S/AS01 that came about in 2015, and PfSPZ that came about in 2016, as discussed later. Having these preventative strategies backed with strong clinical evidence suggests that there is hope in combating malaria.
2.1 Insecticide-treated mosquito nets
In malaria-infected regions, ITNs are an essential public health service that aids in vector control. ITNs appropriately assembled provide almost complete protection from mosquito bites [6, 7]. The insecticides commonly used include pyrethroids, carbamates, and occasionally organochlorine dichlorodiphenyltrichloroethane (DDT) [1]. As most infected mosquitoes feed indoors and at night, sleeping under an ITN can drastically reduce contracting it [8]. In 2018, compared with 2010, 24% more people had access to ITNs [1]. However, it is essential to overcome the limitations of ITNs. The nets usually do not work well in low and unstable transmission areas where mosquitoes bite in the early evening and morning, and some insects have changed their feeding habits in response to the ITNs [8]. Besides, it is vital to educate oneself on the proper use and maintenance of ITNs; the nets have the potential to be a vital tool in the fight against malaria, but their efficacy diminishes by incorrect use [9, 10]. Given this prevention strategy that has been around for some time, their metabolic resistance mechanisms have been detected through molecular assays, thus illustrating limited overall efficacy. Fortunately, researchers are investigating new insecticide alternatives such as neonicotinoid and pyrrole at different dosages determine if they can be incorporated in ITNs [1]. Overall, the use of ITNs effectively can drastically help the odds of controlling the disease, especially for the overnight protection it provides.
2.2 Intermittent Preventative Treatment for Infants
Following a westernized model, infants living in areas with malaria transmission classified as moderate to high risk receive routine immunization. It is scheduled intermittently at three different intervals of 10 weeks, 14 weeks, and nine months but varies depending on the geographic location. Infants will receive this regardless of being infected with the parasite or not to reduce their risk of contracting it. The coverage data is not yet reported for IPTi because, as of 2018, no country has officially adopted it. One of the first countries that began to upscale its adoption of IPTi in 2019 is Sierra Leone [1]. Table 3 lists the combination therapy options paired with IPTi for infants. Pairing these intermittent preventative treatments with their immunization schedules will make it an easy and quick additional vital to ensuring their protection.
Table 3: Summary of the regimens used for infants and their efficacy in the prevention of malaria
|
Regimen |
Standard of Use/Efficacy |
|
IPTi + SP |
Dual action against anemia and clinical malaria |
|
SMC + AQ + SP |
Specifically, for children aged 3 to 59 months, this combination has 75% efficacy against clinical attacks and severe malaria averting thousands of deaths and many more cases. |
2.3 Intermittent Preventative Treatment for Pregnant Women
More than 30 million pregnant women in Africa become exposed to malaria. Therefore, a four-dose scheduled regimen of IPTp1, IPTp2, IPTp3, and IPTp4, each given one month apart, has been adopted by thirty-nine African countries. The data from 2018 currently shows that less and less pregnant women follow through with all four doses. The coverage rates for the first three doses were 60%, 49%, and 31%, respectively [1]. Therefore, follow through for the entire four-dose series is relatively low and is an area for potential adherence education. However, the data from 2018 show better adherence than in 2017 by at least 10%.
Further data shows that pairing IPTp with at least two low doses of sulphadoxine-pyrimethamine (SP) reduces the concerns associated with contracting malaria, including maternal anemia, low birth weight, and perinatal mortality. IPTp gets provided during antenatal care visits following the first trimester. Therefore, it is imperative to malaria transmission control to administer these intermittent preventative therapies to pregnant women.
2.4 Vaccinations
In July of 2015, the first vaccine for malaria, Mosquirix, hit the market. It is a recombinant protein-based vaccine, also referred to as RTS,S/AS01. It was an instrumental discovery as it is the world’s first licensed vaccine for malaria, the first of its kind to be used against a parasitic disease [1]. The vaccine’s efficacy as per conducted clinical trials is listed in the table below [11, 12]. Vaccine efficacy does wane over time. A study conducted a 7-year follow-up and found that its efficacy was 4.4% [13]. Table 4 shows some of the efficacy results associated with vaccine studies.
