Impact of Early Palliative Care on End of life in patients with Advanced Biliary Tract Cancer
Margaux Miralles1, Marie Muller1, Christophe Borg2, Sylvain Manfredi3, Anne Minello3, Olivier Bouché4, Marlène Tambou5, Didier Mutter6, Pascale Chiappa7, Jean-Emmanuel Kurtz7, Amandine Luc8, Cédric Baumann8, and Anthony Lopez*, 1
1Department of Hepato-Gastroenterology, University Hospital of Nancy, Lorraine University, Vandoeuvre-Lès-Nancy, France
2Department of Medical Oncology, University Hospital of Besançon, Besançon, France
3Department of Hepato-Gastroenterology, University Hospital of Dijon, Dijon, France
4Department of Hepato-Gastroenterology, University Hospital of Reims, Reims, France
5Department of Hepato-Gastroenterology, University Hospital of Strasbourg, Strasbourg, France
6Department of Digestive Surgery, University Hospital of Strasbourg, Strasbourg, France
7Department of Medical Oncology, Oncology Centre ICANS, Strasbourg, France
8MPI department, Methodology, data management and statistic Unit, University Hospital of Nancy, Vandœuvre-Lès-Nancy, France
*Corresponding author: Anthony Lopez. Department of Hepato-Gastroenterology, University Hospital of Nancy, Lorraine University, Vandoeuvre-Lès-Nancy, France
Received: 08 August 2022; Accepted: 17 August 2022; Published: 07 January 2023
Article Information
Citation: Margaux Miralles, Marie Muller, Christophe Borg, Sylvain Manfredi, Anne Minello, Olivier Bouché, Marlène Tambou, Didier Mutter, Pascale Chiappa, Jean-Emmanuel Kurtz, Amandine Luc, Cédric Baumann, and Anthony Lopez. Impact of Early Palliative Care on End of life in patients with Advanced Biliary Tract Cancer. Journal of Pharmacy and Pharmacology Research 7 (2022): 01-11.
View / Download Pdf Share at FacebookAbstract
Background: Most of patient with biliary tract cancer (BTC) have not access to surgery because of advanced/metastatic disease at diagnosis (aBTC). They receive palliative chemotherapy and/or palliative care (PC). We studied if early palliative care referral might influence overall survival (OS) and the aggressiveness of end-of-life care.
Participants and Design: We conducted a retrospective multicentric cohort study in patients with non-curative BTC, diagnosed between 2013 and 2019 in 6 hospitals of Eastern France. PC was defined a specialist-delivered palliative care encounter.
Results: 200 patients with aBTC were included. 87 (44%) never received PC, 30 (15%) had very early PC (< 3 months after aBTC diagnosis), 20 (10%) had an early PC (3-6 months), and 63 (32%) had late PC (>6 months). The median time between referral and death was 0.9 to 1.3 months. OS were 12.4 months (no PC), 3.0 months (PC<3 m), 6.4 months (PC 3-6 m), 16 months (PC>6 m). There was no evidence for survival improvement with early PC. PC tended to reduce chemotherapy near death (37% without PC, 30% with PC<3m, 11% with PC 3-6m, 10% with PC>6m), visits in emergency department (ED) during final month (respectively: 36%, 20 %, 15%, and 7%), intensive care unit hospitalizations (ICU) near death (13%, 0%, 0%, 2%). Place of death seemed to be positively impacted by PC (conventional acute unit, respectively: 73%, 21%, 21%, 25% and ICU or ED: 8%, 0%, 5%, 2%).
Conclusion: Referral to PC remains too late in the support of patients with aBTC. Our practice should evolve: all patients with aBTC should be referred to early PC in palliative care unit after diagnosis to improve the management of end-of-life, symptoms, and family needs. Trial registration number: 2019PI274, 05/28/2020.
Keywords
<p>Biliary tract cancer; cholangiocarcinoma; gallbladder carcinoma; palliative care; survival</p>
Article Details
Abbreviations:
BTC: Biliary tract cancer, aBTC: advanced biliary tract cancer, CCA: Cholangiocarcinoma (iCCA : intra hepatic cholangiocarcinoma, eCCA : extra hepatic cholangiocarcinoma, pCCA : peri hilar cholangiocarcinoma), ED: Emergency department, ICU: Intensive care unit, OS: Overall survival, PCU: Palliative care unit, PCT: Palliative care teams, PCR: Palliative care referral, PS: Performans status
1. Introduction
Biliary tract cancer (BTC) are rare primary liver cancers but its incidence is increasing [1]. Prognosis remains poor with overall survival (OS) reaching 6 to 11 months [1, 2]. That poor prognosis is attributed to challenges in early detection (due to delayed symptoms), low opportunity for radical resection and limited response to chemotherapy and/or radiotherapy. Indeed, only 20 to 30% of patients diagnosed with BTC have access to surgery, which is the only curative-intent treatment [3]. Therefore, most of patients receive palliative systemic therapy (chemotherapy, targeted therapy, and immunotherapy) or exclusive supportive care.
