Isolation of A Marine Purple Non-Sulfur Photosynthetic Bacterium With A High Ability of Glycerol Assimilation
Nao Yamauchi1, Aoi Koga1, Hiroshi Okuhata2, Satoshi Tanaka2, Naoki Yamada3, Takaaki Maki3, Shuhei Hayashi1, Shinjiro Yamamoto1, Hitoshi Miyasaka1*
1Department of Applied Life Science, Sojo University, 4-22-1 Ikeda, Nishiku, Kumamoto 860-0082, Japan
2The Kansai Electric Power Co., Environmental Research Center, Keihanna-Plaza, 1-7 Seikacho, Sourakugun, Kyoto 619-0237, Japan
3Matsumoto Institute of Microorganisms Co. Ltd., 2904 Niimura, Matsumoto, Nagano 390-1241, Japan
*Corresponding Author: Dr. Hitoshi Miyasaka, Department of Applied Life Science, Sojo University, 4-22-1 Ikeda, Nishiku, Kumamoto 860-0082, Japan.
Received: 22 November 2019; Accepted: 29 November 2019; Published: 02 December 2019
Article Information
Citation: Nao Yamauchi, Aoi Koga, Hiroshi Okuhata, Satoshi Tanaka, Naoki Yamada, Takaaki Maki, Shuhei Hayashi, Shinjiro Yamamoto, Hitoshi Miyasaka. Isolation of A Marine Purple Non-Sulfur Photosynthetic Bacterium With A High Ability of Glycerol Assimilation. International Journal of Plant, Animal and Environmental Sciences 9 (2019): 214-221.
View / Download Pdf Share at FacebookAbstract
Conversion of glycerol, a by-product of biodiesel production, into value-added materials increases the cost-effectiveness of biodiesel production. Purple nonsulfur photosynthetic bacteria (PNSB) are microorganisms that have been safely applied to various fields, such as agriculture, aquaculture, dairy farming, wastewater treatment, fine chemical production, and bioenergy production. The use of PNSB that efficiently assimilate glycerol is a promising approach to the value-added utilization of glycerol. In this study, we isolated 40 PNSB strains from seashore sediment, and examined their abilities in assimilate glycerol. One strain grew well with glycerol as a sole carbon source, and this strain was designated Rhodovulum sulfidophilum OKHT16 based on its 16S rRNA sequence. R. sulfidophilum OKHT16 showed the highest growth rate between 37 °C and 40 °C with a doubling time of 72 minutes, and the thermo-tolerance of this strain was up to 48 °C.
Keywords
<p>Glycerol; Purple nonsulfur photosynthetic bacteria; Rhodovulum sulfidophilum; Thermo tolerance</p>
Article Details
1. Introduction
Biodiesel production has increased rapidly in the last decade, resulting in a surplus of glycerol, a by-product of this process [1]. Technologies for the conversion of glycerol into value-added materials are, therefore, important for economically feasible biodiesel production, and there have been many studies on chemical [2] and biological [3-4] processes for value-added glycerol utilization.
Purple nonsulfur photosynthetic bacteria (PNSB), constituting a non-taxonomic group of versatile organisms, generally grow photoheterotrophically by anoxygenic photosynthesis, although some of them can also grow photoautotrophically or chemoheterotrophically [5]. Most PNSB are nonpathogenic, and they have been applied to many fields [6], such as agriculture [7], stock raising [8], aquaculture [9-10], wastewater treatment [11- 12], bioremediation [13-14], bioactive compound production [15-16], and hydrogen energy production [17-18]. Thus the use of PNSB that efficiently assimilate glycerol is a promising approach to the value-added utilization of glycerol, but there has been only a few studies on glycerol assimilating PNSB, and the strains were limited to terrestrial (freshwater) strains [19-20]. In this study we isolated 40 PNSB strains from seashore sediment, and examined their abilities in assimilate glycerol.
