Fowl Adenovirus: Pathogenesis and Control
Areayi·Haiyilati1,2, Xiaoqi Li2*, Shijun J Zheng1,2*
1Key Laboratory of Animal Epide Miology of the Ministry of Agriculture, Beijing 100193, China
2College of Veterinary Medicine, China Agricultural University, Beijing 100193, China
*Corresponding Author: Xiaoqi Li, College of Veterinary Medicine, China Agricultural University, Beijing 100193, China
Shijun J Zheng, Key Laboratory of Animal Epide Miology of the Ministry of Agriculture, College of Veterinary Medicine, China Agricultural University, Beijing 100193, China
Received: 15 August 2021; Accepted: 24 August 2021; Published: 16 September 2021
Article Information
Citation: Areayi·Haiyilati, Xiaoqi Li, Shijun J Zheng. Fowl Adenovirus: Pathogenesis and Control. International Journal of Plant, Animal and Environmental Sciences 11 (2021): 566-589.
View / Download Pdf Share at FacebookAbstract
Fowl adenovirus (FAdV), belonging to the Aviade-novirus genus of Adenoviridae family, poses an increasing threat to poultry industry across the globe. In particular, the occurrence of hepatitis hydroper-icardium syndrome (HHS) in flocks in China has caused severe economic losses to stakeholders since 2015. Some serotypes of the FAdVs, especially FAdV-4, -2, -7 and -11, are highly pathogenic to 3- to 6-week-old chickens and responsible for HHS and inclusion body hepatitis (IBH). On the contrary, lines of evidence indicate that adenovirus could serve as an optimal vector to deliver protective antigens of other pathogens in host. This review mainly focuses on the pathogenesis and control of HHS, and discusses over the possible role of FAdV as a vector in controlling other avian diseases.
Keywords
<p>Fowl adenovirus; Pathogenesis; Immune response; Vaccines; Vector</p>
Article Details
1. Introduction
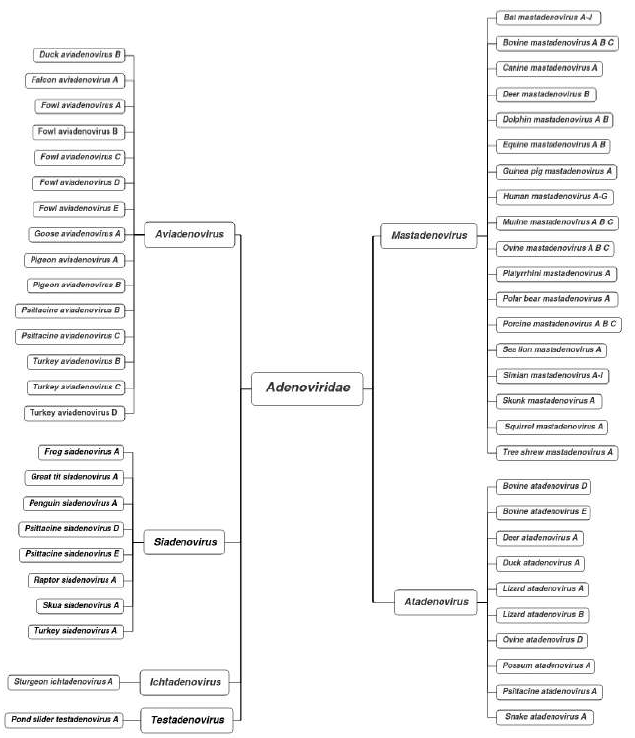
Adenoviruses (AdV), widely-distributed microbes across the globe, can infect varied species of vertebrates including birds, animals and human. In 1949, Vanden Ende et al. accidentally isolated adeno-virus from bovine nodular dermatitis material for the first time [1] and subsequently, several strains of chicken embryo lethal orphan (CELO) virus were isolated from chicken embryos [2] and gallus adeno-like (GAL) virus was obtained from chicken cell culture [3]. In 1952, Olson formally isolated fowl adenovirus named quail bronchitis virus (QBV) from North American quail with bronchitis [4]. It was not until 1953 that the first human AdV was discovered in human adenoid tissue by Wallace Rowe and collea-gues [5], and it belonged to the Mastadenovirus genus Adenoviridae family. Currently, according to the International Committee on Taxonomy of Viruses (ICTV), Adenoviridae can be classified into five genera: Mastadenovirus, Aviadenovirus, Siadeno-virus, Atadenovirus and Ichtadenovirus [6]. Based on phylogenetic distance, restriction fragment length polymerphism, genome organization, host range, pathogenicity and cross-neutralization, the Aviaden-virus are divided into 15 species as shown in Figure 1 [7]. Fowl adenoviruses (FAdVs) are generally divided into 5 species (FAdV-A to FAdV-E) based on their restriction enzyme digestion patterns [8], and 12 serotypes (FAdV1-7, FAdV8a/b, and FAdV9-11) as determined by serum cross-neutralization test [9]. The 12 serotypes of FAdVs are grouped into the 5 species: FAdV-1 belongs to species FAdV-A, FAdV-5 to species FAdV-B, FAdV-4 and-10 to species FAdV-C, FAdV-2, -3, -9 and-11 to species FAdV-D, and FAdV-6, -7, -8a, and -8b to species FAdV-E. Although FAdVs could be isolated from healthy chickens, some serotypes of FAdVs are associated with several notable diseases such as inclusion body hepatitis (IBH) [10], hepatitis hydropericardium syndrome (HHS) (also known as hydropericardium syndrome, HPS) [11], and adenoviral gizzard erosion (AGE) [12] in chickens and other birds. These diseases have caused significant economic losses to the poultry industry around the world. The serotypes FAdV-2, FAdV-11, FAdV-8a and FAdV-8b caused outbreaks of IBH in chickens with 10-30% mortality [13-15]. AGE is mainly induced by FAdV-1 and FAdV-8 and commonly found in slaughtered broiler chickens [16-19]. FAdV-4 serotype is the primary causative agent responsible for HHS at a mortality of 30-80% [14, 20, 21]. In the past few years, the occurrence of HHS in Asia, particularly in China, has attracted much attention, and there even appears a novel natural recombinant FAdV-E that has spread in China recently [22]. Thus, FAdV infection as a serious threat to the poultry industry across the globe has become the focus in terms of poultry disease control. The basic research on the pathogenesis of FAdV infection could provide an important clue to the stipulation of effective measures for the prevention and control of FAdV infection. The topics on avian adenoviruses especially FAdV-4 were very well reviewed [7, 14, 23-26]. This review mainly focuses on our current knowledge of the recent findings about the pathogenesis, prevention and con-trol of FAdV infection.
2. Fowl Adenovirus Characteristics and Diseases
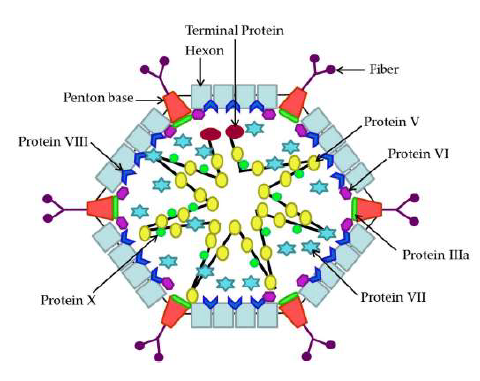
FAdV, a non-enveloped and double-stranded DNA (dsDNA) virus, belongs to the Aviadenovirus genus of Adenoviridae family. The genomes of different serotype FAdVs may vary in size, ranging approximately from 43 to 45 kb, encoding approximately 10 structural proteins and different non-structural proteins. It was reported that the genome of the non-pathogenic FAdV-4 is composed of 23.3% A, 27.7% C, 26.9% G and 22.1% T and a G+C content of 54.6% with 45667 bp in length [27], larger than that of FAdV-8 (45063 bp) [28] and FAdV-1 (43804 bp) [29]. Several studies have illustrated the structures of FAdV by cryo-electron microscopy (CM) [7, 30, 31]. The FAdV capsid is composed of 240 hexon capsomers (protein II), 12 penton bases (protein III) and 1 or 2 fiber proteins (protein IV) per vertex as well as 7 structural proteins (Figure 2) [7, 30]. As shown in Figure 2, hexon, fiber and penton base are the major capsid proteins, which have been mostly investigated so far [32]. As some serotypes of FAdVs caused IBH and HHS in chickens [33], it would be reasonable and important to determine the roles of viral proteins in this pathological process, which would help to elucidate the pathogenesis of FAdV in-fection.