Palliative care teams (PCT) are composed of several actors making possible a multidisciplinary and global comprehensive care : physical, emotional, social (family caregivers…), spiritual (meaning, dignity, faith…), informational (prognosis, illness understanding…) [4]. Palliative care (PC) and systemic palliative therapy are complementary approaches, that should be associated early for more benefits. In 2017, the American Society of Clinical Oncology (ASCO) published recommendations about palliative care, specifying that palliative care should be introduce early in cancer management, at diagnosis and with the beginning of systemic therapy [5]. Despite these recommendations, and probably due to a lack of palliative care teams, patients’ referral to PCT in daily practice is often late (eg: no more therapeutic options, general condition too weak).
Recent studies demonstrated the positive impact of PC on aggressiveness of end-of-life care, that includes chemotherapy, intensive care unit admission, emergency department (ED) visit and acute unit hospitalization, during last month of life [6]. In pancreatic cancer, Jang et al. shown in a retrospective study the positive impact of a PC consultation on these above mentioned end-of-life indicators [7]. These results were confirmed in a prospective multicentric randomized controlled Italian trial including 206 patients with metastatic pancreatic cancer [8]. Recently, the concept of PC has evolved with the issue of the timing of palliative care referral (PCR), and the benefits of an early palliative care. An Australian retrospective study highlighted the use of early PC to improve aggressiveness of end-of-life care, compared to late PC (>90 days before death) in patients with pancreatic cancer [9].
Moreover, a retrospective study among patients in advanced lung cancer shown that early PC (30 to 365 after diagnosis) was significantly associated with an increase of OS (aHR, 0.47; 95% CI, 0.45-0.49) [10]. In 2021, a randomized study of advanced esophagogastric cancer also demonstrated an OS improvement in case of early interdisciplinary supportive care [11]. In the context of aBTC, which prognosis is poor with frequent uncomfortable symptoms as jaundice [12], anorexia, asthenia, abdominal pain, weight loss, psychological suffering ; palliative care team have a crucial role to provide a multidisciplinary take of care. To our knowledge, no study assessed early PCR in aBTC patients. We conducted a retrospective multicentric study to assess the relationship between early PCR and survival (primary outcome) and to explore the impact of early PCR on treatment aggressiveness near the end of life.
2. Patients and Methods
2.1 Study Design
We conducted a retrospective multicentric study including patients with a diagnosis of aBTC (cholangiocarcinoma and gallbladder) between January 1, 2013 and December 31, 2019. We included patients of 6 University hospitals of Eastern France (Nancy University Hospital, Besançon University Hospital, Dijon University Hospital, Reims University Hospital, Strasbourg University Hospital, and Strasbourg ICANS Centre for the Treatment of Cancer). Follow-up data were available until January 2021. Detailed clinical and pathology data were collected for all patients through a custom-made questionnaire providing epidemiological informations, data about aBTC (age at diagnosis, risk factors, treatment) and palliative care support (time to BSC referral, end-of-life acute events and condition). When necessary, we consulted medical files on site. Ethics approval was obtained from the Ethics Committee of Nancy CHU (September 8, 2020), Licence number 272. Patients received an information letter and had 30 days to ask their withdrawal from the study. After this delay, they were considered as participants.
2.2 Cohort Selection
Patients were eligible if they were ≥ 18 years old, had and histologically confirmed, locally advanced or metastatic cholangiocarcinoma or gallbladder adenocarcinoma diagnosed between January 1, 2013 and December 31, 2019. Patients were included regardless their Eastern Cooperative Oncology Groupe (ECOG) performance status
Patients were excluded if they died within a week after diagnosis and if insufficient clinical data were available.
2.3 Exposure
PC was defined as a specialist-delivered palliative care encounter received inpatient or outpatient setting after aBTC diagnosis. It could be with a mobile PCT, in hospitalization (palliative care units, PCU) or in consultation. Data were collected in electronic medical records (DxCare software). Four groups were defined according to the date of the first PCT meeting: patients who met the PCT less than three months after diagnosis (early PC), 3-6 months after aBTC diagnosis, more than 6 months after aBTC diagnosis (late PCC) or no PC.