Table 4: Summary of RTS,S/AS01 efficacy results.
|
Author |
Months after first dose of vaccine |
Incidences of first episode (episodes per person-year) |
Average Efficacy (%) |
|
Penny et al. |
14 |
0.32 |
45 |
|
Agnandji et al. |
14 |
0.31 |
30 |
The side effects that accompany vaccines sometimes occur immediately after the vaccine and some in a few days. According to Ofori-Anyinam et al., in RTS,S/AS01, patients experienced generalized convulsive seizures. These febrile convulsions’ incidences occurred within 7-days of vaccine administration, and its likelihood increased with each subsequent dose administration. Furthermore, there were increased cases of meningitis and cerebral malaria cases. However, this study had analysis limitations as these effects may be due to other vaccines that the patients received simultaneously with the RTS,S/AS01 [14]. Therefore, Mosquirix brought a vaccine to malaria where none existed before. Even though efficacy results have not shown a high efficacy level, they are a step forward in the right direction. At the same time, researchers studied another vaccine for its efficacy and safety profile in adults against Plasmodium falciparum, the Plasmodium falciparum (Pf) sporozoite (SPZ) or PfSPZ vaccine. It is a radiation-attenuated malaria vaccine that, when used against the same strain as in the vaccine, can result in 100% protection against that strain for three weeks after five IV doses, as studies have shown [15, 16]. Table 5 outlines the data for the PfSPZ vaccine. Researchers use controlled human malaria infection (CHMI) to test the vaccine’s efficacy to examine its physiological and immunological response to malaria parasites. PfSPZ is safe, well-tolerated, and easy to administer.
Table 5: Summary of the percentage of protection achieved and thus the efficacy of PfSPZ [15, 17-19]
|
Author |
# Doses |
PfSPZ Dose |
Type of CHMI |
% Protection against CHMI after 3 weeks |
|
Epstein et al. |
5 |
2.7 × 105 |
Heterologous |
92.33 |
|
3 |
4.5 × 105 |
Homologous |
86.7 |
|
|
Lyke et al. |
3 |
9.0 × 105 |
Homologous |
64 |
|
Olotu el al. |
5 |
2.7 × 105 |
Heterologous |
70 |
|
Bastiaens et al. |
3 |
7.5 × 104 |
Heterologous |
60 |
The percentage of efficacy obtained with PfSPZ warrants further clinical trials to study different doses to achieve greater efficacy against heterologous CHMI. Therefore, the safety and efficacy profile of PfSPZ shows great promise in providing prevention against Plasmodium falciparum and other potential strains.
3. Testing and Diagnosis
The diagnosis of malaria is a challenge in countries with the highest number of cases. The challenge presents itself due to the nature of symptomology and their classification. For example, it is common for patients to present with a fever. Before diagnostic testing was made available, it led physicians to prescribe antimalarial drugs unnecessarily when the patient’s fever could have otherwise been due to other febrile illnesses [1]. The lack of hindrance in prescribing antimalarials resulted in resistance to medications used in the early treatment of malaria years ago, which is a problem we are combating today. Other symptoms associated with malaria are minor and include chills, sweats, and headache. As a result, medical personnel that grew accustomed to seeing thousands of malaria cases might skip the testing and immediately administer an antimalarial drug where further testing could have indicated a different diagnosis. The goal is to have medical personnel perform a test right after identifying symptomology to ensure the correct action course. This reflex of testing upon specific symptomology has risen approximately 50% from 2010 [1]. Therefore, malaria diagnosis through parasitological testing, rapid diagnostic tests, or microscopy addresses the challenge of malaria diagnosis with its general symptomology profile.
3.1 Malaria Rapid Diagnostic Tests
In the case that prevention was not sufficient, it is imperative to have robust diagnosis tools in order to track malaria cases quickly and accurately. The sooner a diagnosis is made, the sooner a severe malaria case is prevented, the less likely it is for individuals to die, and transmission risk decreases. Parasite-based or malaria rapid diagnostic tests (mRDTs) are available, and most detect P. falciparum only. It is an easy method for most clinics to utilize and is more cost-effective than other methods such as microscopy [20]. In 2018, 47% more patients suspected of having malaria obtained a test than 2010 [1]. A study composed in 2017 found many concerns that exist around mRDTs, some of which include physicians not prescribing ACTs for all positive diagnostic results or physicians prescribing ACTs for negative diagnostic results. However, as an overall consensus, the use of mRDTs resulted in lower ACT prescribing [21]. These findings are concerning as inappropriate prescribing of anti-malarial and anti-microbial agents can increase resistance to drug therapies globally. These findings also highlight the need for increased formal training for personnel who conduct these tests and, in turn, prescribe antimalarials. Therefore, mRDTs are one of the diagnostic tools distributed to help faster and more efficient diagnosing.
Besides ensuring the adherence to the correct protocol upon mRDTs test performance, it is crucial to ensure enough market access. A randomized trial of 310 dispensers from Tanzania illustrated that having mRDTs available at drug dispensing sites can significantly increase malaria testing and diagnosis [22]. This one-year pilot demonstrates the benefit of having increased market access. If paired with proper and complete training, it can increase the correct course of action as it corresponds to the test results. In providing access to patients, they may be more likely to get tested, diagnosed, and receive treatment promptly.