The first step of FAdV infection is the attachment of virus to host cell by binding to the specific receptors on the cell membrane surface. Coxsackievirus and adenovirus receptor (CAR), a transmembrane protein on the target cell surface, is a specific receptor for FAdV attachment, facilitating the entry of the virus into the host cells via the FAdV’s fiber knob by endocytosis, and the genome is transported to the cell nucleus [34, 35]. A recent report indicates that the D2 domain of CAR (D2-CAR) acts as an active domain responsible for binding to the short fiber of the novel FAdV-4 [36]. In addition to CAR, other AdVs may use different receptors for viral entry, such as sialic acid, which serves as the receptor for bovine adenovirus serotype 3 [37]. Once inside the cell, FAdV will start its replicating program employing cellular machinery, during which the viral components interact with that of host cells (virus-host interaction) at both protein and nucleic acid levels, leading to apoptosis, autophagy, inflammatory cytokine response and so on.
FAdVs are ubiquitously prevalent in poultry, and have been detected and isolated from chickens [38], guinea fowls [39], turkeys [40], pigeons [41], house sparrows [39], geese [42] and ducks [43]. Among these hosts, 3- to 6-week-old broilers are most susceptible to FAdV infection. FAdV-4 was first identified as a causative agent responsible for HHS in 3- to 6-week-old broiler in Pakistan in 1987 [44], and subsequently the outbreaks of HHS occurred in India [45], Middle East [46], Russia [47], South and Central America [48-50], Japan [51], Korea [52], and China [53]. In contrast to mild diseases caused by other FAdV serotypes, the outbreaks of HHS in chickens infected by FAdV-4 in 2015 caused tremendous economic losses to the poultry industry in China [54, 55]. FAdV-4 mainly infects 3- to 6-week-old broilers and occasionally 10- to 20-week-old layers and breeder pullets [56-58]. Notably, the mortality caused by hypervirulent FAdV-4 in China reached as high as 80% [20, 59]. In addition to the occurrence of HHS in chickens, HHS outbreaks have been reported in geese [42], pigeons [41], black kites [60], ducks [43], ostriches [61], and in peacocks [62]. The predominant gross lesions of HHS in chicken is hydropericardium, accumulation of clear or yellowish jelly-like fluid in the pericardial sac as well as a yellow brown-colored and swollen, enlarged, congested and friable liver with foci of hemorrhages and necrosis as shown in Figure 3, and in few cases, hemorrhage in spleens, lungs and kidneys [38, 63] and enlarged bursa of Fabricius could be observed [45]. Histologically, there were fatty changes and focal areas of lymphocytic infiltration in the liver. The conspicuous intranuclear basophilic inclusion bodies were seen in the hepatocytes and many hepatocytes contained large dense hyperchromatic nuclei [57, 64]. Despite the pathogenesis of FAdV has not been unraveled, inflammatory cytokines, autophagy, apoptosis and maternally derived antibodies may play roles in the pathogenesis of FAdVs [24, 65]. Although FAdV-4 seems to be the predominant serotype in China, other serotypes such as FAdV-2 [66, 67], FAdV-8 [68, 69] and FAdV-11 [67, 69] have also been isolated from flocks with clinical signs of IBH [53].
3. Roles of the Major Viral Proteins of FAdV in the Pathogenesis of Viral Infection
The genome of FAdV-4 potentially contains 46 ORFs encoding 10 structural proteins and approximately 32 non-structural proteins. The major structural proteins consist of hexon, penton base, fiber (fiber-1, fiber-2), terminal protein, and proteins V, VI, VII, VIII, IIIa, and X [23], while non-structural proteins include U exon, DBP, 52K, 100K, 22K, pIVaII, pol, 33K and so on [7, 70]. Attached to the DNA genome are terminal protein, proteins X, V, and VII within the virus particles [71]. According to the reports, various genomic deletions were found in some of highly virulent FAdV-4 strains isolated in China, especially a strain with 1966-bp deletion on the right end region of the genome [55, 72]. However, the virulence of hypervirulent FAdV-4 was found to be independent of the 1966-bp deletion [73]. Although the pathogenesis of FAdV infection is still not very clear, remarkable progresses have been made in elucidating the roles of some viral proteins (hexon, fibers, penton base, 100K and PX) of FAdV in the pathogenesis of viral infection.
3.1 Hexon
Hexon, a structural protein of FAdV with a mass of 107 kD, is the most abundant capsid protein, which plays a vital role in the genome organization. Every hexon possesses two distinctive parts: triangular with three towers. The conserved pedestal regions P1 and P2 of hexon are basement created of the particle and taking part in trimer formation and the variable loops L1, L2 and L4, which are in external part of virion, created by hypervariable HVR [74, 75]. The molecular changes of the knobs of hexon L1 and fiber are signs of FAdV infectivity [76], and the pathogenicity of highly pathogenic FAdV-4 also depends on hexon and fiber [77].Specifically, it was recently demonstrated that the amino acid residue at position 188 of the hexon is responsible for FAdV-4 pathogenicity [78]. Usually, serotyping, phylogenetic analysis and potential diagnosis of FAdVs are mainly based on L1 loop of hexon gene [48, 79-81]. Thus, hexon gene was amplified to determine FAdV-4 contamination in live vaccines for poultry use in China [82], and it was also used for molecular typing of isolates in different areas around the world [83-86]. It was reported that high-resolution melting (HRM)-curve analysis based on the conserved loop regions and pedestal regions of hexon divided FAdV strains into 22 sub-groups, which is consistent with phylogenetic analysis [48]. Hexon is an important part of the virion establishing infection [87], containing group-, type- and subtype-specific antigenic determinants [88, 89], against which antibodies are produced [90]. Hexon is also associated with pathogenicity, as modification of hexon gene is involved in attenuation of FAdV virulence [91]. Inter-estingly, our laboratory found that FAdV-4 hexon interacted with chaperonin containing TCP-1 subunit eta (CCT7), enhancing viral replication, thus CCT7 may serve as a potential target for controlling FAdV-4 infection [92]. However, the exact role of hexon in the pathogenesis of FAdV-4 infection is still not very clear. More efforts will be required to investigate the possible roles of hexon in the pathogenesis of FAdV-4 infection.
3.2 Fiber
Fiber, a structural protein of FAdV with a mass of 66 kD, consists of three regions: the tail, shaft and knob [93]. However, some FAdVs possess two fibers based on electron microscopy (EM) [30], such as FAdV-1 and FAdV-4, which harbor two genes encoding the fiber proteins with different length [27, 29]. Previous studies on AdV showed that N-terminal tail region binds noncovalently to the penton base protein, the C-terminal knob region is considered to be the ligand for binding to the host receptor, and the shaft region is the part of connecting N-terminal tail and knob and its length could influence the interaction between knob and cellular receptor [94, 95]. As a major surface-exposed capsid structure, the fiber protein plays different roles in virus neutralization, cellular receptor binding [96], tissue tropism and variations in virulence [95]. It was found that the two fibers have different functions, one for virus attachment and the other for viral assembly or spread at some stage in virus growth [97], suggesting that both fibers are required for viral infection. Among the 12 serotypes of FAdVs, serotypes FAdV-1, FAdV-4 and FAdV-10 have two fiber genes, the others like FAdV-9, FAdV-8, and FAdV-5 only carry one fiber gene [98]. FAdV-1 fiber-1 mediates cell attachment to CAR in both avian and mammalian cells, while fiber-2 mediates attachment to receptor(s) present on avian but not in mammalian cells [97], which was considered as one of possible reasons for viral tropism [27].
A previous study suggested that CELO virus belonging to FAdV-1, might bind to the CAR via fiber-1, but initiate the accessory infection through fiber-2, indicating that fiber-2 might be critical for virus-host interaction at an early stage of infection [97]. It was found that the knob domain of fiber-1 was the key factor for directly triggering the infection of FAdV-4 [99]. Recombinant FAdV-4 fiber-2 protein could act as a protective immunogen against HHS [100, 101]. It was reported that fiber-2 also interacts with host proteins KPNA3/4 efficiently via its N-terminal 1-40aa, which assists the replication of FAdV-4 in LMH cells [102]. However, the exact role of fiber-2 in the pathogenesis of hypervirulent FAdV-4 infection is still not very clear.