2.4 Study Objectives
Primary outcome was OS after aBTC diagnosis according to PCR. We secondary evaluated the impact of early PCR on the aggressiveness of end-of-life care, which included place of death (at home with home hospitalization, at home without home hospitalization, intensive care unit, conventional acute unit, palliative care unit), last-month chemotherapy, last-month ED visit and last-month intensive care unit hospitalization. Continuous variables were summarized by descriptive statistics (number of cases, mean, median, quartile) and categorical variables were summarized with percentages. We also compared the most frequently reported reasons to referral and proposals made by PCT.
2.5 Statistical Analysis
Characteristics of sample were described by percentage for categorical variables and median with inter-quartile range [Q1-Q3] for continued variables. OS was calculated from the date of diagnosis of advanced BTC until the date of death or date of last follow-up and Kaplan-Meier curve was realized for each group. A second analysis was made by calculating the time from the date of first meeting with PC until the date of death or date of last follow-up. To address potential selection bias, we have planned to make a propensity score of receiving palliative care, composed of metastatic stage, age (>75 years old), performans status OMS (2, 3 and 4), Charlson score (>3). This approach minimized selection bias by comparing patients with a similar likelihood of receiving PC. Due to heterogeneity between study groups, propensity score has not been achieved. Clinical characteristics among these four groups were too different to produce a reliable comparison. The significant threshold was fixed at 5%. All analyses were performed with SAS® software version 9.4 (SAS Institute, Cary, NC, USA).
3 Results
3.1 Patients’ Characteristics
Two hundred patients with aBTC were included in our study. Median age at aBTC diagnosis was 67.6 years old (20-91 years old). 61% of the patients were men (Table 1). Most of the tumours were intrahepatic cholangiocarcinoma (iCCA) (51%), then perihilar (pCCA) (24%), gallbladder (18%) and distal CCA (7%). 62.5% patients had distant metastasis at diagnosis and 37.5% were locally advanced. About well-known BTC risk factors, 27% of patients were smokers and 18% had a heavy alcohol consumption, 19% were obese (body mass index ≥ 30 kg/m2), 31% had type II diabetes, 14% had a cirrhosis. Only 2 patients had a primary sclerosing cholangitis. The most common symptoms at diagnosis were abdominal pain (40%), jaundice (33%), asthenia (31%) and anorexia (28%). A quarter (26%) had a prior curative-intent treatment before their relapse (surgery or locoregional treatment) and then a non-curative recurrence. Systemic palliative chemotherapy was prescribed for 82.5% of the patients, and 17.5% received exclusive supportive care.
Among the 165 patients who received chemotherapy, the most frequent (67%) first-line regimen was gemcitabine + oxaliplatine (GEMOX). Other protocols administred as first line palliative therapy where gemcitabine + cisplatin (14%) and gemcitabine alone (10%). More than half of the patients received a second-line chemotherapy (56%), mainly 5FU-based regimen (54% received FOLFIRI).
Table 1: Patients’ characteristics
|
Variable |
Total (n=200) |
|
Age, years (median, IQR) |
67.6 (60.7 – 74.4) |
|
Gender, n (%) |
|
|
Male |
123 (61.5%) |
|
Female |
77 (38.5%) |
|
Performance status, n (%) |
|
|
0-1 |
152 (77.2%) |
|
2 |
29 (14.7%) |
|
3-4 |
16 (8.1%) |
|
missing |
3 |
|
Denutrition (IMC <18.5 or <21 for patients older than 70 years) |
7 (3.7%) |
|
RISK FACTORS of biliary tract cancer |
|
|
Obesity (IMC >30), n (%%) |
36 (18.8%) |
|
Diabetes, n (%) |
62 (31.0%) |
|
Smoker, n (%) |
53 (26.5%) |
|
Chronic alcoholism, n (%) |
36 (18.0%) |
|
Cirrhosis, n (%) |
28 (14.0 %) |
|
Primary sclerosing cholangitis, n (%) |
2 (1.0%) |
|
DIAGNOSIS |
|
|
Primary tumor site, n (%) |
|
|
Intrahepatic |
101 (50.8%) |
|
Perihilar (=Klatskin) |
48 (24.1%) |
|
Gallblader |
