Effects of Fruits of Aqueous Extract of Sarcocephalus Latifolius B. on Gentamicin-Induced Nephrotoxicity in Rats
DA Filkpièrè Léonard*, 1, 3, BALLO Mahamadou2, SOUDRE Albert1, TINDANO Basile3, BAH Sékou2, BAYALA Balé3
1Université Norbert ZONGO, Unité de Formation et de Recherches Sciences et Technologies, Laboratoire Sciences de la Vie et de la Terre ;
2Université des Sciences, des Techniques et des Technologies de Bamako, Mali - Faculté de Pharmacie ;
3Université Joseph KI ZERBO, Unité de formation en Sciences et Technologie, Laboratoire de Sciences de la Vie et de la Terre, Département de Biologie et Physiologie Animale, Laboratoire de Physiologie Animale.
*Corresponding author: DA Filkpièrè Léonard. University of Norbert ZONGO, Laboratoire de Sciences de la Vie et de la Terre, BP: 376, Koudougou, Burkina Faso
Received: 11 January 2023; Accepted: 25 January 2023; Published: 02 February 2023
Article Information
Citation: DA Filkpièrè Léonard, BALLO Mahamadou, SOUDRE Albert, TINDANO Basile, BAH Sékou, BAYALA Balé. Effect of Sarcocephalus Latifolius B. Aqueous Extract Fruits on Gentamicin-Induced Nephrotoxicity in Rats. Journal of Pharmacy and Pharmacology Research 6 (2023): 12-19.
View / Download Pdf Share at FacebookAbstract
Background and Aims: Inflammation is a reaction of the body whenever the integrity of its morphological and biological constants is threatened. All diseases affecting the great vital functions imply in their manifestation inflammation mediators. For the treatment, non-steroidal anti-inflammatory drugs (NSAIDs) and glucocorticoids are used. Although efficient, NSAIDs most often have side effects that hinder their long-term use. In Burkina Faso, medicinal plants combining anti-inflammatory and analgesic activities could be an alternative in anti-inflammatory therapy. The objective of the present study was to evaluate the effect of aquous extract of Sarcocephalus latifolius on gentamicin induced nephrotoxicity in experimental animal models.
Methodology: Nephrotoxicity was induced in wistar rats by intraperitoneal injection of gentamicin (80 mg/kg). The effects of S. latifolius by oral administration during 8 consecutive days with the doses of 100, 250, and 500 mg/kg were evaluated on renal and oxidative markers.
Results: Gentamicin was induced nephrotoxicity marked by biochemical changes, increased lipid peroxidation and reduced antioxidant enzyme activity in renal tissues. Aqueous extracts of S. latifolius were showed a nephroprotective effect by decreasing elevated urea, uric acid, albumin, creatinine, total protein and blood glucose levels and normalized serum Na+, K+, Cl-, Ca2+, Mg2+ and PO42- electrolyte levels in gentamicin intoxicated rats. The extracts were significantly increased the activities of renal antioxidant enzymes (catalase, glutathione) in gentamicin intoxicated rats.
Conclusion: These studies give strong support to the hypothesis that S. latifolius fruits extracts have nephroprotective activity against gentamicin-induced acute nephritis in rats. S. latifolius can therefore be used in preventive applications.
Keywords
Nephrotoxicity, Gentamicin, Sarcocephalus latifolius, Electrolytes, Oxidative stress, Rat
Article Details
1. Introduction
The kidney is an important organ that removes toxic substances from the body. Its major function is to filter and rid the blood of any toxic substance and to retain essential molecules [1]. The kidney regulates blood pressure and extracellular volume. It maintains acid-base and electrolyte balance, hence its role in internal homeostasis [2]. Drug-induced nephropathy is usually kidney damage acute that only persists for the duration of the treatment [3]. In Burkina Faso, medicinal plants combining anti-inflammatory and analgesic activities could be an alternative in anti-inflammatory therapy. This is due to their better accessibility and their lower toxicity in general, compared to classical anti-inflammatory drugs. It is known that many wild plants are part of the traditional dishes cooked in developing countries.