There are, however, limitations with mRDTs. HRP2, a gene expressed on parasites such as P. falciparum, is detected by mRDTs to determine a diagnosis. However, some parasites are no longer expressing some of the genes that the mRDTs were made to detect but would be positive with microscopy. As early as 2010, microscopy detected positive blood samples not detected by the mRDTs due to the absence of pfhrp2/3 [1]. In this case, microscopy would be a more accurate test. However, physicians may not prescribe it unless the patient presents with symptoms that indicate malaria while obtaining a negative mRDT test.
4. Current Treatment Guidelines in WHO Regions:
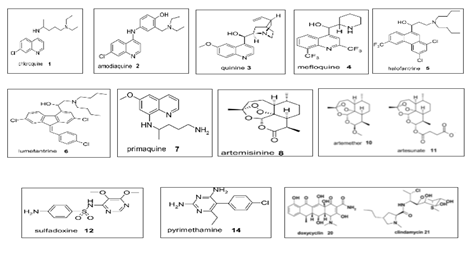
First-line treatments such as AL, AS-AQ, AS-MQ, and DHA-PPQ remain effective with only a 10% failure rate in some WHO regions. However, treatment failure rates tend to be higher in countries like India, ranging from 0% to 24%. Surprisingly, Thailand, which uses DHA-PPQ as its first-line treatment, is experiencing a failure rate of 92.9%, which warranted their first-line treatment to change to AS-PY. Table 6 outlines the diagnosis criteria and medications used by WHO regions for each category [1]. The different drug classes are listed in Table 6 and figure 2 showing the chemical structure of the different classes.
Table 6: Summary of Treatments Used for each species and diagnosis severity
|
Species |
Diagnosis |
Treatments Used |
|
P. falciparum |
Uncomplicated Unconfirmed |
AL, AS + AQ, DHA-PPQ |
|
CQ, AS + SP, AL + PQ |
||
|
Uncomplicated Confirmed |
AL, AS + AQ, DHA-PPQ |
|
|
QN + CL, QN + D, CQ + PQ |
||
|
AL + PQ, AS + MQ |
||
|
AS + MQ + PQ, AS + SP + PQ |
||
|
AS + SP, DHA-PPQ + PQ |
||
|
AS + MQ, PYR |
||
|
Severe |
AS, QN, AM, QN + AS |
|
|
AS + D, QN + D, QN + T |
||
|
QN + CL, AL, AS + MQ |
||
|
AS + AL, PYR, AS + AL + PQ |
||
|
P. vivax |
PQ, AS + AQ + PQ, CQ |
|
|
CQ + PQ, AL, AL + PQ |
||
|
AS + PQ + AQ, DHA-PPQ + PQ |
||
|
AS + MQ + PQ, PQ + PPQ |
||
|
ACTs + PQ, PYR |
ACT: artemisinin-based combination therapy; AL: artemether-lumefantrine; AM: artemether; AQ: amodiaquine; ART: artemisinin; AS: artesunate; AT: atovaquone; CL: clindamycline; CQ: chloroquine; D: doxycycline; DHA: dihydroartemisinin; MQ: mefloquine; NQ: naphroquine; PG: proguanil; PPQ: piperaquine; PQ: primaquine; PYR: pyronaridine; QN: quinine; SP: sulfadoxine-pyrimethamine; T: tetracycline [25-34].
4.1 Drugs introduced before 2000s
The drug classes quinine, chloroquine, proguanil, sulfadoxine-pyrimethamine, mefloquine, and atovaquone were introduced in 1632, 1945, 1948, 1967, 1977, and 1996, respectively. They have been around for quite some time and still being used today despite the resistance cases that have emerged, as seen in Table 6. Chloroquine, the first synthetically developed anti-malarial drug, proved to be an almost magical cure for more than 30 years until the emergence and spread of chloroquine-resistant parasites. However, a study sought to determine whether doubling the chloroquine dose would help combat the resistance. They divided this daily dose into two doses, administrated every day for three days. They were able to show that it was just as efficacious as artemisinin-based combination therapy, so there may still be a place for it in treatment today with slight regimen modification [23]. However, many of the drugs currently used for malaria have been on the market for years. They are not as effective as they need to effectively treat malaria patients.