35 (17.6%) |
|
Distal |
14 (7.0%) |
|
Hepatocholangiocarcinoma |
1 (0.5%) |
|
Missing |
1 |
|
Disease status, n (%) |
|
|
Locally advanced |
75 (37.5%) |
|
Metastatic |
125 (62.5%) |
|
Symptoms at diagnosis (4 most common symptoms for 197 patients with available data) |
|
|
Abdominal pain |
79 (40.1%) |
|
Jaundice |
65 (33.0%) |
|
Asthenia |
61 (31.0%) |
|
Anorexia |
56 (28.4%) |
|
Absence of symptoms |
47 (23.9%) |
|
Albumine median (g/L) (N=175, IQR) |
34 (29 – 39) |
|
CA 19-9 median (N=153, IQR) (kUI/L) |
263 (27 – 1954) |
|
TREATMENT |
|
|
Prior curative-intent treatment, n (%) |
52 (26.0%) |
|
Chemotherapy received, n (%) |
|
|
Yes |
165 (82.5%) |
|
No |
35 (17.5%) |
|
Type of L1 chemotherapy regimen, n (%) |
N=165 (82.5%) |
|
Among 165 patients who received L1 chemotherapy (N=165) |
|
|
GEMOX |
110 (66.7%) |
|
GEMCIS |
23 (13.9%) |
|
GEMCITABINE |
16 (9.7%) |
|
Others or unknow |
16 (9.7%) |
|
Patients treated with L2 chemotherapy, n (%) |
N= 93/165 (56.4%) |
|
FOLFIRI |
42/93 (45.2%) |
|
LV5FU2 Cisplatine |
22/93 (23.7%) |
|
FOLFOX |
8/93 (8.6%) |
|
Others or unknow |
21 (22.6%) |
|
Patients treated with L3 chemotherapy, n (%) |
N= 40/165 (24.2%) |
|
Patients treated with L4 chemotherapy, n (%) |
13/165 (7.9%) |
|
PALLIATIVE CARE |
|
|
No encounter with palliative care |
87 (43.5%) |
|
Palliative care |
113 (56.5%) |
|
HOME HOSPITALIZATION |
|
|
Yes |
39 (19.6%) |
|
No |
160 (80.4%) |
|
Unknow |
1 |
3.2 Palliative Care
Most of included patients (56%) met the PCT at least once (Table 1). The PCT were constituted of at least one specialized doctor and a nurse. The mean time to first PCR was more than 6 months after diagnosis (9.7 months) (Table 2). In most of the 6 centres, patients benefit of supportive care through an encounter with mobile PCT (65%), less through a hospitalization in PC units (26%) or a consultation (9%). In one of the centres (ICANS), we notice there was hospital day care that allow a global take of care, with participation of several stakeholders during one day.
This support by PCT mainly often results of a request by the oncologist. The most frequent reasons for referral were asthenia (58%), decision for place of care (41%) and pain (40%). PCR interventions were “decision for becoming and place of care” (54%), opioids treatment (49%), anxiolytics (36%), mouth care (36%) (not mutually exclusive).
Table 2: Description of Palliative care
|
Variable |
N=113 (palliative care) |
|
Mean time of first PC encounter after diagnosis (months) |
9.7 |
|
Timing of the encounter after diagnosis |
|
|
< 3 months after diagnosis |
30 (26.5%) |
|
3-6 months after diagnosis |
20 (17.7%) |
|
>6 months after diagnosis |
63 (55.7%) |
|
Type of first encounter |
|
|
Mobile palliative care teams |
74 (65.5%) |
|
Palliative care units/beds or hospital day care |
29 (25.7%) |
|
Consultation |
10 (8.8%) |
|
Encounter during the last hospitalization (before death) |
13 (11.5%) |
|
Most frequently reasons for referral |
|
|
among 111 patients with available date |
|
|
Asthenia |
64 (57.7%) |
|
Decision for becoming and place of care |
46 (41.4%) |
|
Pain |
45 (40.5%) |
|
Psychological symptoms |
13 (11.7%) |
|
Following the cessation of active therapeutics |
19/92 (10.9%) |
|
Most frequently treatment/take of care by the PC team |
|
|
among 106 patients with available data |
|
|
Decision for becoming and place of care |
57 (53.9%) |
|
Opioids (start or dose increase) |
52 (48.6%) |
|
Anxiolytics |
38 (35.8%) |
|
Mouth care |
38 (35.8%) |
3.3 Impact of palliative care on overall survival
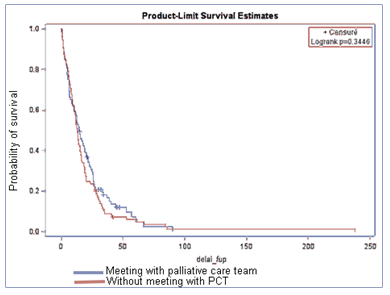
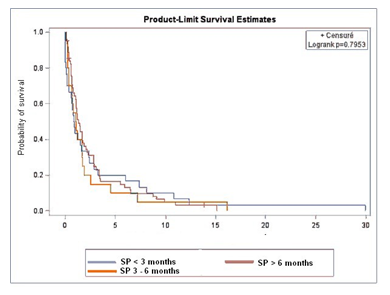
Among the 200 included patients, 188 (94%) died at the end of data collection. 87 (43.5%) did not received PC, 30 (15%) had early PC (<3months after diagnosis), 20 (10%) had PC 3 to 6 months after diagnosis, and 63 (31.5%) had late PC (>6months) (Table 3). Median OS were respectively in the four groups 12.4 months (no PC), 3.0 months (PC < 3months), 6.4 months (PC 3-6 months) and 16.0 months (PC >6 months).
