Drug Use Evaluation (DUE) of Ceftriaxone in Mubende Regional Referral Hospital, Uganda: A Cross-Sectional Survey
Leonard Manirakiza1*, Victoria Nambasa1, Stella Nanyonga2, Allan Serwanga1, Matovu Alphonsus3, Nankola Denis3, Helen Ndagije1
1National Pharmacovigilance Center, National Drug Authority, Uganda
2Department of clinical epidemiology, Makerere University, Kampala, Uganda
3Mubende Regional Referral Hospital, Uganda
*Corresponding Author: Manirakiza Leonard, National Pharmacovigilance Center, National Drug Authority, Uganda
Received: 11 September 2019; Accepted: 27 September 2019; Published: 30 September 2019
Article Information
Citation: Leonard Manirakiza, Victoria Nambasa, Stella Nanyonga, Allan Serwanga, Matovu Alphonsus, Nankola Denis, Helen Ndagije. Drug Use Evaluation (DUE) of Ceftriaxone in Mubende Regional Referral Hospital, Uganda: A Cross-Sectional Survey. J Pharm Pharmacol Res 3 (2019): 105-115.
View / Download Pdf Share at FacebookAbstract
Introduction: Ceftriaxone is a third generation cephalosporin recommended as first line treatment option for a number of diseases in Uganda. However, the National Drug Authority has in the recent past received complaints of suspected treatment failure from clinicians who use different brands of ceftriaxone in Uganda. The main aim of the study was to document the treatment outcome following use of ceftriaxone and evaluating the use of ceftriaxone against the current treatment guidelines in Uganda.
Methods: A descriptive observational, non-intervention study design to document treatment outcomes after administration of Ceftriaxone injection in hospitalized patients was undertaken in Mubende. A total of 100 hospitalized patients treated with ceftriaxone were enrolled.
Results: Overall, Ceftriaxone was used to treat pneumonia in the paediatric ward, presumptive therapy for infection following caesarean section (n=47) and PID in the post-natal ward, while on surgical and medical wards, Ceftriaxone was used to manage upper respiratory infection, bacterial infections and meningitis. There were no Adverse Events reported to have occurred during treatment with ceftriaxone. Of the patients treated with ceftriaxone 18% completed their doses and had regular administration. Majority 60% of the patients had irregular administration with completed doses and 22% did not complete their doses.
Conclusion: There is low treatment outcome during use of Ceftriaxone and the empirically treatment is highly prevalent in the hospital. There is high number of inappropriate drug administration, in which patients usually miss doses or do not complete as prescribed. This practice has an effect of affecting the patient outcomes and aggravating antimicrobial resistance. Ch
Keywords
<p>Ceftriaxone, Drug use, Evaluation, Antibiotics</p>
Article Details
1. Introduction
Ceftriaxone is recommended as first line treatment option in Uganda and is commonly used to treat different types of bacterial infections [1]. It is one of the widely used antibiotic in many health facilities in Uganda, however, some reports of its irrational use have been reported [2]. Irrational use of antibiotics creates a threat of decreased susceptibility and the emergence of multi-drug resistant pathogens, treatment failure, unwanted adverse effects, and increased costs to the health care system [3, 4]. The study conducted in Uganda revealed worsening trends of resistance and diminishing effectiveness of antibiotics in Uganda [5].
Studies have revealed worsening trends of resistance and diminishing effectiveness of antibiotics in Uganda and the associated factors classified as healthcare provider factors, patient factors or drug related factors. Healthcare provider related factors include lack of information, excessive and unnecessary antibiotic prescribing, incorrect dosage or route of administration and antibiotic prescribing for non-bacterial infections. Patient related factors include failure to complete doses and self-medication whereas drug related factors include ineffective drugs [6-8]. In Uganda, it has been reported that clinician practices that increase the threat of irrational use of antibiotics included selling of antibiotics over-the-counter without a prescription which was associated with under-dosing and prescription of antibiotics to patients with no clinical indications for antibiotic therapy [9, 10]. Due to the above factors, NDA conducted a DUE to establish the effectiveness of Ceftriaxone and to identify any irrational use, which could be affecting the realisation of the expected treatment outcomes when using Ceftriaxone in Mubende Regional Referral Hospital.