The fruits, when edible, are excellent sources of nutrients in vitamins, minerals and carbohydrates. The fruit of Sarcocephalus latifolius is used in traditional medicine, it is also consumed by local populations. Ethnobotanical studies have revealed in Ghana, Ivory Coast and Burkina Faso that the roots of S. latifolius are prescribed against malaria [4]. In Burkina Faso, its leaves are prescribed in case of abscesses, fractures or chicken pox (Adjanohoun et al., 1989). In association with the roots, the leaves are administered in cases of general fatigue, stomach aches, itching, pimples and sores [5]. However, few studies in Burkina Faso are interested in the pharmacological effects of the fruits of S. latifolius, hence our attention for this plant. The objective of this study is to show that in wistar rats pretreated with the aqueous extract of the fruits of S. latifolius, the renal damage induced by gentamicin is restored.
2. Materials and Methods
2.1 Collection and Authentication of Plant
Sarcocephalus latifolius (Smith) Bruce fruits were harvested in Gaoua located in the south-west of Burkina Faso, 415 km from Ouagadougou. The plant was identified at the “Laboratoire de Biologie et Écologie Végétale” of Université Joseph KI-ZERBO under number 18028.
2.2 Extraction
The harvested fruits were washed, cut into small pieces and dried under ventilation at room temperature. These dried fruits were pulverized and 400 g of this powder was macerated in 1000 ml of distilled water for 24 hours. The filtrate was centrifuged at 2000 rpm for 10 min. The supernatant was frozen at -23°C and lyophilized at -52°C and the aqueous extract of S. latifolius fruits obtained, was stored at - 4 ° C. The extraction yield was 24.23%.
2.3 Experimental Animals
Three-months old Wistar rats of either sex weighing 200-250 g were used for the study. The animals were purchased from Department of Traditional Medicine in Bamako. Animals were housed at a temperature of 24 ± 2 ºC. A 12/12 h light and dark cycle was followed. All animals were fed on standard balanced diet and provided with water ad libitum.
2.4 Gentamicin Induced Kidney Damage
The methods described by [6, 7] were used. Wistar rats of either sex (200-250g) were used. All the animals were divided into six groups; each group consisted of five animals. They were treated daily for 8 days as follows:
Group I: Neutral control (10 ml/kg Saline p.o.);
Group II: Gentamicin (negative control group): 80 mg/kg i.p.;
Group III: Vitamin C (positive control group) at 200 mg/kg + gentamicin 80 mg/kg;
Group IV: Aqueous extract of S. latifolius (100 mg/kg p.o.) + gentamicin 80 mg/kg;
Group V: Aqueous extract of S. latifolius (250 mg/kg p.o.) + gentamicin 80 mg/kg;
Group VI: Aqueous extract of S. latifolius (500 mg/kg p.o.) + gentamicin 80 mg/kg.
2.5 Biochemical, Hematological Estimation
At the end of the treatment, the rats were anesthetized with ether after 16 h of fasting and sacrificed. Blood was collected in dry tubes. After centrifugation at 3000 rpm for 15 minutes the serum was recovered and then stored at -20°C for biochemical analysis (blood glucose, urea, uric acid, creatinine, total protein, albumin) and ionogram (calcium, phosphate, potassium, sodium, chlorine, magnesium, iodine). Blood was collected in EDTA tubes for CBC count. After dissection the kidneys were removed, rinsed and weighed [6, 7].
2.6 Preparation of Kidney Homogenate
Kidney (0.20 g) was crushed in a mortar to which Tris-HCl 50 mM (1 mL) was added. The homogenate (20%) was centrifuged at 3600 rpm for 20 minutes. The supernatant was used for the determination of oxidative stress parameters.
2.7 Assessment of Oxidant/Antioxidant Activity
The method described by [25] was used for the determination of reduced glutathione (GSH) in the kidney. In each tube was introduced homogenate (0.02 mL) and Ellman's reagent (3 mL). After vortexing, the staining was allowed to develop staining for 60 minutes at room temperature. In the control tube were introduced Tris-HCl 50 mM, KCl 150 mM, pH 7.4 (0.02 mL) and Ellman's reagent (3 mL). The absorbance of each tube was then read at 412 nm against the blank.