Figure 1 represents OS with or without PC. OS was not significantly different between PC and no PC groups (p=0.34). Figure 2 represents survival according to the first PCR. No significant difference was observed between groups (p=0.79). Time between PCT contact and death were 0.9 months (PC < 3months), 1 month (PC 3-6months) and 1.3 months (PC >6months).
3.4 Impact of Palliative Care on Aggressiveness in End-Of-Life Care
Place of Death
Place of death seemed to be influenced by PC (not significant), especially when PCR was early (<3 months after diagnosis). PC was associated with less death in conventional acute unit, intensive care unit and ED (respectively 72% no PC vs 21% early PC and 8% no PC versus 0% early PC) and more death in palliative care unit (Table 3).
Last-month Chemotherapy
PC was associated with less chemotherapy during last month of life (non-significant): 36% (no PC), 30% (PC <3months), 10% (PC 3-6months), 10% (PC >6months).
Last-Month Emergency Department Visit
Results were similar for ED visits near death. PC was associated with less visits in ED, However the support of early PCR was not significant (20% PC<3months, 15% PC 3-6months, 7% PC >6months ; versus 35% no PC)
Last-Month Intensive Care Unit Hospitalization
Hospitalizations in intensive care unit seemed to be impacted by PC: 10 patients in the no PC group were admitted in ICU versus 1 patient in the PC group.
Table 3: Impact of palliative care on survival and aggressiveness of end-of-life care:
Comparison between the 4 groups (No PC , PC < 3months after aBTC diagnosis, PC 3-6 months after aBTC diagnosis, PC > 6 months after aBTC diagnosis)
|
Variable |
No PC |
PC <3months |
PC |
PC >6months |
All PC |
|
|
(N=200 patients) |
N=87 (43.5%) |
N=30 (15.0%) |
N=63 (31.5%) |
N=113 |
||
|
3-6months |
||||||
|
N=20 (10.0%) |
-56.70% |
|||||
|
DESCRIPTION OF THE GROUPS |
||||||
|
Age ≥75 years old |
22 |
7 |
6 |
12 |
25 |
|
|
-25.30% |
-24.10% |
-30.00% |
-19.00% |
-27.20% |
||
|
Performans status ≥2 |
19 |
18 |
3 |
5 |
26 |
|
|
-21.80% |
-62.10% |
-15.80% |
||||
|
-8.10% |
-23.60% |
|||||
|
Unknow |
1 |
1 |
3 |
|||
|
1 |
||||||
|
Charlson ≥2 |
31 (35.6%) |
14 (46.7%) |
9 (45.0%) |
20 (31.7%) |
43 |
|
|
-38% |
||||||
|
Metastasis |
56 |
19 |
14 |
38 |
71 |
|
|
-65.10% |
-63.30% |
-70.00% |
-60.30% |
|||
|
-62% |
||||||
|
1 |
||||||
|
Unknow |
||||||
|
Chemotherapy received |
73 |
12 |
19 |
61 |
92 |
|
|
-83.90% |
-40.00% |
-95.00% |
-96.80% |
-81.40% |
||
|
Death |
78 |
30 |
20 |
60 |
110 (97.3%) |
|
|
-89.70% |
-100% |
-100% |
-95.20% |
|||
|
SURVIVAL (N=188 patients dead) |
||||||
|
Overall survival |
||||||
|
in months (median) |
12.4 |
|||||
|
3 |
6.4 |
16 |
||||
|
Delay between PC contact and death |
||||||
|
in months (median) |
||||||
|
0.9 |
1 |
1.3 |
||||
|
COMPARISON OF END-OF-LIFE CARE (N=188 patients dead) |
||||||
|
Place of death |
N=188 |
No PC |
PC <3m |
PC 3-6m |
PC >6m |
All PC |
|
N=78 |
N=30 |
N=20 |
N=60 |
N=110 |
||
|
Home |
26 (13.8%) |
7 (9.6%) |
4 |
2 |
13 |
19 |
|
-13.80% |
-10.50% |
-22.00% |
||||
|
-17.30% |
||||||
|
Home hospitalization |
12 (6.4%) |
7 (9.6%) |
1 |
0 |
4 |
5 |
|
-3.40% |
-6.80% |
|||||
|
-4.50% |
||||||
|
Conventional acute unit |
78 (41.5%) |
53 (72.6%) |
6 |
4 |
15 |
25 |
|
-20.70% |
-21.10% |
-25.40% |
||||
|
-22.70% |
||||||
|
Palliative care unit |
56 (29.8%) |
- |
18 |
12 |
26 |
56 |
|
-62.10% |
-63.20% |
-44.10% |
||||
|
-50.90% |
||||||
|
Intensive care unit |
8 |
6 (8.2%) |
0 |
1 |
1 |
2 |
|
or emergency department |
-4.30% |
0% |
-5.30% |
-1.70% |
||
|
-1.80% |
||||||
|
Unknow |
8 |
5 |
1 |
1 |
1 |
3 |
|
Cause of death (3 most frequents) |
||||||
|
Deterioriation of general condition |
39 |
27 |
17 |
50 |
94 |
|
|
-59.10% |
-93.10% |
-89.50% |
-92.60% |
|||
|
-85.40% |
||||||
|
Sepsis |
16 |
2 |
0 |
1 |
3 |
|
|
-24.20% |
-6.90% |
0% |
-1.90% |
|||
|
-2.70% |
||||||
|
Gastro intestinal bleeding |
3 (4.5%) |
0 (0%) |
0 (0%) |
0 (0%) |
0% |
|
|
Unknow |
12 |
1 |
1 |
6 |
8 |
|
|
Chemotherapy near death (last month) (among the 157 dead patients who received chemotherapy) |