3.3 Penton base
Penton base, a structural protein with a mass of 87 kD, holding a morphologically prominent position at the vertex capsomers in the AdV particle [103], participates in the internalization of virus into an endosome of host cell [71, 104]. The internalization of AdV by host cell involves the recognition of cellular integrins by the RGD peptide of the penton [105]. Each penton unit consists of a penton base anchored in capsid and a projection (fiber) at the distal end. Fiber is noncovalently linked to the pentameric penton base [94]. However, the specific role of penton base protein in FAdV infection is not fully studied. Further investigation will be required for the role of this viral protein in the pathogenesis of viral infection in the future.
3.4 100K
100K, an important nonstructural protein (NSP) of AdV of subgroups B and C with a mass of 292 kDa [106], showing symmetrical, dumbbell shaped molecule consisting of two terminal globular domains linked by a rod like connecting domain by EM, assists in trimerization and nuclear localization of hexon during viral replication in host cells [107]. It was considered that the 100K protein acts as a scaffold protein for the hexon folding and self-assembly into capsomer in insect cells [108]. It was found that antibodies specific for the 100K NSP could be detected in chickens experimentally infected with live FAdVs, but not in chickens vaccinated with inactivated FAdVs [109]. Thus, 100K protein might be used as an antigen for the development of diagnostic kits to examine specific antibodies, differentiating the infected chickens from the vaccinated ones (DIVA). It was reported that recombinant 100K protein only had little protection (40%) to the immunized chicken against pathogenic viral challenge and it is not exposed on the surface of the virus at any stage [110].
3.5 PX
In human adenovirus (HAdV), PX, also known as Mu, a structural protein of FAdV, plays an important role in attaching the linear double-stranded DNA genome to the capsid during replication [111]. Our laboratory recently reported that PX acts as a major viral factor inducing apoptosis in FAdV-4 infected LMH cells [112]. Interestingly, we found that all these Chinese FAdV-4 strains (HNJZ, SDDZ, SXCZ, AHBZ, JSXZ and HuBWH) had the same spon-taneous amino acid substitutions at residues 11 (T to A) and 129 (T to A) in the PX when compared with non-pathogenic FAdV-4 ON1 strain. Surprisingly, mutation of A11T, A129T or both A11/129T back, referring to the PX sequence of non-pathogenic FAdV-4 ON1, dramatically reduced apoptosis in plasmid transfected cells compared to that of virulent FAdV-4 PX transfected controls, indicating that alanines 11 and 129 are critical for PX-induced apoptosis [112].
It seems that PX may serve as a virulence factor contributing to the pathogenicity of FAdV-4 isolates because inhibition of apoptosis by caspase inhibitors significantly reduced the viral growth in LMH cells. Thus, PX-induced apoptosis contributes at least in part to FAdV-4-induced apoptosis, which facilitates viral replication. Furthermore, the fact that FAdV-4-induced apoptosis facilitates viral replication in LMH cells suggests that PX may serve as a virulence factor for FAdV-4 infection. Further investigation into the roles of viral components in the pathogenesis of FAdV-4 infection will be definitely required.
4. Immune Response to FAdV
The immune system of vertebrates has evolved varied mechanisms to defend themselves from external insults including virus infection. As the first line of defense of the host immune system, innate immunity plays a pivotal role in resisting the pathogenic infection. However, the information regarding the immune response of fowls to FAdV infection is quite limited.
As important innate immune molecules, defensins play important roles in innate immunity against pathogenic infection. It was found that most avian β-defensins (AvBDs) were upregulated in the specific tissues of chickens with FAdV-4 infection and that there was a positive correlation between FAdV-4 genome load and the mRNA expression levels of AvBDs (AvBD5, 7, 8, 9, and 13), implicating the potential role of AvBDs during FAdV-4 infection [113]. It seems that AvBDs are involved in innate immune responses against FAdV-4 infection, but their exact roles in combating viral infection are still not very clear.
In response to virus infection, host cell senses viral components (pathogen-associated molecular patterns, PAMPs) via pattern-recognition receptors (PRRs), which initiates immune response and eventually induces the expression of anti-virus factors. FAdV-4 infection activated several immune related pathways, including Toll-like receptors (TLRs) signaling pathway and cytokine cytokine receptor interaction pathway, and MyD88 mediated FAdV-4-induced inflammation [114, 115]. TLR1, TLR2A, TLR3, TLR4, TLR5 and TLR21 were found to be involved in FAdV-4 infection [113, 116], suggesting that these TLRs play roles in host response to FAdV-4 infection.
Cytokines are protein or glycoproteins secreted by cells that are involved in immune signaling. Generally, type I IFNs and pro-inflammatory cytokines are rapidly expressed to restrict virus infections and stimulate the immunity response. FAdV infection surely results in the up-regulated expressions of pro-inflammatory cytokines [117]. It was reported that the expressions of IFN-α, IFN-γ and interleukin (IL)-12 increased in the liver, spleen and bursa of Fabricius of chickens infected with FAdV-9 or FAdV-9∆4, while the expression of IL-10 decreased or didn’t change, depending on tissues [118]. During FAdV-8 infection, mRNA expression of IFN-γ in the spleen of infected chickens was significantly upregulated while IL-10 down-regulated [119]. FAdV-4 infection also upregulated the mRNA expressions of IL-1β, IL-6, IL-8 and TNF-α in vivo and in vitro [120], and the expressions of some cytokines (IL-1β, -2, -6, -8, and -18, and IFN-γ) were upregulated in the spleen and bursa of Fabricius [113]. These findings suggest that FAdV infection induces host innate immune response. However, more efforts will be required to investigate the response in chickens with FAdV infection.
Adaptive immunity, the process by which the immune system produces specific antibodies and immune effector cells under the stimulation of antigens, includes two major aspects: humoral immune response and cellular immune response [121]. Although humoral immunity is an essential component of the adaptive immune response of host to viral infection, cell-mediated immunity to FAdVs seems to play important roles in virus clearance [116]. The overall T-lymphocyte population in chickens infected with FAdV-1 increased and was important to combat viral infection [122]. It was found that infection with virulent FAdV-4 isolate (AG234) caused a decrease of CD3+, CD4+ and CD8+ T-cells in the spleen and a decrease of CD4+ and CD8+ T-lymphocytes in the thymus associated with severe depletion of lymphocytes in the bursa of Fabricius [123], suggesting that FAdV-4 infection affects adaptive immune response of chickens. Some observations suggest that there might be an associa-tion of host genetic background with FAdV-induced pathogenesis [124, 125]. For instance, it was found that the mortalities of specific pathogen free (SPF) broilers infected with strains belonging to species FAdV-E and FAdV-D were 100% and 96%, respectively, which were much higher than that of SPF layers, displaying 20% and 8% respectively [125]. This information indicates a marked difference between broilers and layers in terms of their resistance to FAdV infection.
It was reported that FAdV infections in poultry, even in SPF chickens, could evade the immune response of host resulting in latent or persistent infections [126, 127]. Chickens with FAdV-4 infection caused depletion of lymphocytes and growth impairment in the thymus and bursa of Fabricius. Along with histopathological changes in lymphoid organs, FAdV-4 infection resulted in suppression of the humoral immune response [123]. It seems that FAdV-4 in-fection induced apoptosis in lymphocytes, leading to the damages to lymphoid organs. Although PX proteins were found responsible for FAdV-induced apoptosis [112], the exact mechanisms underlying FAdV-induced apoptosis remain elusive. It was found that depletion of lymphocyte in the lymphoid organs by virulent FAdV (serotypes 1, 4 and 8) infection was associated with suppression of immune response [125, 128]. However, the molecular mechanism underlying hypervirulent FAdV-4-induced immunosuppression is still unclear. The mixed infections of more than one serotype might also be a contributing factor that prolongs the persistence and excretion of FAdV-4 [129]. Further exploration of the molecular basis of FAdV-4-induced immunosuppression in host will be essential to elucidate the pathogenesis of FAdV-infection.