2.8 Determination of Lipid Peroxidation
The determination of malondialdehyde (MDA) was done following the protocol of [24]. In the test tubes was introduced the homogenate (2ml) and in the control tube, the buffer Tris-HCl 50 mM, KCl 150 mM, pH 7.4 (1ml). To each tube was added respectively trichloroacetic acid (TCA) 20% (1ml) and thiobarbituric acid (TBA) 0.67% (2ml). The tubes were capped and incubated for 10 minutes at 90°C in a water bath. They were then cooled and centrifuged at 3000 rpm for 15 minutes. The supernatant was decanted and the absorbance was read at 530 nm against the blank.
2.9 Determination of Catalase
Homogenate (50 µL) and phosphate buffer (0.1 M; pH 7.5) (750 µL) were introduced into each tube. Add hydrogen peroxide (50 mM) (200 µL) and 1 minute later add the dichromate/acetic acid solution (2 mL). In the control tube was introduced the homogenate (50 µL) and the phosphate buffer (0.1M, pH 7.5) (800 µL). The whole set was heated at 100 °C for 10 minutes. After cooling, the optical density was read at 570 nm. For each tube, the amount of hydrogen peroxide remaining in the solution after addition of the acid was evaluated using the calibration curve [8].
2.10 Statistical Analysis
The data were expressed as mean ± S.E.M. One-factor analysis of variance followed by Dunnett's post-test was used to analyze the data. The control group was compared with the test groups and p < 0.05 was considered significant.
3. Results
The effects of the extract on relative kidney weight are described in Table 1. Daily intraperitoneal injection of gentamicin to rats caused a significant (p < 0.05) increase in relative kidney weight. Daily pretreatment of rats with the extract at all doses did not cause a decrease in kidney weight.
Table 1: Effects of gentamicin and aqueous extract of Sarcocephalus latifolius on the relative weights of the kidneys
|
NaCl 0.9% |
Gentamicin 80 mg/kg |
|||||
|
NaCl 0.9% |
Vitamin C 200 mg/kg |
EASL 500 mg/kg |
EASL 250 mg/kg |
EASL 100 mg/kg |
||
|
kidneys (g) |
0,78 ± 0.09 |
0.95 ± 0.05 ** |
0.68± 0.07 |
0.55 ± 0.04 |
0.67± 0.03 |
0.7 ± 0.05 * |
Values are expressed as mean ± SEM; n=5; ≠p < 0.05 when compared to normal control group; *p < 0.05 when compared to gentamicin treated group.
The effects of the extract and gentamicin on the body weight of rats are described in Table 2. Gentamicin caused a significant decrease in rat growth compared to the neutral control (p < 0.05). This decrease is marked by a loss of body weight of the rats. Daily pretreatment of rats with the extract at all doses caused growth in rats (P>0.05). Growth marked by weight gain of rats.
Table 2: Effects of gentamicin and aqueous extract of Sarcocephalus latifolius on Weight gain
|
Weight of rats (kg) |
Weight of rats (kg) |
Weight gain (%) |
|
|
Day 0 |
Day 8 |
||
|
NaCl 0.9% |
137.7 ± 18.7 |
150.90 ± 19.55 |
8.62 ± 3.0 |
|
Gentamicin 80 |
177.3 ± 16.7 |
160.90 ± 15.43 |
-10,7 ± 6.1 ≠≠ |
|
Vitamin C + Gentamicin 80 |
145.5 ± 4.00 |
154.58 ± 4.87 |
5.80 ± 1.50 * |
|
EASL 500 + Gentamicin 80 |
164.0 ± 3.80 |
171.87 ± 3.88 |
4.52 ± 2.10 |
|
EASL 250 + Gentamicin 80 |
140.0 ± 9.70 |
145.12 ± 15.31 |
2.50 ± 3.20 |
|
EASL 100 + Gentamicin 80 |
233.3 ± 20.6 |
235.27 ± 20.56 |
2.93 ± 2.71 |
Values are expressed as mean ± SEM; n=5; ≠p < 0.05 when compared to normal control group; *p < 0.05 when compared to gentamicin treated group
Gentamicin-induced nephrotoxicity significantly increased the WBC, RBC, monocytes and hemoglobin count. Plant extract doses (500 and 250 mg/kg) significantly improved both the WBC, RBC, monocytes and hemoglobin count compared with the gentamicin group (Table 3).