5. Current Clinical Trials
We must have more advancement in the prevention and treatment strategies for malaria. A novel drug, SJ733, is an orally bioavailable inhibitor of P. falciparum ATP4. This is the first time this drug has been studied in humans. It recently completed a phase 1a/b trial where pharmacokinetics, safety, tolerability, and antimalarial activity were investigated in humans. Fasted participants were infected with the blood-stage P. falciparum and then treated with SJ733. They gave them two doses, 150 mg followed by 600 mg with only one confirmed side effect, mild bilateral foot paresthesia. The trial results were favorable and have a future of being incorporated in antimalarial therapy [24]. There are many more advancements coming to malaria, and Table 7 identifies the current clinical trials and the interventions studied to further prevention and treatment strategies. These trials hold great promise in bringing in new interventions for vaccines, prophylaxis, and treatment. We must move forward in the fight against malaria so that we may reduce transmission cases.
Table 7: Summary of current clinical trials seeking to enhance prevention and treatment of malaria
|
Area of Focus |
Intervention |
Subjects |
Primary Outcome |
|
Vaccine |
SB257049 |
5 to 17 months old |
P. falciparum asexual parasitemia > 5000 parasites/microliters and presence of fever ≥ 37.5 ? |
|
Prophylaxis |
Artemether-lumefantrine with multivitamins |
16 to 65 years old |
28-day PCR positivity rate of Plasmodium infections of any species and proportion of participants with confirmed clinical malaria of any species reported between day 0 and 28 |
|
Prevention and Treatment |
Meplazumab |
18 to 55 years old |
Presence of anti-bodies in 71 ± 3 days and presence of adverse events |
|
Treatment |
KAF156 |
6 months to < 18 years old |
% of participants with PCR-corrected and uncorrected adequate clinical and parasitological response (ACPR) at Day 29 |
|
Vaccine |
Pfs230D1M-EPA/AS01 |
1 to 99 years |
Safety and reactogenicity of vaccine administration |
|
Treatment |
KAF156 + Lumefantrine Solid Dispersion |
2 years and older |
PCR-corrected adequate clinical and parasitological response (ACPR) |
|
Treatment |
New Artemether-lumefantrine dispersible tablet in infants and neonates |
Infants and Neonates < 5 kg |
ART Cmax concentrations at 1 and 2 hours after the first dose |
|
Prophylaxis and Malnutrition |
SMC + Nutrients Supplementation |
6 to 59 months |
The relative risk of the incidence of clinical malaria, mid-upper arm circumference gain, weight gain and prevalence of anemia |
|
Prevention in Pregnant Women |
Azithromycin and Sulphadoxine-pyrimethamine |
18 years and older |
Number of pregnant women with maternal peripheral blood parasitemia during pregnancy among pregnant women who use sulfadoxine pyrimethamine and azithromycin for malaria prevention in pregnancy, |
|
Vaccine |
ChAd63/ MVA PvDBP |
18 to 45 years |
Efficacy of the two vaccines, assessed by a parasite multiplication rate in vaccinated subjects |
4.1 Common links between Malaria and Covid 19
Among the common links reported include the followings: (a) the low prevalence of COVID-19 in malaria-endemic countries, (b) the similarity between malaria and COVID-19 symptoms, (c) the role of ACE2 in malaria and COVID-19, (d) the roles of interferons and the neutralizing antibodies in malaria and COVID-19, and (e) the use of hydroxychloroquine and chloroquine in COVID 19 [25].
5. Conclusion
Malaria takes millions of lives every year but through the efforts of the World Health Organization, there are strategic plans in place to decrease malaria transmission cases. In the last decade, diagnostics tests have been developed that are more accurate and provide an avenue of diagnosis for physicians, which aids in the administration of treatments correctly and only when indicated. In addition, funding for research has increased and many clinical trials are underway to bring new vaccines, preventions, and treatments.
Ethics approval and consent to participate:
N/A
Consent for publication:
N/A
Availability of data and material:
N/A
Competing interests:
No competing interest to be declared
Funding:
No funding
Authors' contributions:
Nardeen Perko updated the latest literatures and write up, Tewodros Kebede put together initial draft, and Shaker A. Mousa supervised and guided the content as well as the revisions and submission of the article.
Acknowledgements:
Special thanks to the Pharmaceutical Research Institute in supporting the development of this timely review.
References
- World Health Organization, World malaria report. World Health Organization: Geneva, Switzerland. P, volumes.
- Greenwood BM, et al. Malaria: progress, perils, and prospects for eradication. J Clin Invest 118 (2008): 1266-76.
- Wellems TE, Hayton K, Fairhurst RM. The impact of malaria parasitism: from corpuscles to communities. J Clin Invest 119 (2009): 2496-505.
- Ejigiri I and Sinnis P. Plasmodium sporozoite-host interactions from the dermis to the hepatocyte. Curr Opin Microbiol 12 (2009): 401-7.