5. Prevention and Control
FAdVs, highly resistant to inactivation, persistently exist in the environment for a long period of time and are transmitted both horizontally and vertically. Co-infection of FAdVs with other highly infectious viruses such as infectious bursal disease virus (IBDV), avian leukosis virus (ALV) and chicken anemia virus (CAV) makes the control and prevention of FAdV infection more difficult and complex. Good management practices and biosecurity measures are very important to the prevention and control of infectious diseases.
Therefore, vaccination against FAdV infection is generally recommended and practiced with promising results. Inactivated vaccines, attenuated live vaccines, and recombinant vaccines have been used to control HHS, and these vaccines have been proven to be effective in protecting chickens against FAdV infec-tion.
5.1 Inactivated vaccines
Inactivated vaccines, different from live vaccines, can induce antibody response in pullets with high and uniform titres persisting for an extended period, resulting in long-lasting immunity [130]. The first successful protection of chickens by vaccination against IBH was reported over 40 years ago [131]. In some countries, vaccines prepared from liver homogenates of infected chickens are commonly used against HHS/IBH [129, 132, 133]. Dual vaccinations of breeders with killed FAdV-4 and CAV provide effective protection of the progeny of chickens against HHS/IBH [134, 135]. It was reported that oil-adjuvanted cell culture IBH vaccine provided better protection when compared to the autogenous vaccine [136]. Autogenous vaccines, derived from formalin-inactivated liver homogenates, are generally used to control HHS [91].This type of vaccines contain the local circulating viruses and readily induce neutralizing antibodies, and can be used to control FAdV infection with satisfactory success.
It was reported that the inactivated oil-emulsion FAdV-4 vaccine could pro-vide broad cross-protection against various serotypes of FAdVs in not only vaccinated birds, but also the progenies of vaccinated breeder [137], suggesting that this type of FAdV-4 vaccine could be effective in controlling the spread of FAdV-4 as well as other serotypes of FAdVs. Inactivated and live bivalent fowl adenovirus (FAdV8b + FAdV11) breeder vaccines could provide broad-spectrum protection in chicks against IBH [138], supporting the previous report on the efficacy of a bivalent autogenous FAdV vaccine [139]. Simi-larly, IBH could be effectively controlled by vaccination of broiler breeders with a bivalent vaccine containing live FAdV8a-TR59 and FAdV11-1047 [140]. These observations suggest that multivalent vaccines could be clinically used with satisfactory outcomes.
5.2 Attenuated live vaccines
Virulent FAdV-4 isolates were attenuated by adapting to growth in a quail fibroblast cell line (QT35) or in SPF chicken embryos and applied as vaccines, which were capable of reducing the immunopathology induced by a severe challenge [123]. Mansoor and colleagues developed a live attenuated vaccine against HHS by propagation of chicken embryo-adapted FAdV-4 [91]. They adapted a field isolate of HHS virus to embryonated chicken eggs via four blind passages and continuously passaged the virus up to 12 times in SPF chicken embryos for further attenuation. They inoculated 14-day-old broilers orally and parenterally with attenuated virus and used liver homogenate vaccine as a control. The results show that the antibody response significantly increased in broilers vaccinated with the attenuated virus 7, 14 and 21 days post-immunization, and the broilers immunized with 16th-passage attenuated virus were conferred with significantly higher protection (95%) compared to that (55% protection) of the broilers immunized with the liver homogenate vaccine [136]. In comparison with liver homogenate vaccine, the attenuated vaccine showed better protection of chickens from HHS [45, 91, 141]. The advantage of attenuated live virus over the inactivated vaccine is that live virus could elicit not only humoral immune response but also cell-mediated immunity. Thus, the attenuated vaccine was more effective against HHS in broilers. However, the disadvantage of employing attenuated live vaccine is the potential risk of reversion to virulence as well as the generation of new virus strains via recombination with circulating field virus.
5.3 Recombinant and subunit vaccines
The recombinant DNA technology has been widely used to develop vaccines for animals as well as human. The viral proteins of FAdV, such as hexon, fi-ber-2 and penton base, have the potential of being used for the development of subunit vaccines. It was reported that a portion of fiber protein had been used as an immunogen for the vaccine development with some success [142], and the penton base protein expressed by prokaryotic-expression system had been used as subunit vaccines for protecting broilers from HHS, achieving 90% protection for the vaccinated chickens against challenge with virulent virus [143]. Schachner and colleagues compared the immune-genicity of two viral recombinant fiber proteins (fiber-1 and fiber-2). They expressed FAdV-4 fi-ber-2 by eukaryotic expression system (Baculovirus expression system), inoculated 1-day-old chicks with purified recombinant fiber-2 by intramuscular injection, and found that fiber-2 protein could effectively reduce the mortality of chicks to 3.5% after challenge with a virulent virus [100], suggesting that fiber-2 could serve as a protective antigen. A follow-up study by another research group indicated that recombinant fiber-2 protein could provide 100% protection against challenge with virulent FAdV-4 strain HB1501, neither clinical signs nor gross lesions were observed in vaccinated chickens after challenge, and that immunization of SPF chickens with recom-binant fiber-2 protein could induce quicker and stronger immune response than the inactivated oil-emulsion FAdV-4 vaccine [101]. Recently, it has been reported that the highly conserved epitopes (Asp348-Phe369) on the surface of hexon used as virus-like-particle (VLP) vaccine can provide 90% protection against the challenge with the virulent FAdV-4 isolate in chickens [144]. These observations provide important information about the development of subunit vaccines for the potential application to the clinical control of HHS in chickens.
5.4 FAdV as vector
HAdV vectors represent one of the most efficient systems for gene delivery in vivo for vaccine application. As potential vaccine vectors, AdVs have been developed for preventing different infectious diseases such as HIV [145], In-fluenza [146], Ebola [147], Dengue [148] et al. Similarly, FAdVs have such a potential to be used as vectors for gene delivery in vivo to elicit an immune response against pathogenic infections in poultry. Most FAdV vectors were constructed by replacement of non-essential DNA sequences with foreign genes at the right end genomic region of FAdV-1, FAdV-8, FAdV-9 and FAdV-10. It was reported that the CELO could be used as a vector expressing the capsid protein VP2 of IBDV to confer chickens with complete protection against a lethal infection with very virulent IBDV (vvIBDV) strain [149]. FAdV-10 recombinant expressing VP2 of IBDV also induced protective im-munity against bursal disease [150]. FAdV-8 has been constructed to express antibody fragments scFv, which targets and neutralizes IBDV [151]. It was reported that recombinant FAdV expressing the S1 gene from infectious bronchitis virus (IBV) was sufficient to protect chickens at the trachea, the primary site of infection by IBV [152]. FAdV-9 could serve as a suitable vaccine vector due to large stretches of nonessential DNA sequences in their genomes, and chickens immunized with FAdV-9 recombinant viruses developed antibodies against the foreign antigens [153-155].
Generation of infectious clones based on a nonpathogenic FAdV-4 or dele-tion/mutation of virulence genes of circulating viral strain by reverse genetic manipulation technique will no doubt facilitate the development of effective live vaccines, avoid risking the reversion to virulence in attenuated live vac-cines developed by blind-passages, and thus represent the future trends in vaccine development. Meanwhile, similar to HAdV, FAdV could also serve as a useful vector for gene delivery when appropriately constructed [156]. Thus, FAdV-4 has a great potential of being constructed as a useful vector for gene-deliver as well as an attenuated live vaccine for prevention and control of HHS and other avian diseases.
6. Conclusion
Although HAdVs are well studied and have served as gene transfer vectors for gene therapy, the current understandings of the pathogenesis of FAdV infection are still insufficient. FAdVs are distributed worldwide, causing enormous economic losses to the poultry industry across the global. Vaccination is the most successful and economically effective strategy to reduce the mortality and morbidity of infectious diseases. Therefore, there is and will be an urgent need to develop effective vaccines against diseases caused by FAdVs using modern molecular techniques. Elucidation of the pathogenesis of FAdV infection will be definitely required to provide helpful clues to the development of novel vaccines. In addition to the three major structural proteins (hexon, fiber and penton base), more viral proteins of FAdV should be investigated for their possible roles in pathogenesis of FAdV infection and for their potential use as subunit vaccine candidates. Maternally derived neutralizing antibodies are important in protecting chicks against FAdV infection and development of clinical disease. Currently, the protection of chickens by vaccination with inactivated vaccines, attenuated vaccine or recombinant vaccines is still not completely satisfactory. Thus, more efforts will be required to investigate the pathogenesis of FAdV infection and develop more effective vaccines for clinical use. Of note, recent years FAdV-4 has attracted much attention as a potential vector for foreign gene-delivery, which may serve as a recombinant live vaccine for the prevention and control of HHS and other avian diseases.