2. Materials and Methods
Isolation of photosynthetic bacterial strains from seashore sediment
Glutamate malate (GM) medium with 3% NaCl was used to isolate PNSB from seashore sediment samples. The components of the GM medium included 3.8 g (22 mM) sodium glutamate, 2.7 g (14 mM) sodium DL-malate, 2.0 g yeast extract, 0.5 g KH2PO4, 0.5 g K2HPO4, 0.8 g (NH4) 2HPO4, 0.2 g MgSO4.7H2O, 0.053 g CaCl2.2H2O, 0.0012 g MnSO4·5H2O, 5 mg nicotinic acid, 5 mg thiamine hydrochloride, 5 mg biotin, and deionized water up to 1,000 ml. The pH was adjusted to 6.8. For the isolation of PNSB strains, about 0.1 g of sediment sample was inoculated into 10 ml of GM medium with 3% NaCl in a test tube, and cultured at room temperature under continuous light with incandescent lamps (light intensity ca. 100 mM photon/sec/m2) for 7 to 14 days. After the appearance of purple, pink, red, or brown colors in the culture, the culture broth was streaked onto a GM agar plate containing 3% NaCl, and cultured under the same conditions. Streak cultures were repeated until pure monocultures of PNSB strains were obtained.
3. Reference PNSB strains
The reference PNSB strains, Rhodobacter sphaeroides strain S [20], and Rhodovulum sulfidophilum DSM1374 (type culture of R. sulfidophilum) used in this study are the kind gifts from Professor Ken Sasaki of Hiroshima Kokusai Gakuin University, and Dr. Umekage So and Dr. Nobuyoshi Nagao of Toyohashi University of Technology, respectively. Rhodobacter sphaeroides is the widely used commercial PNSB strain in Japan, and the 16S rRNA sequence of this strain (data not shown) showed 100% similarity to Rhodobacter sphaeroides ATCC 17025 (GenBank accession number CP000661).
Screening for PNSB that efficiently assimilate glycerol
To screen PNSB strains, the carbon sources in the GM medium (glutamate, malate, and yeast extract) were replaced with 5 g/L (54 mM) of glycerol. A loopful of inoculum from the liquid culture stocks of 40 PNSB strains was inoculated into 3 ml of the medium in a test tube, and cultured under dark and aerobic conditions with shaking (80 rpm) for 7 days. Growth of the cells was measured at an optimal density of 660 (OD660). Glycerol concentrations in the medium were determined with F-kit glycerol (Roche Diagnostics).
Identification of PNSB strains based on 16S rRNA sequences
Total DNA was extracted from the PNSB cells with Instagene Matrix (Bio-Rad, Hercules, CA, USA) according to the manufacturer's instructions. The 16S rRNA sequences of the PNSB were amplified by PCR using genomic DNA as the template and primers 8F (5′-AGAGTTTGATCCTGGCTCAG- 3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′). DNA sequences were analyzed using a commercial DNA sequence analysis service (Eurofins Genomics Inc., Tokyo, Japan). A homology search of 16S rRNA sequences was performed using the BLAST program. The GenBank/EMBL/DDBJ accession number for the 16S rRNA gene sequences of Rhodovulum sulfidophilum OKHT16 is LC037397.
Growth rate under various temperature conditions
Twenty milliliter cultures in 100 ml flask were started at initial cell density of OD660 = 0.05, and cultured under light (light intensity ca. 100 mM photon/sec/m2) with shaking (80 rpm) under various temperature conditions (25, 30, 33, 35, 37, 38, 40, 41, 42, 43, 44, 45, 46, 47, 48 and 49 °C). Growth of the cells was measured by measuring OD660, and the specific growth rates (m) were calculated in the logarithmic growth phases of the cultures from the slope of the equation of lnX = m t + lnX0 (X: OD660 value, X0: OD660 value at t = 0).