- West PA, et al. Enhanced protection against malaria by indoor residual spraying in addition to insecticide treated nets: is it dependent on transmission intensity or net usage? PLoS One 10 (2015): e0115661.
- Steketee RW and Campbell CC. Impact of national malaria control scale-up programmes in Africa: magnitude and attribution of effects. Malar J 9 (2010): 299.
- Koudou BG, et al. The use of insecticide-treated nets for reducing malaria morbidity among children aged 6-59 months, in an area of high malaria transmission in central Cote d'Ivoire. Parasit Vectors 3 (2010): 91.
- Govella NJ, Okumu FO and Killeen GF. Insecticide-treated nets can reduce malaria transmission by mosquitoes which feed outdoors. Am J Trop Med Hyg 82 (2010): 415-9.
- Korenromp EL, et al. Monitoring mosquito net coverage for malaria control in Africa: possession vs. use by children under 5 years. Trop Med Int Health 8 (2003): 693-703.
- Afolabi BM, et al. Household possession, use and non-use of treated or untreated mosquito nets in two ecologically diverse regions of Nigeria--Niger Delta and Sahel Savannah. Malar J 8 (2009): 30.
- Rts SCTP, et al. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med 367 (2012): 2284-95.
- Penny MA, Pemberton-Ross P and Smith TA. The time-course of protection of the RTS,S vaccine against malaria infections and clinical disease. Malar J 14 (2015): 437.
- Olotu A, et al. Seven-Year Efficacy of RTS,S/AS01 Malaria Vaccine among Young African Children. N Engl J Med 374 (2016): 2519-29.
- Guerra Mendoza Y, et al. Safety profile of the RTS,S/AS01 malaria vaccine in infants and children: additional data from a phase III randomized controlled trial in sub-Saharan Africa. Hum Vaccin Immunother 15 (2019): 2386-2398.
- Epstein JE, et al. Protection against Plasmodium falciparum malaria by PfSPZ Vaccine. JCI Insight 2 (2017): e89154.
- Jongo SA, et al. Safety, Immunogenicity, and Protective Efficacy against Controlled Human Malaria Infection of Plasmodium falciparum Sporozoite Vaccine in Tanzanian Adults. Am J Trop Med Hyg 99 (2018): 338-349.
- Lyke KE, et al. Attenuated PfSPZ Vaccine induces strain-transcending T cells and durable protection against heterologous controlled human malaria infection. Proc Natl Acad Sci U S A, 114 (2017): 2711-2716.
- Olotu A, et al. Advancing Global Health through Development and Clinical Trials Partnerships: A Randomized, Placebo-Controlled, Double-Blind Assessment of Safety, Tolerability, and Immunogenicity of PfSPZ Vaccine for Malaria in Healthy Equatoguinean Men. Am J Trop Med Hyg 98 (2018): 308-318.
- Bastiaens GJH, et al. Safety, Immunogenicity, and Protective Efficacy of Intradermal Immunization with Aseptic, Purified, Cryopreserved Plasmodium falciparum Sporozoites in Volunteers Under Chloroquine Prophylaxis: A Randomized Controlled Trial. Am J Trop Med Hyg 94 (2016): 663-673.
- Hansen KS, et al. Cost-effectiveness of malaria diagnosis using rapid diagnostic tests compared to microscopy or clinical symptoms alone in Afghanistan. Malar J 14 (2015): 217.
- Bruxvoort KJ, et al. The Impact of Introducing Malaria Rapid Diagnostic Tests on Fever Case Management: A Synthesis of Ten Studies from the ACT Consortium. Am J Trop Med Hyg 97 (2017): 1170-1179.
- Maloney K, et al. Expanding access to parasite-based malaria diagnosis through retail drug shops in Tanzania: evidence from a randomized trial and implications for treatment. Malar J 16 (2017): 6.
- Ursing J, et al. High-Dose Chloroquine for Treatment of Chloroquine-Resistant Plasmodium falciparum Malaria. J Infect Dis 213 (2016): 1315-21.
- Gaur AH, et al. Safety, tolerability, pharmacokinetics, and antimalarial efficacy of a novel Plasmodium falciparum ATP4 inhibitor SJ733: a first-in-human and induced blood-stage malaria phase 1a/b trial. Lancet Infect Dis 20 (2020): 964-975.
- Hussein MIH, et al. Malaria and COVID-19. Unmasking their ties. Malar J 19 (2020): 1-10.


 Impact Factor: * 3.3
Impact Factor: * 3.3 Acceptance Rate: 74.39%
Acceptance Rate: 74.39%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 