2. Materials and Methods
2.1 Study design
This was a descriptive observational and non-intervention study designed to document treatment outcomes after administration of Ceftriaxone injection in hospitalized patients managed with ceftriaxone in Mubende Regional Referral Hospital.
2.2 Setting
Following signed informed consent, all patients who were treated with ceftriaxone were enrolled. The data collection tool was pre tested at Mubende hospital (see Additional file #1).Two data collection assistants were trained intently for one week on data collection prior to the data collection process by a member of the NPC. All activities were conducted through the Medicines and Therapeutics Committees (MTC) of Mubende hospital. The MTC was brought on board because it is the mandate of the MTC to conduct DUE in the hospital.
2.3 Participants
Data was collected from 100 hospitalized patients admitted on the medical ward and treated with ceftriaxone during the study period of 45 days. All adult patients above 18 years admitted to the medical ward during the study period with ability and willingness to participate by giving signed informed consent were considered eligible for this study.
2.4 Variables
Data on administered medicine (s) including the medicine brand, diluent used, indication, dose prescribed, and dose administered, duration of treatment, concomitant medications, treatment outcome as well as data on laboratory investigation obtained was collected. Where routine practice permitted, data on culture and sensitivity was collected.
2.5 Data sources
The medication records of all the patients in different wards of the hospital who received ceftriaxone.
2.6 Statistical methods
Statistical analysis was performed using Microsoft Excel. A detailed statistical analysis plan was generated within three months of study start. This plan was revised during the course of the study in order to take into account protocol amendments, and to address potential issues occurring during the study, that could affect data analysis. Data was summarized using frequencies and percentages.
3. Results
3.1 Description of study respondents
Majority of the patients involved in the study were females (n=76, 76%), with average weight of 53.2 kilograms and majority falling in the age bracket of below 40 years. Majority of patients enrolled were from post-natal unit (47%, n=47), followed by paediatric (20%, n=20), medical ward (17%, n=17) and surgical ward 16%, n=16) (Table 1).
3.2 Common indicators
The most common indications of Ceftriaxone were infections and infestations (57%) and respiratory, thoracic and mediastinal disorders (20%) (Table 2).
3.3 Drug characteristics and treatment outcome
Sterile water was the most used diluent (74%) to reconstitute ceftriaxone followed by normal saline 20 mls 5% and normal saline 10 mls 8%. Overall, half of the patients (53%, n=100) prescribed ceftriaxone responded to treatment and were discharged with having recovered from the diagnosed illness, whereas (35%) of patients required change of medication. Other outcomes included referral (4%), run away (4%), unknown outcome (3%) and one patient (1%) died (Table 3).
3.4 Occurrence of adverse events (AEs)
There were no Adverse Events reported to have occurred during treatment with ceftriaxone.
3.5 Dose adherence and completion
Of the patients treated with ceftriaxone 18% completed their doses and had regular administration. Majority 60% of the patients had irregular administration with completed doses and 22% did not complete their doses (Table 4).
3.6 Culture and sensitivity test
Culture and sensitivity test was not done in most of the patients (93%). Of the 7 (7%) cases from paediatric ward in which test was done, growth and resistance of organisms was not determined and therefore not reported.
3.7 Dosing and duration of ceftriaxone use
In 74 (74%) cases, ceftriaxone was dosed as 2 g/day. The result of this study revealed that among all the patients the prescribed frequency for ceftriaxone was OD (100%) that is once a day.The mean duration of ceftriaxone use was 3.9 days (ranging 1 day to 8 days), with the majority (88%) taking 2-7 days on Ceftriaxone treatment. The average duration of stay in the hospital was 7.1 days (ranging from 1 day to 27 days), with most of the patients (70%) staying for 1-7 days (Table 5).
3.8 Comorbidity condition of patients
Out of total 100 patients enrolled in the study 89 (89%) patients did not have any co-morbid condition whereas 6 (6%) had co-morbid condition. The most common co-morbidity were Sickle cell disease, Diabetes mellitus, hypertension, Asthma and Epilepsy (Table 6).
3.9 Adherence to Uganda clinical guidelines (UCG)
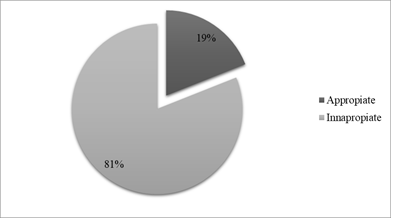
More than four-fifth of Ceftriaxone use (n=81, 81%) were reported to be inappropriately administered (Figure 1).