23/63 (36.5%) |
12-Mar |
19-Feb |
Jun-58 |
Nov-89 |
|
|
Unknow |
-30.00% |
-10.50% |
-10.30% |
-12.40% |
||
|
1 |
||||||
|
2 |
- |
- |
2 |
|||
|
Emergency department visit near death (last month) |
27 (35.5%) |
6 (20.0%) |
3 (15.0%) |
4 (6.8%) |
13 (11.8%) |
|
|
Unknow |
||||||
|
2 |
- |
- |
1 |
1 |
||
|
Intensive care unit hospitalization near death (last month) |
10 (12.8%) |
0 (0%) |
0 (0%) |
1 (1.7%) |
1 (0.9%) |
|
|
Unkown |
||||||
|
2 |
- |
- |
1 |
1 |
||
4. Discussion
Because of its advanced/metastatic stage at diagnosis and its poor prognosis, the management of aBTC should be inseparable from the initiation of early palliative care, as soon as systemic palliative therapy is initiated. To our knowledge, the present study is the first which provides real-world data about PC settings and which assess the impact of this support on survival in patients with aBTC. Indeed, there is scarce literature available about biliary tract cancer and PC. Therefore, we will compare our data with available literature regarding advanced/metastatic pancreatic ductal adenocarcinoma, which prognosis is similar.
In our study, 17% of patients were not able to received systemic treatment, which is different from other published studies (63% of iCCA received only PC in a study published by Neuzillet et al. in 2020 [13], which included 3650 iCCA patients). Such a difference might be partly explained, by the fact that in this above mentioned study, all patients with advanced or metastatic BTC were included, even those without accessible histological evidence, making it feared that their general condition was too poor at diagnosis to obtain histological evidence. In the same way, more than a half (56%) of chemotherapy-treated patients had a second-line chemotherapy, which is more than other studies (less than one third [14]).
Our main objective was to assess if early PC could improve OS. We found no evidence that OS was better in PC group in comparison to no PC group. It could be explained by the heterogeneity between groups. Indeed, groups were not comparable: patients who were referred earlier to PCT had more comorbidities and more advanced BTC. The group of patients who met PCT early after aBTC diagnosis had the worst survival due to several poor prognosis factors: high performans status (≥ 2 for 62% of them), more comorbidities (Charlson Index Score ≥2 for 47% of them), and only one third of them was able to receive palliative chemotherapy. Consequently, causes of death seem to be also different between groups. Because patients from early PC groups had more frailties and comorbidities, related-cause of death were mainly due to deterioration of general conditions (93% for early PC vs 59% no PC). Differences in the rate of acute events were also noticed, as sepsis (7% early PC vs 24% no PC) or gastro-intestinal bleeding (0% early PC vs 4% no PC). On the contrary, patients who never receive PC died more from acute complication as sepsis or bleeding, with more hospitalizations in intensive care unit.
Differences are also due to the retrospective character of our study, which reflects our daily clinical practice, with patients referred to PCT 0.9 to 1.3 months before death, usually when systemic treatments are no more possible. The early PC group is representative of these practices: patients who were referred early to PCT after aBTC diagnosis had symptomatic advanced or metastatic BTC cancer and presented altered general conditions. This is probably the main explanation for poor OS (3 months) in that group. In such situations, PCT cannot build strong relationship with patient and their family. However, important times for exchanges should be established, as interruption of systemic chemotherapy, end-of-life preferences, and meeting family members to have a global action. These results are similar to a French study published in 2017, about all patients addressed to PCT of 25 Parisians hospitals: median survival time after first PCT meeting was 31 days [15], and 18% of the patients first met PCT 3 days before death.