Results and Discussion
Isolation of a marine PNSB Rhodovulum sulfidophilum OKHT16 that efficiently assimilates glycerol
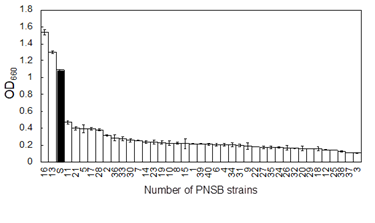
There have been several reports on glycerol assimilating PNSB strains, but no report on marine PNSB strain. To isolate marine PNSB strains that efficiently assimilate glycerol, 40 PNSB strains (strain numbers 1 to 40) were isolated from the seashore sediment samples collected from the Osaka Bay area in Japan and were purified to monocultures. These 40 PNSB strains were then grown in basal media containing 5 g/L (54 mM) glycerol as the sole carbon source. Figure 1 shows the results. The freshwater PNSB Rhodobacter sphaeroides strain S, which assimilates glycerol well [20], was used as a reference strain. Among the 40 PNSB strains, numbers 16 and 13 showed growth rates comparable to that of Rhodobacter sphaeroides strain S. The 16S rRNA sequences of these strains were determined, and a DNA database search revealed that the sequences of both strains showed 100% identity to Rhodovulum sulfidophilum strain P5 (GenBank accession no. JF794560) and Rhodovulum sp. MB263 (GenBank accession no. D32246). We designated our strains Rhodovulum sulfidophilum strains OKHT13 and OKHT16. These strains were isolated from sediment samples collected from the same sampling site, and we assumed these strains might be identical strains. Therefore, detailed investigations were carried out only with R. sulfidophilum OKHT16. Generally glycerol is not a good carbon source for the cultures of microorganisms. As shown in Figure 1, the majority of PNSB strains isolated in the present study showed quite low ability in glycerol assimilation. One of the main metabolic pathways for glycerol is phosphorylation of glycerol to glycerol-3-phosphate (G3P) by glycerol kinase, and then dehydrogenation of G3P to dihydroxyacetone phosphate (DHAP) by G3P-dehydrogenase. DHAP is metabolized through the pentose phosphate pathway (PPP). One possible reason for the low ability in glycerol assimilation observed among some kinds of microorganisms including PNSB is supposed to be the low enzymatic activity of glycerol kinase [21-22].
The PNSB cells were cultured in GM medium containing 5 g/L of glycerol as a sole carbon source under dark and aerobic conditions for 7 days. The growth of the reference strain, Rhodobacter sphaeroides strain S which well assimilates glycerol [20], is indicated as the black bar. Data are means + SD (n = 3).
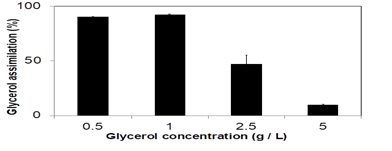
We also examined the glycerol assimilation ability of R. sulfidophilum OKHT16 by culturing the cells in the medium containing 0.5, 1.0, 2.5 or 5 g/L of glycerol (Figure 2). The cells were cultured for 5 days under light and aerobic conditions, and the percentage of the assimilated glycerol was examined. When the initial concentrations of glycerol were 0.5 or 1.0 g/L, the cells assimilated more than 90% of the glycerol in the medium, while about 47% and 10% were assimilated in the media containing 2.5 and 5.0 g/L of glycerol, respectively.
The cells of R. sulfidophilum OKHT16 were cultured in GM medium containing 0.5, 1.0, 2.5, or 5.0 g/L of glycerol under light and aerobic conditions for 5 days. The assimilation ability is shown as percent of the assimilated glycerol. Data are means + SD (n = 3).
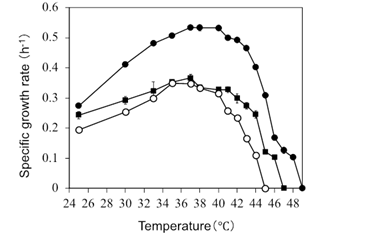
Since the growth rate at high temperatures and thermo-tolerance of PNSB are important factors in the practical application of PNSB, especially for applications that rely on solar energy for outdoor cultivation [23], the growth of R. sulfidophilum OKHT16 under various temperature conditions was examined. The growth of Rhodovulum sulfidophilum DSM1374 (type culture of Rhodovulum sulfidophilum) and the freshwater PNSB Rhodobacter sphaeroides ATCC 17025, which has many practical applications in Japan [24], were used as the reference strains. Figure 3 shows the specific growth rate of the R. sulfidophilum OKHT16, R. sulfidophilum DSM1374, and R. sphaeroides cultured under various temperature conditions (from 25 °C to 49 °C). R. sulfidophilum OKHT16 showed the highest growth rate between 37 °C and 40 °C with a specific growth rate of 0.53 hr-1(doubling time: 72 minutes), while R. sulfidophilum DSM1374 and R. sphaeroides showed the highest growth rate between 35 °C and 37 °C with a specific growth rate of 0.37 hr-1 (doubling time: 109 minutes) and 0.35 hr-1 (doubling time: 110 minutes), respectively. The thermo-tolerance of R. sulfidophilum OKHT16 was up to 48 °C and those for R. sulfidophilum DSM1374 and R. sphaeroides were up to 46 °C and 42 °C, respectively. The much faster growth at higher temperatures and the high thermo-tolerance of R. sulfidophilum OKHT16 are advantageous for its application in high temperature areas such as tropical regions.