3.10 Reasons for inappropriateness
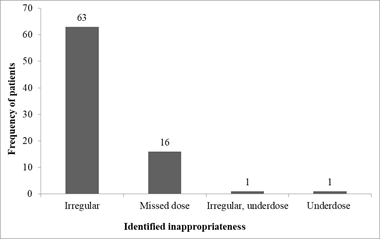
Majority of unjustified Ceftriaxone administration arose from irregular administration (63%) and missing dose (16%) (Figure 2).
3.11 Factors associated with inappropriate administration of ceftriaxone
Using bivariate logistic regression, factors that were associated with inappropriate use of ceftriaxone in the study population included patient age, days of hospital stay and weight of the patient. Having other variables controlled, days of hospital stay (aOR=5.7, 95% CI: 1.3-24.6, P=0.02) and weight of the patient (aOR=36.8, 95% CI: 3.7-366.7, P=0.002) remained to be significant in the multivariate logistic model (Table 7).
|
Variables |
Frequency (%) |
|
Demographic characteristics |
|
|
Sex |
|
|
Female |
76 (76%) |
|
male |
24 (24%) |
|
Age category |
|
|
0-20 years |
42 (42%) |
|
21-40 years |
43 (43%) |
|
Over 40 years |
15 (15%) |
|
Weight |
|
|
Average (±SEM) |
53.2 (± 2.4) |
|
Patient ward |
|
|
Medical |
17 (17%) |
|
Paediatric |
20 (20%) |
|
Post-natal |
47 (47%) |
|
Surgical |
16 (16%) |
Table 1: Social and demographic characteristics of respondents.
|
Indication |
Frequency (N=100) |
|
Infections and infestations |
57 |
|
Respiratory, thoracic and mediastinal disorders |
20 |
|
Reproductive system and breast disorders |
9 |
|
Nervous system disorders |
3 |
|
Gastrointestinal disorders |
2 |
|
Injury, poisoning and procedural complications |
2 |
|
Neoplasms benign, malignant and unspecified (incl cysts and polyps) |
2 |
|
Blood and lymphatic system disorders |
1 |
|
Metabolism and nutrition disorders |
1 |
|
Musculoskeletal and connective tissue disorders |
1 |
|
Vascular disorders |
1 |
|
No indication |
1 |
Table 2: Indications for Ceftriaxone use.
|
Variable |
Frequency (%) |
|
Dilution used |
|
|
Sterile water 20% |
72 (72%) |
|
Sterile water 10% |
15 (15%) |
|
Normal saline 10% |
8 (8%) |
|
Normal saline 20% |
5 (5%) |
|
Treatment outcome |
|
|
Discharged on recovery |
53 (53%) |
|
Changed medication |
35 (35%) |
|
Run away |
4 (4%) |
|
Referred |
4 (4%) |
|
Unknown |
3 (3%) |
|
Died |
1 (1%) |
Table 3: Drug characteristics and treatment outcome.
|
Dose completion status and action taken |
Frequency of patients |
|
Dose completed and administered irregularly |
60 |
|
Discharged |
56 |
|
Referred |
1 |
|
Run away |
1 |
|
Still on medication |
1 |
|
Transferred |
1 |
|
Dose completed and administered regularly |
18 |
|
Discharged |
17 |
|
Run away |
1 |
|
Dose incomplete |
22 |
|
Discharged |
16 |
|
Referred |
2 |
|
Died |
1 |
|
Run away |
2 |
|
Still on medication |
1 |
Table 4: Dose adherence and completion.
|
Variable |
Frequency (%) |
|
Dose |
|
|
<1 gm |
11 (11%) |
|
1 gm |
14 (14%) |
|
2 gm |
74 (74%) |
|
>2 gm |
1 (1%) |
|
Daily dose |
|
|
Once daily |
100 (100%) |
|
Duration (days) on Ceftriaxone |
|
|
1 |
7 (7%) |
|
2-7 |
88 (88%) |
|
8-14 |
5 (5%) |
|
Average (Min-Max) |
3.9 (1-8) |
|
Duration of stay in hospital |
|
|
1-7 days |
70 (70%) |
|
8-14 days |
24 (24%) |
|
Over 14 days |
6 (6%) |
|
Average (Min-Max) |
7.1 (2-27) |
Table 5: Dosing and duration of Ceftriaxone use.