Table 3: Effects of gentamicin and aqueous extract of Sarcocephalus latifolius on white blood cells, red blood cells, monocytes, hemoglobin
|
NaCl 0.9% |
Gentamicine 80 mg/Kg |
|||||
|
NaCl |
Vitamin C |
EASL |
EASL |
EASL |
||
|
0.90% |
200 mg/kg |
500 mg/kg |
250 mg/kg |
100 mg/kg |
||
|
WBC x 109/L |
2.57 ± 0.19 |
8.95 ± |
4.75 ± |
5.92 ± |
7.32 ± |
6.05 ± |
|
0.27 ≠≠≠ |
0.16*** |
0.25*** |
0.18** |
0.17*** |
||
|
MID x 109 /L |
0.27 ± 0.02 |
1.00 ± |
0.4 ± |
0.47 ± |
0.57 ± |
0.62 ± |
|
0.06 ≠≠≠ |
0.03*** |
0.02*** |
0.02*** |
0.02*** |
||
|
RBC x 1012/L |
6.84 ± 0.14 |
10.27± 0.18≠≠≠ |
7.9 ± |
8.53 ± |
8.88 ± |
9.19 ± |
|
0.30*** |
0.19** |
0.13* |
0.3 |
|||
|
HGB g/dl |
9.8 ± 0.4 |
15.95± 0.31≠≠≠ |
13.22 ± |
13.67 ± |
15.48 ± |
13.72 ± |
|
0.35** |
0.56* |
0.39 |
0.25* |
|||
Values are expressed as mean ± SEM; n=5; ≠p < 0.05 when compared to normal control group; *p < 0.05 when compared to gentamicin treated group.
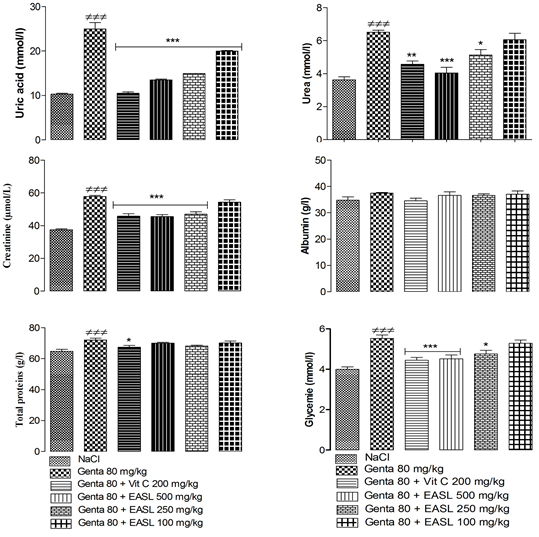
The results showed a significant (P<0.05) increase in serum uric acid, urea, creatinine, total proteins and glycemia concentrations and no significant increase in serum albumin concentration in rats treated with gentamicin compared with control group. Pretreated of S. latifolius with gentamicin, at all doses, significantly (P<0.05) decreased serum uric acid, urea, creatinine concentration compared to gentamicin group (Figure 1).
Values are expressed as mean ± SEM; n=5; ≠p < 0.05 when compared to normal control group; *p < 0.05 when compared to gentamicin treated group.
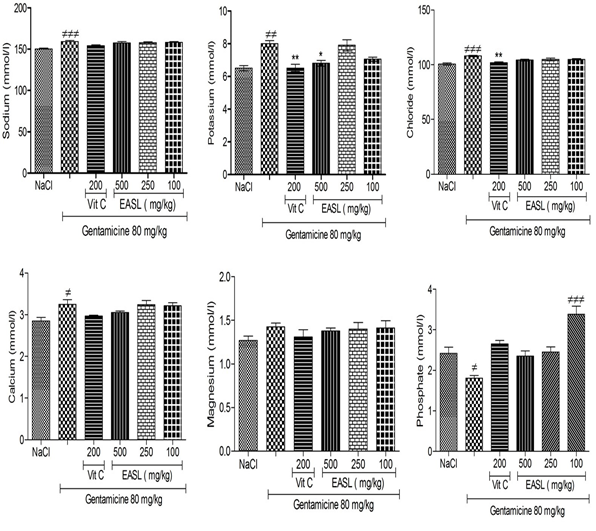
The results showed a significant (P<0.05) increase in serum sodium, potassium, chloride, calcium concentrations and no significant increase (P>0.05) in serum magnesium concentration in rats treated with gentamicin compared with control group. For the same types of comparison, the serum concentration of Phosphate significantly decreased. Co-administration of Sarcocephalus latifolius with gentamicin, at all doses, no significantly (P>0.05) decreased serum sodium, chloride, calcium and magnesium concentration compared to gentamicin group (Figure 2).