The cells of R. sulfidophilum OKHT16 (closed circle), R. sulfidophilum DSM1374 T (closed square) and R. sphaeroides (open circle) were cultured in GM medium (with 3% NaCl for R. sulfidophilum OKHT16 and R. sulfidophilum DSM1374 T), in light (100 mM photon/sec/m2) with shaking (80 rpm) under various temperature conditions (25 °C to 48 °C). The initial cell density of the culture was OD660 = 0.05, and the specific growth rates (m) were calculated in the logarithmic growth phases of the cultures from the slope of the equation of lnX = m t + lnX0 (X: OD660 value, X0: OD660 value at t = 0). Data are means + SD (n = 3).
The marine PNSB R. sulfidophilum OKHT16 isolated in the present study grows well with glycerol as a sole carbon source, shows a high growth rate (doubling time: 72 min) at high temperatures (37 °C to 40 °C), and also tolerates high temperature (up to 48 °C). For the practical application of R. sulfidophilum OKHT16, the use of this strain for the feed supplement of aquaculture of shrimp is under progress, because R. sulfidophilum for aquaculture supplement has been reported previously [25].
Conclusions
Rhodovulum sulfidophilum OKHT16 isolated in the present study is the first example of marine PNSB with a high ability of glycerol assimilation. This strain shows a high growth rate (doubling time: 72 min) at high temperature (37 °C to 40 °C), and also tolerates higher temperature (up to 48 °C).
Acknowledgements
We thank late Professor Ken Sasaki of Hiroshima Kokusai Gakuin University for his kind gift of Rhodobacter sphaeroides strain S. We also thank Dr. Umekage So and Dr. Nobuyoshi Nagao of Toyohashi University of Technology for their kind gift of Rhodovulum sulfidophilum DSM1374 (type culture of R. sulfidophilum).
Disclosure of potential conflicts of interest
The authors declare that they have no conflict of interest.
References
- Almeida, João RM, Léia CL Fávaro, and Betania F. Quirino. Biodiesel biorefinery: opportunities and challenges for microbial production of fuels and chemicals from glycerol waste. Biotechnology for biofuels 5 (2012): 48.
- Johnson, Duane T., and Katherine A. Taconi. The glycerin glut: Options for the value?added conversion of crude glycerol resulting from biodiesel production. Environmental Progress 26 (2007): 338-348.
- Li, Cheng, Keaton L. Lesnik, and Hong Liu. Microbial conversion of waste glycerol from biodiesel production into value-added products. Energies 6 (2013): 4739-4768.
- Yazdani, Syed Shams, and Ramon Gonzalez. Anaerobic fermentation of glycerol: a path to economic viability for the biofuels industry. Current opinion in biotechnology 18 (2007): 213-219.
- Bryant, Donald A., and Niels-Ulrik Frigaard. Prokaryotic photosynthesis and phototrophy illuminated. Trends in microbiology 14 (2006): 488-496.
- Del Socorro, Magdalene Mae L., et al. Purple Nonsulfur Bacteria (PNSB) Isolated from Aquatic Sediments and Rice Paddy in Iligan City, Philippines. Journal of Multidisciplinary Studies 1 (2013): 45-58.
- Harada, Naoki, et al. Effects of inoculation of phototrophic purple bacteria on grain yield of rice and nitrogenase activity of paddy soil in a pot experiment. Soil Science & Plant Nutrition 51 (2005): 361-367.
- Salma U, et al. Effect of dietary Rhodobacter capsulatus on cholesterol concentration and fatty acid composition in broiler meat. Poultry Science 86 (2007): 1920-1926.
- Banerjee S, Azad SA, Vikineswary S, Selvaraj OS and Mukherjee TK. Phototrophic bacteria as fish feed supplement, Asia-Aus J Anim Sci 13 (2000): 991-994.