|
Variable |
Frequency (%) |
|
Comorbidity presence |
|
|
Yes |
6 (6%) |
|
No |
89 (89%) |
|
Unknown |
5 (5%) |
|
Comorbidities identified |
|
|
Sickle cell disease |
2 (2%) |
|
Asthma |
1 (1%) |
|
Diabetes Mellitus |
1 (1%) |
|
Diabetes Mellitus and Hypertension |
1 (1%) |
|
Epilepsy |
1 (1%) |
Table 6: Comorbidity conditions of patients.
|
Variables |
Appropriateness |
Crude analysis |
Adjusted analysis |
|||||
|
Yes |
No |
cOR |
CI of OR |
P-Value |
aOR |
CI of OR |
P-Value |
|
|
Age (Years) |
||||||||
|
0-20 |
11 (11%) |
31 (31%) |
1.0 |
- |
- |
1.0 |
- |
- |
|
21-40 |
4 (4%) |
39 (39%) |
3.5 |
1.0-11.9 |
0.049 |
0.35 |
0.03-4.3 |
0.42 |
|
Over 40 |
4 (4%) |
11 (11%) |
0.98 |
0.26-3.71 |
0.97 |
0.15 |
0.01-1.8 |
0.13 |
|
Bed days |
||||||||
|
1-4 |
14 (14%) |
20 (20%) |
1.0 |
- |
- |
1.0 |
- |
- |
|
Over 4 |
4 (4%) |
58 (58%) |
10.2 |
2.99-34.4 |
0.0001 |
5.7 |
1.3-24.6 |
0.02 |
|
Weight (Kgs) |
||||||||
|
<50 |
14 (14%) |
12 (12%) |
1.0 |
- |
- |
1.0 |
||
|
>=50 |
4 (4%) |
65 (65%) |
18.9 |
5.3-67.5 |
0.0001 |
36.8 |
3.7-366.7 |
0.002 |
|
Sex |
||||||||
|
Female |
7 (7%) |
17 (17%) |
1.0 |
- |
- |
- |
- |
- |
|
Male |
12 (12%) |
64 (64%) |
2.2 |
0.75-6.43 |
0.15 |
- |
- |
- |
|
Ward |
||||||||
|
Pediatric |
10 (10%) |
10 (10%) |
1.0 |
- |
- |
- |
- |
- |
|
Medical |
4 (4%) |
13 (13%) |
3.3 |
0.78-13.48 |
0.104 |
- |
- |
- |
|
Surgical |
4 (4%) |
12 (12%) |
3.0 |
0.72-12.55 |
0.132 |
- |
- |
- |
|
Post natal |
1 (1%) |
46 (46%) |
6.0 |
2.27-14.4 |
0.142 |
- |
- |
- |
Table 7: Factors associated with inappropriate use of Ceftriaxone.
4. Discussion
Ceftriaxone is considered to have superior activity against Enterobacteriaceae but its activity is challenged by several factors, including resistance and inappropriate use [11]. The current study, therefore sought to determine therapeutic outcomes of ceftriaxone among the 100 randomly selected patients admitted at Mubende RRH. In this study, 53% of the patients successfully responded to Ceftriaxone treatment. Out of total 100 patients enrolled in the study, 89 (89%) patients did not have any co-morbid condition, whereas 6 (6%) had co-morbid condition. The most common co-morbidity were Sickle cell disease, Asthma, Diabetes mellitus, hypertension and Epilepsy. The finding of this study was similar other studies [12]. The results of this study showed that 93% of the patients were treated without culture and sensitivity testing. This finding was consistent with the findings of the related study [13, 14]. Mubende RRH had only 7% patients who conducted culture and sensitivity tests. These cultures are supported under a program for Acute Febrile Illnesses for paediatric patients study. The rate of performing culture and drug sensitivity test was very low compared to other related studies [15-18]. The difference may be due to the difference in sample sizes and high cost of culture and drug sensitivity which few patients could afford.