In 2017, the American Society of Clinical Oncology (ASCO), published recommendations about palliative care, and especially mentioned that patients with advanced cancer should receive dedicated palliative care services early in the disease course and concurrent with active treatment [5]. In our study, 56% of the patients have met at less once the PCT, which is similar that a retrospective study in pancreatic cancer patients (52%) [7]. However, time-to-referral seems too late for impacting prognosis. Average time between diagnosis and PCR was 9.7 months, although median OS fluctuates from 3 to 16 months. Among 11% of the patients who met PCT, this meeting happened during the last hospitalization (a few days before death), which is too late.
The most common reasons for PCR in our study were asthenia (58%), “decision for becoming and place of care” (41%), pain (40%), psychological symptoms (12%) and then following the cessation of active therapeutics (11%). As a comparison, Vinant et al. [15] demonstrated in their study that the 3 major reasons for PCR (for all cancer types) were pain (57%), early encounter (24%), decision for place of care (18%) and then in 6th position “decision to withhold or withdraw treatments” (9.3%). In our study, patients were mostly referred to PCT for difficulties for end-of-life management. Late PCR does not allow PCT to be a part of the decision about active treatment, which should be discussed in a multidisciplinary team.
In our study, meeting PCT is associated with an impact on place of death (Table 3). When they received early PC, fewer patients died in conventional, emergency or intensive departments. In a retrospective study published in 2015, conducted in more than 500000 patients with advanced cancer, proportion of death in PC units was 10% [16]. Vinant et al. showed that this proportion reached 30% when patients were referred to PCT [15]. It reflects the positive impact of PC on the place of death, with less deaths in acute care units. Death in appropriate and dedicated department is important for a better management of end-of-life. In palliative care units or home hospitalization, PCT are familiar with those situations, and are able to anticipate future needs for patients, and to discuss of their preferences in order to head off difficult situations of end-of-life. In a retrospective study performed in patients with pancreatic adenocarcinoma, chemotherapy was prescribed during last month in 17% in case of early PCR and 25% in case of late PCR (not significant, p=0,22) (9). In our study, 18% of patients received palliative chemotherapy within the last month of life (36% no PC, 10-30% with PC), which is similar to previous studies ([16]). PC seems to reduce end-life chemotherapy. Chemotherapy at end-of-life does not bring any benefits for patients, leading to a worsening of the side effects of systemic drugs (eg: asthenia, nausea, infections leading to hospitalizations). Such situations could be avoided with early PCT meeting.
In the Australian study focusing on advanced/metastatic pancreatic cancer patients [9], early PC allows a significant reduction of last-month visit in ED (24%) in comparison to late PSC (42%) (p=0,003). In our study, in the PC group, ED visit proportions varied from 7 to 20%, compared with 35% in the no PC group. Our data also suggests that PC reduces ED visits during the last month of life. In 2005, Earl et al. proposed some objectives to evaluate aggressiveness in end-of-life care [6]: - Less than 10% of patients should receive chemotherapy in the last 14 days of life. In our study we collected last-month chemotherapy rate (36% without PC vs 10 to 30% with PC) - Less than 4% of emergency room visits. In our study we had 35% no PC group and 7 to 20% PC group - Less than 17% death in an acute care hospital. We had 8% death in ICU in non-PC group vs 0 to 5% in PC group. In conventional acute unit we had 73% in non PC group vs 21 to 25% in PC group. - Less than 4% of admission in ICU. In our study this objective is achieved for the PC group (0-2%) but no for the no PC group (13%)
One major limitation of our study is its retrospective character leading to heterogeneity into groups and therefore a bias especially for overall survival in the four groups. Another important limitation of our study is the differences of accessibility and support by the PCT. Indeed, although recommendations are established, referral to PCT is not standardized in France. Human and financial resources allocated to palliative care are different in France and vary from one centre to another. This is well illustrated in our work by the presence of a day hospital dedicated to palliative care in only one of the 6 centers studied (Strasbourg, ICANS).
5. Conclusion
This study provides important data about end-of-life management, and partnerships with PCT for patients suffering from aBTC. Although no evidence for improvement of OS with early PCR was demonstrated, PC are introduced too late in the patient’s care pathways to improve prognosis. However, PC have a positive impact on end-of-life with limitation of futile systemic chemotherapy, fewer visit in ED, fewer hospitalization in ICU, and fewer death in a conventional acute unit or ICU.