Values are expressed as mean ± SEM; n=5; ≠p < 0.05 when compared to normal control group; *p < 0.05 when compared to gentamicin treated group.
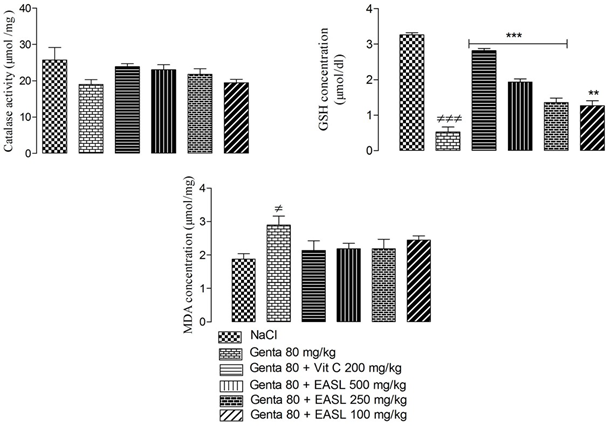
The results showed a significant (P<0.05) increase in tissue MDA concentration, a significant (P<0.05) decrease in GSH concentration and catalase activity in gentamicin-treated rats compared with the control group. Co-administration of S. latifolius and gentamicin, at all doses, resulted in a significant (P <0.05) increase in serum GSH concentration and no significant increase in catalase activity (P >0.05) compared with gentamicin group. The extract, at all doses, caused a no significant increase in tissue MDA concentration compared with gentamicin group. (Figure 3).
Values are expressed as mean ± SEM; n=5; ≠p < 0.05 when compared to neutral control group; *p < 0.05 when compared to gentamicin treated group.
4. Discussion
Gentamicin-induced renal injury is related to its preferential accumulation in the proximal convoluted tubules of the kidney. This results in loss of brush border integrity [9]. Mechanisms of gentamicin-induced nephrotoxicity include generation of free radicals, increased lipid peroxidation, and decreased activity of endogenous antioxidants, renal inflammation characterized by macrophage infiltration and subsequent release of pro-inflammatory cytokines associated with stress-induced NF-κB activation, acute tubular necrosis, and glomerular congestion, resulting in decreased glomerular filtration rate and renal dysfunction [10, 11].
Electrolytes are essential ions in the blood for homeostasis. However, impaired renal function due to exposure to toxic substances can lead to irregularities in body electrolyte levels [12]. Gentamicin interferes with G protein-coupled receptors and prevents cAMP formation in the kidney. Thus, gentamicin reduces the expression of aquaporin-2 channels via the cAMP/arginine vasopressin signaling pathway. It inhibits various transporters located in the cell membranes of the brush border, but also of the basolateral membrane. This causes electrolyte abnormalities. Transport inhibition affects both tubular reabsorption and cell viability. Hence necrosis or apoptosis [13, 14]. This will result in the retention of plasma ions such as sodium and potassium. The consequence is an increase in plasma sodium [11]. Aqueous extract of S. latifolius contains phenolic antioxidants that are believed to increase the activity of Na+/K+ ATPase pumps and thus regulate sodium and potassium excretion [15]. The beneficial effects of S. latifolius extract on renal function can be attributed to these flavonoids. Aqueous extract of S. latifolius (250 and 500 mg/ kg) improved the serum levels of the electolytes measured.
Gentamicin administration stimulated catalase activity, increased malondialdehyde and total protein levels, and decreased serum glutathione levels. The elevated levels of malondialdehyde and total protein indicate increased free radical generation and lipid peroxidation in gentamicin-induced nephrotoxicity. These levels were significantly decreased in rats treated simultaneously with S. latifolius extract and gentamicin. The extract increased glutathione levels and inhibited catalase activity. Similar results have also been reported by other researchers [16, 17]. It seems that these results are related to the antioxidant property of the extract.