The duration of therapy was found to be high in the range of 2-7 days (88%). The mean duration of use was found to be 3.9 days. This is the appropriate duration of ceftriaxone use. The finding of this study is similar to the retrospective cross sectional study conducted in Ethiopia [19]. The mean duration of ceftriaxone therapy used was found to be 3.9 days in this study, which is less than what was reported by a similar study [20, 21]. This is an important factor because the number of days in which an antibiotic is used is associated with its resistance prevalence [20]. Overall, the prescription of ceftriaxone was given as per the current treatment guidelines. All patients received a once daily dosing of ceftriaxone, which is the recommended dosing by WHO as reported by a similar study [19]. The daily prescribed dose was found to be 2gm per day (74%) in most patients in the study, which is the correct daily prescribed dose for ceftriaxone as given by WHO and this was consistent with results of similar studies [20, 21].
The average number of hospital stay for patients was 7.1 days. This is higher than the average 6 days reported by a study in a private hospital in India [22]. Furthermore, our finding shows 24% of the patients stayed longer than 8-14 days. Logistic regression showed that patients who stayed longer than 4 days were 6 times more associated with inappropriate administration of Ceftriaxone (aOR=5.7, 95% CI: 1.3-24.6, P=0.02). Another study reported that staying longer than 10 days is 3 times more likely to result in antibiotic use problems than when staying less than 10 days [23]. Prolonged hospital stay is also associated with higher treatment costs, the emergence of resistant microorganisms and increased risk of ADR and drug-drug interaction [24-26]. We found that 6% of the patients stayed longer than 14 days, which could result in increased risk of antibiotic use problems. This study had some limitations. The sample size was small and the difference between the observed results in relation to other studies is relatively high. Also, poor record keeping in the patient medical records could not enable the evaluation for adverse drug Events during the use of Ceftriaxone.
5. Conclusion
The study revealed low treatment outcome during use of Ceftriaxone. There is high number of inappropriate drug administration, in which patients usually miss doses or do not complete as prescribed. This practice increases a threat of antimicrobial resistance. Choice of ceftriaxone use is not guided by culture and sensitivity. A more structured drug utilization study is recommended to acquire more concrete clinical practices on ceftriaxone use.
Declarations
Ethics approval and consent to participate
Approval was obtained from Mubende Hospital Research and Ethics Committee and from the Mildmay Uganda Research Ethics Committee.
Consent for publication
Not applicable
Availability of data and material
The datasets generated and analysed are available.
Author’s Contribution
VN generated the idea, designed protocol and supervised the study. MA and ND conducted data collection. ML analyzed and interpreted the results and prepared the draft manuscript. AS involved in the supervision of the study. HN involved in manuscript preparations and editorial proof reading. All authors have complete access to study data that support the publication. All authors read and approved the final manuscript.
Competing Interests
Victoria Nambasa, Matovu Alphonsus, Nankola Denis, Leonard Manirakiza, Helen Ndagije, and Allan Serwanga have no conflicts of interest that are directly relevant to the content of this study.
Acknowledgements
The authors acknowledge the study participants, data collectors and the staffs of Mubende Regional Referral Hospital for the support rendered during data collection and manuscript compilation.
References
- Ministry of Health. Uganda Clinical Guidelines: National Guidelines for Management of Common Conditions. In: Health Mo, ed (2016).
- Barlam TF, Cosgrove SE, Abbo LM. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 62 (2016): 51-77.
- Gross R, Morgan AS, Kinky DE, et al. Impact of a hospital-based antimicrobial management program on clinical and economic outcomes. Clin Infect Dis 33 (2001): 289-295.
- White AC Jr, Atmar RL, Wilson J, et al. Effects of requiring prior authorization for selected antimicrobials: expenditures, susceptibilities, and clinical outcomes. Clin Infect Dis 25 (1997): 230-239.
- Otage S. Antibiotic resistance on the rise, say doctors. Daily Monitor (2016).
- Harbarth S, Albrich W, Brun-Buisson C. Outpatient antibiotic use and prevalence of antibiotic resistant Pneumococci in France and Germany: A sociocultural perspective. Emerg Infect Dis 8 (2002): 1460-1467.
- Vazquez-Lago J, Lopez-Vazquez P, López- Durán A, Taracido-Trunk M, Figueiras A.Attitudes of primary care physicians to the prescribing of antibiotics and antimicrobial resistance: a qualitative study from Spain. Fam Pract 29 (2011): 352-360.