Acknowledgements
This work was supported by China Agriculture Research System of MOF and MARA (#CARS-40).
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Van DEM, Don PA, Kipps A. The isolation in eggs of a new filterable agent which may be the cause of bovine lumpy skin disease. Journal of general microbiology 3 (1949): 174-183.
- Yates VJ, Fry DE. Observations on a chicken embryo lethal orphan (CELO) virus. American Journal of Veterinary Research 18 (1957): 657-660.
- Burmester BR, Sharpless GR, Fontes AK. Virus Isolated from Avian Lymph-omas Unrelated to Lympho-matosis Virus. Journal of the National Cancer Institute 6.
- Olson NO. A respiratory disease (bron-chitis) of quail caused by a virus. Veterinary medicine 46 (1951): 22.
- Rowe WP, Huebner RJ, Gilmore LK, et al. Isolation of a cytopathogenic agent from human adenoids undergoing spont-aneous degeneration in tissue culture. Proc Soc Exp Biol Med 84 (1953): 570-573.
- Benko M, Harrach B. Molecular evolu-tion of adenoviruses. Curr Top Microbiol Immunol 272 (2003): 3-35.
- Nagy E, Corredor JC. Avian Virology-Current Research and Future Trends. Samal SK, editor. Norfolk, UK: Caister Academic Press (2019): 283-344.
- Zsak L, Kisary J. Characterisation of adenoviruses isolated from geese. Avian Pathol 13 (1984): 253-264.
- Hess M. Detection and differentiation of avian adenoviruses: a review. Avian Pathol 29 (2000): 195-206.
- Mohamed MHA, El-Sabagh IM, Abde-laziz AM, et al. Molecular charac-terization of fowl aviadenoviruses speci-es D and E associated with inclusion body hepatitis in chickens and falcons indicates possible cross-species transmission. Avian Pathol 47 (2018): 384-390.
- Xia J, Yao KC, Liu YY, et al. Isolation and molecular characterization of prevalent Fowl adenovirus strains in southwestern China during 2015-2016 for the development of a control strategy. Emerg Microbes Infect 6 (2017): e103.
- Grafl B, Prokofieva I, Wernsdorf P, et al. Infection with an apathogenic fowl adenovirus serotype-1 strain (CELO) prevents adenoviral gizzard erosion in broilers. Vet Microbiol 172 (2014): 177-185.
- Niczyporuk JS. Phylogenetic and geo-graphic analysis of fowl adenovirus field strains isolated from poultry in Poland. Arch Virol 161 (2016): 33-42.
- Schachner A, Matos M, Grafl B, et al. Fowl adenovirus-induced diseases and strategies for their control - a review on the current global situation. Avian Pathol 47 (2018): 111-126.
- Schachner A, Marek A, Grafl B, et al. Detailed molecular analyses of the hexon loop-1 and fibers of fowl aviadenoviruses reveal new insights into the antigenic relationship and confirm that specific genotypes are involved in field outbreaks of inclusion body hepatitis. Veterinary Microbiology 186 (2016): 13-20.
- Mirzazadeh A, Asasi K, Schachner A, et al. Gizzard Erosion Associated with Fowl Adenovirus Infection in Slaughtered Broiler Chickens in Iran. Avian Dis 63 (2019): 568-576.
- Garmyn A, Bosseler L, Braeckmans D, et al. Adenoviral Gizzard Erosions in Two Belgian Broiler Farms. Avian Dis 62 (2018): 322-325.
- Schade B, Schmitt F, Bohm B, et al. Adenoviral gizzard erosion in broiler chickens in Germany. Avian Dis 57 (2013): 159-163.
- Okuda Y, Ono M, Shibata I, et al. Pathogenicity of serotype 8 fowl adeno-virus isolated from gizzard erosions of slaughtered broiler chickens. J Vet Med Sci 66 (2004): 1561-1566.
- Li H, Wang J, Qiu L, Han Z, Liu S. Fowl adenovirus species C serotype 4 is attributed to the emergence of hepatitis-hydropericardium syndrome in chickens in China. Infect Genet Evol 45 (2016): 230-41.
- Li L, Wang J, Chen P, et al. Pathogenicity and molecular character-ization of a fowl adenovirus 4 isolated from chicken associated with IBH and HPS in China. BMC Vet Res 14 (2018): 400.
- Lv L, Lu H, Wang K, et al. Emerging of a novel natural recombinant fowl adeno-virus in China. Transbound Emerg Dis 68 (2021): 283-288.
- Li PH, Zheng PP, Zhang TF, et al. Fowl adenovirus serotype 4: Epidemiology, pathogenesis, diagnostic detection, and vaccine strategies. Poult Sci 96 (2017): 2630-2640.
- Wang Z, Zhao J. Pathogenesis of Hypervirulent Fowl Adenovirus Sero-type 4: The Contributions of Viral and Host Factors. Viruses 11 (2019).
- Fitzgerald SD, Rautenschlein S, Mahsoub HM, et al. Adenovirus Infec-tions. Diseases of Poultry (2020): 321-363.
- Schachner A, Grafl B, Hess M. Spotlight on avian pathology: fowl adenovirus (FAdV) in chickens and beyond unresolved host-pathogen interplay. Avian Pathol 50 (2021): 2-5.
- Griffin BD, Nagy E. Coding potential and transcript analysis of fowl adeno-virus 4: insight into upstream ORFs as common sequence features in adenoviral transcripts. J Gen Virol 92 (2011): 1260-1272.
- Ojkic D, Nagy E. The complete nucleotide sequence of fowl adenovirus type 8. J Gen Virol 81 (2000): 1833-1837.
- Chiocca S, Kurzbauer R, Schaffner G, et al. The complete DNA sequence and genomic organization of the avian adenovirus CELO. J Virol 70 (1996): 2939-2949.
- Gelderblom H, Maichle-Lauppe I. The fibers of fowl adenoviruses. Arch Virol 72 (1982): 289-298.
- Hess M, Cuzange A, Ruigrok RW, et al. The avian adenovirus penton: two fibres and one base. J Mol Biol 252 (1995): 379-385.
- San Martin C. Latest insights on adeno-virus structure and assembly. Viruses 4 (2012): 847-877.
- Chen L, Yin L, Zhou Q, et al. Epidemiological investigation of fowl adenovirus infections in poultry in China during 2015–2018. BMC Veterinary Research 15 (2019): 271.
- Bergelson JM, Cunningham JA, Droguett G, et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 275 (1997): 1320-1323.
- Wolfrum N, Greber UF. Adenovirus signalling in entry. Cell Microbiol 15 (2013): 53-62.
- Pan Q, Wang J, Gao Y, et al. Identification of chicken CAR homo-logy as a cellular receptor for the emer-ging highly pathogenic fowl adenovirus 4 via unique binding mechanism. Emerg Microbes Infect 9 (2020): 586-596.
- Li X, Bangari DS, Sharma A, Mittal SK. Bovine adenovirus serotype 3 utilizes sialic acid as a cellular receptor for virus entry. Virology 392 (2009): 162-168.
- Sun J, Zhang Y, Gao S, et al. Pathogenicity of fowl adenovirus sero-type 4 (FAdV-4) in chickens. Infect Genet Evol 75 (2019): 104017.
- Manzoor S, Hussain Z, Rahman SU, et al. Identification of antibodies against hydropericardium syndrome in wild birds. Br Poult Sci 54 (2013): 325-328.
- Gjevre AG, Kaldhusdal M, Eriksen GS. Gizzard erosion and ulceration syndrome in chickens and turkeys: a review of causal or predisposing factors. Avian Pathol 42 (2013): 297-303.