Gentamicin reduces urine concentration by suppressing Na+/K+ ATPase. This leads to leukocyte infiltration (white blood cells, monocytes) and necrosis of renal tubular cells [11]. Blood contains red blood cells and in case of anemia, the number of red blood cells decreases. This pathology is a consequence of renal failure. Indeed, the kidneys produce erythropoietin (EPO) which stimulates the production of red blood cells, and therefore hemoglobin. When kidney function decreases, the body produces less and less EPO. The growth of red blood cells is inhibited and anemia sets in.
Our results showed that serum urea, uric acid and creatinine, albumin, glycemia and relative kidney weights were significantly reduced in the groups of rats given the extract compared to the gentamicin treated group. These data suggest that the extract is effective in reducing blood urea, creatinine and uric acid in rats with gentamicin-induced nephrotoxicity. The extract could have a diuretic effect. The elevated blood uric acid level associated with nephrotoxicity is due to stimulation of xanthine oxidase and stimulation of renal reabsorption of urate The protective role of the extract can be explained by an inhibitory action on urate in the rat kidney. The extract would inhibit the effect of xanthine oxidase and free radical scavenging [18, 20]. [21] showed that fruits are good sources of macro and micro nutrients. This could explain the weight gain seen by rats treated with Sarcocephalus latifolius fruits extract. [22] showed that the fruits of Sarcocephalus latifolius contain vitamins A, C and E with a predominance of vitamin C. The vitamin C confers nutritional and even medicinal properties to the plants. Indeed, vitamins C and E are known to be good antioxidants. Consumption of the fruit could therefore be useful in combating oxidative stress. [23] showed that the ethanolic extract of Sarcocephalus latifolius fruits and leaves each contain triterpenes, tannins, flavonoids, alkaloids, steroids and saponins. The treatment of nephritis in our study is explained by the presence of tannins used against inflammation. The anti-oxidant power of the extract would be due to flavonoids which are known for their antioxidant property.
Conclusion
The present study demonstrated the nephroprotective effect of the aqueous extract of Sarcocephalus latifolius against the toxicity of gentamicin. The study confirms the use of the fruits of this plant by the local populations in the treatment of renal diseases. The nephroprotective effect of the aqueous extract of S. latifolius would be due to its antioxidant and anti-inflammatory capacity. The fruits of S. latifolius could be an alternative in the fight against acute kidney diseases. Future experimental studies in toxicity and renal histology could be investigated to confirm the present study.
Acknowledgments
The authors thank CHU of Point G in Bamako, Mali. We also thank the Polyclinic Laboratory of the Army in Bamako. We thank the State of Burkina Faso through its Ministry of Education and Scientific Innovation.
Conflicts of Interest
The authors report no financial or any other conflicts of interest in this work
Reference
- Reichl FX. Guide pratique de toxicologie.2 eme Edition. Edition DeBoeck et Larcier. Bruxelles 16 (2004).
- Tarloff JB, Wallace AD. Nephrotoxicity: A Textbook of Moder Toxicology. Hoboken New Jersey (2010): 291-302.
- Gueguen Yann, Caroline Rouas, François A. Leblond. Les biomarqueurs d’atteintes rénale. Néphrologie & thérapeutique 8 (2012): 146–155.
- Adjanohoun E, Ahyi MRA, Ake Assi L. Médecine traditionnelle et pharmacopée: contribution à l’étude ethnobotanique et floristique au Togo. Agence de Coopération Culturelle et Technique (ACCT) (1986).
- Cournac V. Evaluation in vitro de l’activité anti malarique d’extraits de Nauclea latifolia Sm. Th D Pharm, Montpellier (1997).
- Poormoosavi SM, Behmanesh MA & Najafzadeh H. Effect of cimetidine on gentamicin-losartan induced -nephrotoxicity in rats. African Journal of Pharmacy and Pharmacology 4 (2010): 341-345.