- Chumpol S, Kantachote D, Rattanachuay P, Vuddhakul V, Nitoda T, Kanzaki H. In vitro and in vivo selection of probiotic purple nonsulphur bacteria with an ability to inhibit shrimp pathogens: acute hepatopancreatic necrosis disease?causing Vibrio parahaemolyticus and other vibrios. Aquaculture research 48 (2017): 3182-3197.
- Kantachote D, Torpee S, and Umsakul K. The potential use of anoxygenic phototrophic bacteria for treating latex rubber sheet wastewater. Electr J Biotechnol 8 (2005): 314–323
- Kim MK, Choi KM, Yin CR, Lee KY, Im WT, Lim JH, Lee ST. Odorous swine wastewater treatment by purple non-sulfur bacteria, Rhodopseudomonas palustris, isolated from eutrophicated ponds. Biotechnology letters 26 (2004): 819-822.
- Sasaki K, Morikawa H, Kisibe T, Takeno K, Mikami A, Harada T, Ohta M. Simultaneous removal of cesium and strontium using a photosynthetic bacterium, Rhodobacter sphaeroides SSI immobilized on porous ceramic made from waste glass. Advances in Bioscience and Biotechnology 4 (2013): 6.
- Sharma N, Doerner KC, Alok PC, Choudhary M. Skatole remediation potential of R hodopseudomonas palustris WKU?KDNS 3 isolated from an animal waste lagoon. Letters in applied microbiology 60 (2015): 298-306.
- Sasaki K, Watanabe M, Suda Y, Ishizuka A, Noparatnaraporn N. Applications of photosynthetic bacteria for medical fields. Journal of bioscience and bioengineering 100 (2005): 481-488.
- Suzuki H, Ando T, Umekage S, Tanaka T, Kikuchi Y. Extracellular production of an RNA aptamer by ribonuclease-free marine bacteria harboring engineered plasmids: a proposal for industrial RNA drug production. Appl. Environ. Microbiol 76 (2010): 786-793.
- Ghosh D, Sobro IF, Hallenbeck PC. Stoichiometric conversion of biodiesel derived crude glycerol to hydrogen: response surface methodology study of the effects of light intensity and crude glycerol and glutamate concentration. Bioresource technology 106 (2012): 154-160.
- McKinlay JB, Harwood CS. Photobiological production of hydrogen gas as a biofuel. Current opinion in biotechnology 21 (2010): 244-251.
- Sabourin-Provost G, Hallenbeck PC. High yield conversion of a crude glycerol fraction from biodiesel production to hydrogen by photofermentation. Bioresource technology 100 (2009): 3513-3517.
- Sasaki K, Morii H, Nishizawa Y, Nagai S. Denitrifying and photoheterotrophic growth of Rhodobacter sphaeroides S under anaerobic-dark and-light conditions. Journal of fermentation technology 66 (1988): 27-32.
- Jung WS, Kang JH, Chu HS, Choi IS, Cho KM. Elevated production of 3-hydroxypropionic acid by metabolic engineering of the glycerol metabolism in Escherichia coli. Metabolic engineering 23 (2014): 116-122.
- Muto M, Tanaka M, Liang Y, Yoshino T, Matsumoto M, Tanaka T. Enhancement of glycerol metabolism in the oleaginous marine diatom Fistulifera solaris JPCC DA0580 to improve triacylglycerol productivity. Biotechnology for biofuels 8 (2015): 4.
- Mahakhan P, Chobvijuk C, Ngmjarearnwong M, Trakulnalermsai S, Bucke C, Svasti J, Kanlayakrit W, Chitradon L. Molecular hydrogen production by a thermotolerant Rubrivivax gelatinosus using raw cassava starch as an electron donor. Sci. Asia 31 (2005): 415-424.
- Maki T. Applications of Rhodobacter capsulata in agriculture, stock raising, environmental technologies, and aquaculture. Seibutsu-kogaku kaishi 89 (2011): 113-116.
- Loo PL, Chong VC, Vikineswary S. R hodovulum sulfidophilum, a phototrophic bacterium, grown in palm oil mill effluent improves the larval survival of marble goby O xyeleotris marmorata (Bleeker). Aquaculture Research 44 (2013): 495-507.


 Impact Factor: * 4.1
Impact Factor: * 4.1 Acceptance Rate: 75.32%
Acceptance Rate: 75.32%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 