- Hess M, Prusas C, Vereecken M, et al. Isolation of fowl adenoviruses serotype 4 from pigeons with hepatic necrosis. Berl Munch Tierarztl Wochenschr 111 (1998): 140-142.
- Wei Z, Liu H, Diao Y, et al. Patho-genicity of fowl adenovirus (FAdV) serotype 4 strain SDJN in Taizhou geese. Avian Pathol 48 (2019): 477-485.
- Chen H, Dou Y, Zheng X, et al. Hydropericardium Hepatitis Syndrome Emerged in Cherry Valley Ducks in China. Transbound Emerg Dis 64 (2017): 1262-1267.
- Anjum AD, Sabri MA, Iqbal Z. Hydro-pericarditis syndrome in broiler chickens in Pakistan. Vet Rec 124 (1989): 247-248.
- Kumar R, Chandra R, Shukla SK, et al. Hydropericardium syndrome (HPS) in India: a preliminary study on the causa-tive agent and control of the disease by inactivated autogenous vaccine. Trop Anim Health Prod 29 (1997): 158-164.
- abdul-Aziz TA, al-Attar MA. New syndrome in Iraqi chicks. Vet Rec 129 (1991): 272.
- Borisov V, Borisov A, Gusev A. Hydropericardium syndrome in chickens in Russia. Proc Proceedings of the Tenth International Congress of World Veter-inary Poultry Association; Buda-pest, Hungary (1997).
- Marek A, Gunes A, Schulz E, et al. Classification of fowl adenoviruses by use of phylogenetic analysis and high-resolution melting-curve analysis of the hexon L1 gene region. J Virol Methods 170 (2010): 147-154.
- Toro H, Prusas C, Raue R, et al. Characterization of fowl adenoviruses from outbreaks of inclusion body heap-titis/hydropericardium syndrome in Chile. Avian Dis 43 (1999): 262-270.
- Cowen BS, Lu H, Weinstock D, et al. Pathogenicity studies of fowl adeno-viruses isolated in several regions of the world. International Symposium on Adenovirus Infection in Poultry (1996): 79-88.
- Abe T, Nakamura K, Tojo H, et al. Histology, immunohistochemistry, and ultrastructure of hydro-pericardium syn-drome in adult broiler breeders and broiler chicks. Avian Dis 42 (1998): 606-612.
- Kim JN, Byun SH, Kim MJ, et al. Outbreaks of hydropericardium syn-drome and molecular characterization of Korean fowl adenoviral isolates. Avian Dis 52 (2008): 526-530.
- Zhao J, Zhong Q, Zhao Y, et al. Pathogenicity and Complete Genome Characterization of Fowl Adeno-viruses Isolated from Chickens Associated with Inclusion Body Hepatitis and Hydro-pericardium Syndrome in China. PLoS One 10 (2015): e0133073.
- Liu Y, Wan W, Gao D, et al. Genetic characterization of novel fowl aviade-novirus 4 isolates from outbreaks of hepatitis-hydropericardium syndrome in broiler chickens in China. Emerg Microbes Infect 5 (2016): e117.
- Ye J, Liang G, Zhang J, et al. Outbreaks of serotype 4 fowl adenovirus with novel genotype, China. Emerg Microbes Infect 5 (2016): e50.
- Jaffery MS. A treatise on Angara disease (hydropericardium-pulmonary oedema-hepatonephritis syndrome. Journal of the Pakistan Veterinary Medical Association 34 (1988): 1-33.
- Jianqiang Ye, Guangchen Liang, Jianjun Zhang, et al. Outbreaks of serotype 4 fowl adenovirus with novel genotype, China. Emerging microbes and infec-tions 5 (2016): e50.
- Yin D, Xue M, Yang K, et al. Molecular characterization and pathogenicity of highly pathogenic fowl adenovirus serotype 4 isolated from laying flock with hydropericardium-hepatitis syn-drome. Microb Pathog 147 (2020): 104381.
- Niu YJ, Sun W, Zhang GH, et al. Hydropericardium syndrome outbreak caused by fowl adenovirus serotype 4 in China in 2015. J Gen Virol 97 (2016): 2684-2690.
- Kumar R, Kumar V, Asthana M, et al. Isolation and identification of a fowl adenovirus from wild Black Kites (Milvus migrans). J Wildl Dis 46 (2010): 272-276.
- Changjing L, Haiying L, Dongdong W, et al. Characterization of fowl adeno-viruses isolated between 2007 and 2014 in China. Vet Microbiol 197 (2016): 62-67.
- Wang X, Li D, Deng Y, et al. Molecular characterization and pathogenicity of a fowl adenovirus serotype 4 isolated from peacocks associated with hydroperi-cardium hepatitis syndrome. Infect Genet Evol 90 (2021): 104766.
- Wu N, Yang B, Wen B, et al. Patho-genicity and Immune Responses in Specific-Pathogen-Free Chickens during Fowl Adenovirus Serotype 4 Infection. Avian Dis 64 (2020): 315-323.
- Asrani RK, Gupta VK, Sharma SK, et al. Hydropericardium-hepatopathy syn-drome in Asian poultry. Vet Rec 141 (1997): 271-273.
- Niu Y, Sun Q, Zhang G, et al. Fowl adenovirus serotype 4-induced apop-tosis, autophagy, and a severe infla-mmatory response in liver. Vet Microbiol 223 (2018): 34-41.
- Mase M, Nakamura K, Minami F. Fowl adenoviruses isolated from chickens with inclusion body hepatitis in Japan, 2009-2010. J Vet Med Sci 74 (2012): 1087-1089.
- Ojkic D, Martin E, Swinton J, et al. Genotyping of Canadian isolates of fowl adenoviruses. Avian Pathol 37 (2008): 95-100.
- Morshed R, Hosseini H, Langeroudi AG, et al. Fowl Adenoviruses D and E Cause Inclusion Body Hepatitis Out-breaks in Broiler and Broiler Breeder Pullet Flocks. Avian Dis 61 (2017): 205-210.
- Steer-Cope P, Sandy J, O'Rourke D, et al. Chronologic Analysis of Gross and Histologic Lesions Induced by Field Strains of FAdV-1, FAdV-8b, and FAdV-11 in Six-Week-Old Chickens. Avian Dis 61 (2017): 512-519.
- Davison AJ, Benko M, Harrach B. Genetic content and evolution of adeno-viruses. J Gen Virol 84 (2003): 2895-2908.
- Nemerow GR, Pache L, Reddy V, et al. Insights into adenovirus host cell inter-actions from structural studies. Virology 384 (2009): 380-388.
- Mo KK, Lyu CF, Cao SS, et al. Pathogenicity of a FAdV-4 isolate to chickens and its genomic analysis. J Zhejiang Univ Sci B 20 (2019): 740-752.
- Zhang Y, Liu R, Tian K, et al. Fiber2 and hexon genes are closely associated with the virulence of the emerging and highly pathogenic fowl adenovirus 4. Emerg Microbes Infect 7 (2018): 199.
- Roberts MM, White JL, Grutter MG, et al. Three-dimensional structure of the adenovirus major coat protein hexon. Science 232 (1986): 1148-1151.
- Niczyporuk JS. Deep analysis of Loop L1 HVRs1-4 region of the hexon gene of adenovirus field strains isolated in Poland. PLoS One 13 (2018): e0207668.
- Norfitriah MS, Hair-Bejo M, Omar AR, et al. Hexon and fiber gene changes in an attenuated fowl adenovirus isolate of Malaysia in chickens embryonated eggs and its infectivity in chickens. Journal of veterinary science (Suwon-si, Korea) (2018).
- Zhang Y, Liu R, Tian K, et al. Fiber2 and hexon genes are closely associated with the virulence of the emerging and highly pathogenic fowl adenovirus 4. Emerging Microbes and Infections 7 (2018).
- Zhang Y, Liu A, Wang Y, et al. A Single Amino Acid at Residue 188 of Hexon Protein is Responsible for the Pathogenicity of the Emerging Novel Fowl Adenovirus 4. J Virol (2021): JVI0060321.
- Raue R, Hess M. Hexon based PCRs combined with restriction enzyme analysis for rapid detection and differ-rentiation of fowl adenoviruses and egg drop syndrome virus. J Virol Methods 73 (1998): 211-217.