- Jumana M, Yousef, Gong Chen, Prue A. Hill A, Roger L. Nation and Jian Li, 2011. Ascorbic acid protects against the nephrotoxicity and apoptosis caused by colistin and affects its pharmacokinetics. J Antimicrob Chemother 67 (2012): 452 – 459.
- Sinha AK. Colorimetric assay of catalase. Analytical Biochemistry 47 (1972): 389-394.
- Lopez-Novoa JM, Quiros Y, Vicente L, Morales AI, and Lopez-Hernandez FJ. New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney International 79 (2011): 33–45.
- Kalayarasan S, Prabhu PN, Sriram N, Manikandan R, Arumugam M, Sudhandiran G. Diallyl sulfide enhances antioxidants and inhibits inflammation through the activation of Nrf2 against gentamicin-induced nephrotoxicity in Wistar rats. European Journal of Pharmacology 606 (2009): 162–171.
- Lee IC, Kim SH, Lee SM. Melatonin attenuates gentamicin-induced nephrotoxicity and oxidative stress in rats. Archives of Toxicology 86 (2012): 1527–1536.
- Alabi QK, Akomolafe RO, Adefisayo MA, Olukiran OS, Nafiu AO, Fasanya MK, et al. Kolaviron attenuates diclofenac-induced nephrotoxicity in male Wistar rats. Appl Physiol Nutr Metab 43: 956-968.
- Rai D, Bhatia G, Sen T, Palit G. Anti-stress effects of Ginkgo biloba and Panax ginseng: a comparative study. J. Pharmacol. Sci 93 (2003): 458–464
- Raghavan V, Weisz OA. Discerning the role of mechanosensors in regulating proximal tubule function. Am. J. Physiol. Renal Physiol 310 (2016): F1–F5
- Al-Numair KS, Veeramani C, Alsaif MA, Chandramohan G. Influence of kaempferol, a flavonoid compound, on membrane-bound ATPases in streptozotocin-induced diabetic rats. Pharm Biol 53 (2015): 1372-1378
- Karadeniz A, Yildirim A, Simsek N, Kalkan Y, Celebi F. Spirulina platensis protects against gentamicin-induced nephrotoxicity in rats. Phytother Res 22 (2008): 1506.
- Farombi EO, Ekor M. Curcumin attenuates gentamicininduced renal oxidative damage in rats. Food Chem Toxicol 44 (2006): 1443-8.
- Rott KT and Agudelo CA. Gout. J. of the Am. Med. Assoc 289 (2003): 2857-2860.
- Yu Z, Fong WP and Cheng CH. The dual actions of morin (3,5,7,2',4'- pentahydroxyflavone) as a hypouricemic agent: Uricosuric effect and xanthine oxidase inhibitory activity. J. of Pharmacol. Exp. Ther 316 (2006): 169-175.
- Venkatesan RS, Sadiq AM, Kumar JS, Lakshmi GR and Vidhya R. Effect of morin on mercury chloride induced nephrotoxicity. An Inter. Quarterly J. of Environ. Sci 4 (2010): 193-196.
- Yesufu HB et Hussaini IM. Studies on dietary mineral composition of the fruit of Sarcocephalus lantifolius (Smith) bruce (Rubiaceae). J Nutr food Sci 88 (2014).
- Omale J, HarunaHU. Hypocholesterolemic effects of Nauclea latifolia (Smith) fruit studied in albinos rats. Americam Jounal of Tropical Medicine et Public Health 1 (2011): 11-21.
- Eze SO, Obinwa E. Phytochemical and nutrient evaluations of the leaves and fruits of Nauclea latifolia (Uvuru-ilu). Communications in Applied Sciences 2 (2014): 8-24.
- Wilbur KM, Bernheim F, Shapiro OW. Determination of lipid peroxidation. Archives of Biochemistry 24 (1949): 305-310.
- Ellman GL. Tissue sulfhydryl group. Archives of Biochemistry and Biophysics 82 ((1959): 70-77.
- Lee J, Yoo KS, Kang DG, Kim SW, Choi KC. Gentamicin decreases the abundance of aquaporin water channels in rat kidney. J Pharmacol Sci 85 (2001): 391-398.


 Impact Factor: * 3.3
Impact Factor: * 3.3 Acceptance Rate: 74.39%
Acceptance Rate: 74.39%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 