- Cadieux G, Tamblyn R, Dauphinee D, et al. Predictors of inappropriate antibiotic prescribing among primary care physicians. CMAJ 177 (2007): 877-883.
- Mukonzo JK, Namuwenge PM, Okure G, et al. Over-the-counter suboptimal dispensing of antibiotics in Uganda. J Multidiscip Health 6 (2013): 303-310.
- Means AR, Weaver MR, Burnett SM, et al. Correlates of inappropriate prescribing of antibiotics to patients with malaria in Uganda. PLoS One 9 (2014): 90179.
- Kiguba R, Karamagi C, Bird SM. Extensive antibiotic prescription rate among hospitalized patients in Uganda: but with frequent missed-dose days. The Journal of antimicrobial chemotherapy 71 (2016): 1697-1706.
- Babu JJ. Drug utilization evaluation of cephalosporins in general medicine units of rural tertiary care hospital. Int J Curr Pharm Res 4 (2012): 88-91.
- Sileshi A, Tenna A, Feyissa M, et al. Evaluation of ceftriaxone utilization in medical and emergency wards of TikurAnbessa specialized hospital: a prospective cross-sectional study. BMC Pharmacology and Toxicology 17 (2016): 7.
- Lee H, Jung D, Yeom JS, et al. Evaluation of ceftriaxone utilization at multi-center study. Korean J Intern Med 24 (2009): 374-380.
- Bajimaya S, Kansakar I, Sharma BR, et al. Outcome of cluster endophthalmitis in Western plain region of Nepal. Kathmandu Univ Med J 8 (2010): 102-108.
- Furqan S, Paracha SA. Frequency of Streptococcus pneumonia and Haemophilus influenza in acute exacerbation of chronic obstructive airway disease and their sensitivity to levofloxacin. J Pak Med Assoc 64 (2014): 399-402.
- Iqbal MM, Sattar H, Islam MN, et al. Spectrum of organisms causing peritonitis in peritoneal dialysis patients: experience from Bangladesh. AdvPerit Dial 24 (2008): 40-43.
- Alemayehu S, Admasu T, Mamo F, et al. Evaluation of ceftriaxone utilization in medical and emergency wards of TikurAnbessa specialized hospital: a prospective cross-sectional study. BMC PharmacolToxicol 17 (2016): 7.
- Wade WE, McCall CY. Pharmacoeconomic impact of a drug use evaluation of ceftriaxone in an acute-care medical center. ClinTher 17 (1995): 973-976.
- Abebe FA, Berhe DF, Berhe AH, et al. Drug use evaluation of Ceftriaxone: The Case of Ayder Referral Hospital, Mekelle, Ethiopia. Int J Pharm Sci Res 3 (2012): 2191-2195.
- Ayinalem GA, Gelaw BK, Belay AZ, et al. Drug use evaluation of ceftriaxone in medical ward of Dessie Referral Hospital, North East Ethiopia. International Journal of Basic & Clinical Pharmacology 2 (2013): 711-717.
- Landstedt K, Sharma A, Johansson F, et al. Antibiotic prescriptions for in-patients having non-bacterial diagnosis at medicine departments of two private sector hospitals in Madhya Pradesh, India: a cross-sectional study. BMJ Open 1 (2017): 1.
- Tanaka A, Yano A, Watanabe S, et al. Impact of switching from intravenous to oral linezolid therapy in Japanese patients: a retrospective cohort study. J Pharm Policy Pract 9 (2016): 35.
- Strengthening Pharmaceutical Systems. How to investigate antimicrobial use in hospitals: selected indicators. Published for the U.S. Agency for International Development by the Strengthening Pharmaceutical Systems Program. Arlington: Management Sciences for Health (2012).
- Yadesa TM, Gudina EK, Angamo MT. Antimicrobial use-related problems and predictors among hospitalized medical in-patients in southwest Ethiopia: prospective observational study. PLoS ONE (2015).
- Gentile I, Landolfo D, Buonomo AR, et al. A survey on antibiotic therapy knowledge among physicians of a tertiary care and university hospital. Le Infezioni in Medicina 1 (2015): 12-17.


 Impact Factor: * 3.3
Impact Factor: * 3.3 Acceptance Rate: 74.39%
Acceptance Rate: 74.39%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 