- Guy, Meulemans, Bernard, et al. Phylogenetic analysis of fowl adeno-viruses. Avian Pathology (2010).
- Pizzuto MS, De Battisti C, Marciano S, et al. Pyrosequencing analysis for a rapid classification of fowl adenovirus species. Avian Pathology 39 (2010): 391-398.
- Li Y, Fu J, Chang S, et al. Isolation, identification, and hexon gene characterization of fowl adenoviruses from a contaminated live Newcastle disease virus vaccine. Poultry Science 96 (2016): 1094.
- Kaján GL, Affranio I, Bistyák AT, et al. An emerging new fowl adenovirus genotype. Heliyon 5 (2019): e01732.
- Mase M, Hiramatsu K, Nishijima N, et al. Identification of specific serotypes of fowl adenoviruses isolated from diseased chickens by PCR. J Vet Med Sci 83 (2021): 130-133.
- Lai VD, Min K, Lai HTL, et al. Epidemiology of fowl adenovirus (FAdV) infections in South Korean chickens during 2013-2019 following introduction of FAdV-4 vaccines. Avian Pathol 50 (2021): 182-189.
- Choi KS, Kye SJ, Kim JY, et al. Epidemiological investigation of out-breaks of fowl adenovirus infection in commercial chickens in Korea. Poult Sci 91 (2012): 2502-2506.
- Russell WC. Adenoviruses: update on structure and function. J Gen Virol 90 (2009): 1-20.
- Xu L, Benson SD, Burnett RM. Nanoporous crystals of chicken embryo lethal orphan (CELO) adenovirus major coat protein, hexon. J Struct Biol 157 (2007): 424-431.
- Ganesh K, Suryanarayana V, Raghavan R, et al. Nucleotide sequence of L1 and part of P1 of hexon gene of fowl adenovirus associated with hydroperi-cardium hepatitis syndrome differs with the corresponding region of other fowl adenoviruses. Vet Microbiol 78 (2001): 1-11.
- McFerran JB, Adair BM. Avian adeno-viruses--a review. Avian Pathol 6 (1977): 189-217.
- Mansoor MK, Hussain I, Arshad M, et al. Preparation and evaluation of chicken embryo-adapted fowl adenovirus sero-type 4 vaccines in broiler chickens. Trop Anim Health Prod 43 (2011): 331-338.
- Gao J, Zhao M, Duan X, et al. Requirement of Cellular Protein CCT7 for the Replication of Fowl Adenovirus Serotype 4 (FAdV-4) in Leghorn Male Hepatocellular Cells Via Interaction with the Viral Hexon Protein. Viruses 11 (2019).
- Chroboczek J, Ruigrok RW, Cusack S. Adenovirus fiber. Curr Top Microbiol Immunol 199 ( Pt 1) (1995): 163-200.
- Valentine RC, Pereira HG. Antigens and structure of the adenovirus. Reprinted from J. Mol. Biol. 1965; 13: 13-20. Rev Med Virol 13 (2003): 71-82.
- Pallister J, Wright PJ, Sheppard M. A single gene encoding the fiber is responsible for variations in virulence in the fowl adenoviruses. J Virol 70 (1996): 5115-5122.
- Roelvink PW, Lizonova A, Lee JG, et al. The coxsackievirus-adenovirus rec-eptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J Virol 72 (1998): 7909-7915.
- Tan PK, Michou AI, Bergelson JM, et al. Defining CAR as a cellular receptor for the avian adenovirus CELO using a genetic analysis of the two viral fibre proteins. J Gen Virol 82 (2001): 1465-1472.
- Grgic H, Krell PJ, Nagy E. Comparison of fiber gene sequences of inclusion body hepatitis (IBH) and non-IBH strains of serotype 8 and 11 fowl adeno-viruses. Virus Genes 48 (2014): 74-80.
- Wang W, Liu Q, Li T, et al. Fiber-1, Not Fiber-2, Directly Mediates the Infection of the Pathogenic Serotype 4 Fowl Adenovirus via Its Shaft and Knob Domains. Journal of Virology 94 (2020).
- Schachner A, Marek A, Jaskulska B, et al. Recombinant FAdV-4 fiber-2 protein protects chickens against hepatitis-hydropericardium syndrome (HHS). Vaccine 32 (2014): 1086-1092.
- Ruan S, Zhao J, Yin X, et al. A subunit vaccine based on fiber-2 protein provides full protection against fowl adenovirus serotype 4 and induces quicker and stronger immune responses than an inactivated oil-emulsion vaccine. Infect Genet Evol 61 (2018): 145-150.
- Xie Q, Wang W, Li L, et al. Domain in Fiber-2 interacted with KPNA3/4 signi-ficantly affects the replication and pathogenicity of the highly pathogenic FAdV-4. Virulence 12 (2021): 754-765.
- Sheppard M, Trist H. Characterization of the avian adenovirus penton base. Virology 188 (1992): 881-886.
- Smith JG, Wiethoff CM, Stewart PL, et al. Curr Top Microbiol Immunol 343 (2010): 195-224.
- Chigbu DI, Labib BA. Pathogenesis and management of adenoviral keratocon-junctivitis. Infect Drug Resist 11 (2018): 981-993.
- Shah MA, Ullah R, March M, et al. Overexpression and characterization of the 100K protein of Fowl adenovirus-4 as an antiviral target. Virus Res 238 (2017): 218-225.
- Yan J, Dong J, Wu J, et al. Interaction between hexon and L4-100K determines virus rescue and growth of hexon-chimeric recombinant Ad5 vectors. Sci-entific Reports 6 (2016): 22464.
- Hong SS, Szolajska E, Schoehn G, et al. The 100K-chaperone protein from adenovirus serotype 2 (Subgroup C) assists in trimerization and nuclear localization of hexons from subgroups C and B adenoviruses. J Mol Biol 352 (2005): 125-138.
- Xie Z, Luo S, Fan Q, et al. Detection of antibodies specific to the non-structural proteins of fowl adenoviruses in infected chickens but not in vaccinated chickens. Avian Pathol 42 (2013): 491-496.
- Shah MS, Ashraf A, Khan MI, et al. Molecular cloning, expression and characterization of 100K gene of fowl adenovirus-4 for prevention and control of hydropericardium syndrome. Bio-logicals 44 (2016): 19-23.
- Lee TWR, Lawrence FJ, Dauksaite V, et al. Precursor of human adenovirus core polypeptide Mu targets the nucleolus and modulates the expression of E2 proteins. J Gen Virol 85 (2004): 185-196.
- Zhao M, Duan X, Wang Y, et al. A Novel Role for PX, a Structural Protein of Fowl Adenovirus Serotype 4 (FAdV4), as an Apoptosis-Inducer in Leghorn Male Hepatocellular Cell. Viruses 12 (2020).
- Zhao W, Li X, Li H, et al. Fowl adenoviruse-4 infection induces strong innate immune responses in chicken. Comp Immunol Microbiol Infect Dis 68 (2020): 101404.
- Li R, Li G, Jing L, et al. Fowl Adenovirus Serotype 4 SD0828 Infec-tions Causes High Mortality Rate and Cytokine Levels in Specific Pathogen-Free Chickens Compared to Ducks. Front Immunol 9 (2018): 49.
- Zhao W, Li X, Li H, et al. Fowl adenoviruse-4 infection induces strong innate immune responses in chicken. Comparative Immunology, Microbio-logy and Infectious Diseases 68 (2019): 101404.
- Zhang J, Zou Z, Huang K, et al. Insights into leghorn male hepatocellular cells response to fowl adenovirus serotype 4 infection by transcriptome analysis. Vet Microbiol 214 (2018): 65-74.
- Grgic H, Poljak Z, Sharif S, et al. Pathogenicity and cytokine gene expre-ssion pattern of a serotype 4 fowl adenovirus isolate. PLoS One 8 (2013): e77601.
- Deng L, Sharif S, Nagy E. Oral inoculation of chickens with a candidate fowl adenovirus 9 vector. Clin Vaccine Immunol 20 (2013): 1189-1196.
- Grgic H, Sharif S, Haghighi HR, et al. Cytokine patterns associated with a sero-type 8 fowl adenovirus infection. Viral Immunol 26 (2013): 143-149.