Despite ASCO recommendations of 2017 in favour of early PCR in cancer course (concomitant to active therapy), we observed that patients are mainly referred to PCT at end-of-life clinical in daily practise. Efforts should be done for a better education and access about PCR role in a multidisciplinary approach in cancer. Introduction of early PC should endorse a central role in aBTC cancer, with many positive consequences on end-of-life preferences, discontinuation of useless chemotherapy, better management of symptoms and family needs. PC should also evolve for each patient according to disease, prognosis, comorbidities, and symptoms, moving towards a “personalized early PC”.
Acknowledgment
The authors would like to acknowledgethe statistician team of University Hospitals of Nancy, especially Amandine Luc and Dr Baumann. Thanks to the 6 hospitals of Eastern France that agreed to participate to this study.
Reference
- Al Mahjoub A, Bouvier V, Menahem B, Bazille C, Fohlen A, Alves A, et al. Epidemiology of intrahepatic, perihilar, and distal cholangiocarcinoma in the French population. European Journal of Gastroenterology & Hepatology juin 31 (2019): 678-84.
- Flemming JA, Zhang-Salomons J, Nanji S, Booth CM. Increased incidence but improved median overall survival for biliary tract cancers diagnosed in Ontario from 1994 through 2012: A population-based study. Cancer (15) 2016: 2534-43.
- Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma — evolving concepts and therapeutic strategies. Nat Rev Clin Oncol févr 15 (2018): 95-111.
- Hui D, Hannon B, Zimmermann C, Bruera E. Improving Patient and Caregiver Outcomes in Oncology: Team-Based, Timely, and Targeted Palliative Care. CA Cancer J Clin sept 68 (2018): 356-76.
- Ferrell BR, Temel JS, Temin S, Smith TJ. Integration of Palliative Care into Standard Oncology Care: ASCO Clinical Practice Guideline Update Summary. J Oncol Pract 13 (2017): 119-21.
- Earle CC, Neville BA, Landrum MB, Souza JM, Weeks JC, Block SD, et al. evaluating claims-based indicators of the intensity of end-of-life cancer care. Int J Qual Health Care déc 17 (2005): 505-9.
- Jang RW, Krzyzanowska MK, Zimmermann C, Taback N, Alibhai SMH. Palliative Care and the Aggressiveness of End-of-Life Care in Patients with Advanced Pancreatic Cancer. JNCI: Journal of the National Cancer Institute (2015).
- Maltoni M, Scarpi E, Dall’Agata M, Zagonel V, Bertè R, Ferrari D, et al. Systematic versus on-demand early palliative care: results from a multicentre, randomised clinical trial. Eur J Cancer 65 (2016): 61-8.
- Michael N, Beale G, O’Callaghan C, Melia A, DeSilva W, Costa D, et al. Timing of palliative care referral and aggressive cancer care toward the end-of-life in pancreatic cancer: a retrospective, single-center observational study. BMC Palliat Care 18 (2019): 13.
- Sullivan DR, Chan B, Lapidus JA, Ganzini L, Hansen L, Carney PA, et al. Association of Early Palliative Care Use With Survival and Place of Death Among Patients With Advanced Lung Cancer Receiving Care in the Veterans Health Administration. JAMA Oncol (2019).
- Lu Z, Fang Y, Liu C, Zhang X, Xin X, He Y, et al. Early Interdisciplinary Supportive Care in Patients With Previously Untreated Metastatic Esophagogastric Cancer: A Phase III Randomized Controlled Trial. J Clin Oncol 39 (2021): 748-56.
- Blechacz B, Gores GJ. Cholangiocarcinoma: Advances in pathogenesis, diagnosis, and treatment. Hepatology 48 (2008): 308-21.
- Neuzillet C, Emery C, Tessier C, Bouée S, Liévre A. Épidémiologie et parcours de soins des cholangiocarcinomes intrahépatiques (iCCA) en France : données de vie réelle issues du Programme de médicalisation des systèmes d’information. Revue d’Épidémiologie et de Santé Publique 68 (2020): S77.
- Lamarca A, Hubner RA, David Ryder W, Valle JW. Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol 25 (2014): 2328-38.
- Vinant P, Joffin I, Serresse L, Grabar S, Jaulmes H, Daoud M, et al. Integration and activity of hospital-based palliative care consultation teams: the INSIGHT multicentric cohort study (2017).
- Morin L, Aubry R, Beaussant Y, Rochigneux P, Goldwasser F, Tournigand C. Burden of inpatient care and treatments in terminally-ill cancer patients Results from a population-based, retrospective study from administrative data in France In (2015).


 Impact Factor: * 3.3
Impact Factor: * 3.3 Acceptance Rate: 74.39%
Acceptance Rate: 74.39%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 