- Niu Y, Sun Q, Liu X, et al. Mechanism of fowl adenovirus serotype 4-induced heart damage and formation of pericarp-dial effusion. Poult Sci 98 (2019): 1134-1145.
- Zheng SJ. Veterinary Molecular Immunology. Ist ed. Peking,China: China Agricultural Press; (2015): 133.
- Lal B, Maiti NK, Oberoi MS, Sharma SN. Cell mediated immune response of chicks following fowl adenovirus type-1 infection. Comp Immunol Microbiol Infect Dis 14 (1991): 55-8.
- Schonewille E, Singh A, Gobel TW, Gerner W, Saalmuller A, Hess M. Fowl adenovirus (FAdV) serotype 4 causes depletion of B and T cells in lymphoid organs in specific pathogen-free chic-kens following experimental infection. Vet Immunol Immunopathol 121 (2008): 130-9.
- Schachner A, Marek A, Grafl B, Hess M. Detailed molecular analyses of the hexon loop-1 and fibers of fowl aviadenoviruses reveal new insights into the antigenic relationship and confirm that specific genotypes are involved in field outbreaks of inclusion body hepatitis. Vet Microbiol 186 (2016): 13-20.
- Matos M, Grafl B, Liebhart D, Hess M. The outcome of experimentally induced inclusion body hepatitis (IBH) by fowl aviadenoviruses (FAdVs) is crucially influenced by the genetic background of the host. Vet Res 47 (2016): 69.
- McFerran JB, Smyth J. Avian adenoviruses. Revue scientifique et tech-nique (International Office of Epi-zootics) 19 (2000): 589-601.
- Girshick T, Crary CK, Luginbuhl RE. Serologic detection of adenovirus infections in specific-pathogen-free chickens. Avian Dis 24 (1980): 527-531.
- Saifuddin M, Wilks CR. Effects of fowl adenovirus infection on the immune system of chickens. J Comp Pathol 107 (1992): 285-294.
- Asthana M, Chandra R, Kumar R. Hydropericardium syndrome: current state and future developments. Arch Virol 158 (2013): 921-931.
- Dhama K, Singh SD, Barathidasan R, et al. Emergence of Avian Infectious Bron-chitis Virus and its variants need better diagnosis, prevention and control strate-gies: a global perspective. Pak J Biol Sci 17 (2014): 751-767.
- Fadly AM, Winterfield RW. Antigenic characterization of the inclusion body hepatitis virus. Am J Vet Res 36 (1975): 532-534.
- Munir K, Muneer MA, Tiwari A, et al. Effects of polyether ionophores on the protective immune responses of broiler chickens against Angara disease and Newcastle disease viruses. Vet Res Commun 31 (2007): 909-929.
- Balamurugan V, Kataria JM. The hydropericardium syndrome in poultry--a current scenario. Vet Res Commun 28 (2004): 127-148.
- Toro H, Gonzalez C, Cerda L, et al. Prevention of inclusion body heap-titis/hydropericardium syndrome in progeny chickens by vaccination of breeders with fowl adenovirus and chicken anemia virus. Avian Dis 46 (2002): 547-554.
- Toro H, Gonzalez O, Escobar C, et al. Vertical induction of the inclusion body hepatitis/hydropericardium syndrome with fowl adenovirus and chicken anemia virus. Avian Dis 45 (2001): 215-222.
- Shah MS, Ashraf A, Khan MI, et al. Fowl adenovirus: history, emergence, biology and development of a vaccine against hydropericardium syndrome. Arch Virol 162 (2017): 1833-1843.
- Kim MS, Lim TH, Lee DH, et al. An inactivated oil-emulsion fowl Adeno-virus serotype 4 vaccine provides broad cross-protection against various serotypes of fowl Adenovirus. Vaccine 32 (2014): 3564-3568.
- Gupta A, Popowich S, Ojkic D, et al. Inactivated and live bivalent fowl adenovirus (FAdV8b+FAdV11) breeder vaccines provide broad-spectrum protect-tion in chicks against inclusion body hepatitis (IBH). Vaccine 36 (2018): 744-750.
- Alvarado IR, Villegas P, El-Attrache J, et al. Genetic characterization, pathogenicity, and protection studies with an avian adenovirus isolate associated with inclusion body hepatitis. Avian Dis 51 (2007): 27-32.
- Popowich S, Gupta A, Chow-Lockerbie B, et al. Broad spectrum protection of broiler chickens against inclusion body hepatitis by immunizing their broiler breeder parents with a bivalent live fowl adenovirus vaccine. Res Vet Sci 118 (2018): 262-269.
- Schonewille E, Jaspers R, Paul G, et al. Specific-pathogen-free chickens vaccinated with a live FAdV-4 vaccine are fully protected against a severe challenge even in the absence of neutralizing antibodies. Avian Dis 54 (2010): 905-910.
- Fingerut E, Gutter B, Gallili G, et al. A subunit vaccine against the adenovirus egg-drop syndrome using part of its fiber protein. Vaccine 21 (2003): 2761-2766.
- Shah MS, Ashraf A, Rahman M, et al. A subunit vaccine against hydroperi-cardium syndrome using adenovirus penton capsid protein. Vaccine 30 (2012): 7153-7156.
- Tufail S, Shah MA, Zafar M, et al. Identification of potent epitopes on hexon capsid protein and their evalua-tion as vaccine candidates against infections caused by members of Adeno-viridae family. Vaccine 39 (2021): 3560-3564.
- Fuchs JD, Bart PA, Frahm N, et al. Network NHVT. Safety and Immuno-genicity of a Recombinant Adenovirus Serotype 35-Vectored HIV-1 Vaccine in Adenovirus Serotype 5 Seronegative and Seropositive Individuals. J AIDS Clin Res 6 (2015).
- Webby RJ, Weaver EA. Centralized Consensus Hemagglutinin Genes Induce Protective Immunity against H1, H3 and H5 Influenza Viruses. PLoS One 10 (2015): e0140702.
- Ledgerwood JE, Costner P, Desai N, et al. A replication defective recombinant Ad5 vaccine expressing Ebola virus GP is safe and immunogenic in healthy adults. Vaccine 29 (2010): 304-313.
- Holman DH, Wang D, Raviprakash K, et al. Two complex, adenovirus-based vaccines that together induce immune responses to all four dengue virus serotypes. Clin Vaccine Immunol 14 (2007): 182-189.
- Francois A, Chevalier C, Delmas B, et al. Avian adenovirus CELO recom-binants expressing VP2 of infectious bursal disease virus induce protection against bursal disease in chickens. Vaccine 22 (2004): 2351-2360.
- Sheppard M, Werner W, Tsatas E, et al. Fowl adenovirus recombinant express-ing VP2 of infectious bursal disease virus induces protective immunity against bursal disease. Arch Virol 143 (1998): 915-930.
- Greenall SA, Tyack SG, Johnson MA, et al. Antibody fragments, expressed by a fowl adenovirus vector, is able to neutra-lize infectious bursal disease virus. Avian Pathol 39 (2010): 339-348.
- Johnson MA, Pooley C, Ignjatovic J, et al. A recombinant fowl adenovirus expressing the S1 gene of infectious bronchitis virus protects against chall-enge with infectious bronchitis virus. Vaccine 21 (2003): 2730-2736.
- Pei Y, Griffin B, de Jong J, et al. Rapid generation of fowl adenovirus 9 vectors. J Virol Methods 223 (2015): 75-81.
- Corredor JC, Nagy E. Antibody res-ponse and virus shedding of chickens inoculated with left end deleted fowl adenovirus 9-based recombinant viruses. Avian Dis 55 (2011): 443-446.
- Corredor JC, Pei Y, Nagy E. Fowl Adenovirus-Based Vaccine Platform. Methods Mol Biol 1581 (2017): 29-54.
- Pei Y, Corredor JC, Griffin BD, et al. Fowl Adenovirus 4 (FAdV-4)-Based Infectious Clone for Vaccine Vector Development and Viral Gene Function Studies. Viruses 10 (2018).



 Impact Factor: * 4.1
Impact Factor: * 4.1 Acceptance Rate: 75.32%
Acceptance Rate: 75.32%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 