Occurrence of Recurrent Mutations in SARS-CoV-2 Genome and its Implications in the Drug Designing Strategies
Niti Yashvardhini1, Deepak Kumar Jha2,*
1Department of Microbiology, Patna Women’s College, Patna, 800 001, India
2Department of Zoology, P. C. Vigyan College, Chapra, 841 301, India
*Corresponding Author(s): Deepak Kumar Jha, Department of Zoology, P. C. Vigyan College, Chapra, 841 301, India
Received: 26 September 2020; Accepted: 08 October 2020; Published: 12 October 2020
Article Information
Citation: Niti Yashvardhini, Deepak Kumar Jha. Occurrence of Recurrent Mutations in SARS-CoV-2 Genome and its Implications in the Drug Designing Strategies. Journal of Pharmacy and Pharmacology Research 4 (2020): 96-102.
View / Download Pdf Share at FacebookAbstract
Abstract
Objectives: The objective of this study was to explore mutational sites in the genome of SARS-CoV-2 of Indian isolates through multiple sequence alignment and comparing it with the reference sequence of Wuhan SARS-CoV-2. These mutational studies generate crucial insights in designing of antiviral agents against COVID-19 infections.
Methods: Sequence downloaded from NCBI virus database and multiple sequence alignment done using CLUSTAL Omega online platform, viewed using Jalview.
Results: A total of 64 recurrent mutations were detected from Indian isolates of SARS-CoV-2 which were phylogenetically distant from that of Wuhan type.
Conclusion: Occurrence of new mutations at different locations supports viral evolbability and provides a crucial insight into the development of effective vaccine and drug against COVID-19.
Keywords
Pandemic; SARS-CoV-2; COVID-19; Wuhan; Mutations
Pandemic articles; SARS-CoV-2 articles; COVID-19 articles; Wuhan articles; Mutations articles
Article Details
Introduction
SARS-CoV-2 is a single stranded, (+) sense RNA virus and the etiolating agents of present pandemic COVID-19. This pandemic emerged from Wuhan, China, is a highly contagious disease, spreading rapidly across the globe [1]. Its transmission occurs primarily through human to human contacts or droplets of an infected person. Due to absence of proper medication against this pandemic more than millions of people getting life threatening risk daily. Its outbreak has been reported from almost all 5 major continents. By 26 September 2020, WHO has reported 32,981,206 confirmed positive cases of COVID-19, including 996,589 deaths worldwide.
Frequent mutations altered protein structure of these RNA viruses by altering their amino acid sequences. Due to high rates of mutation, members of RNA viruses showed high genetic variability, consequently, acquiring drug resistance [2]. The genome size of SARS-CoV-2 is approximately30 kb which encodes 14 open reading frame (ORFs and), including 29 proteins as well as four structural proteins like S (spike), E (envelope), M (membrane), and N (nucleocapsid) proteins. Inspite of these proteins, viral genome contain 16 non structural proteins (nsp) including 9 accessory proteins [3,4].
Previous studies have documented that adaptive potential of these RNA viruses must be given weightage while designing antiviral therapeutics. In the present study, various recurrent mutations have been find out observed in the viral protein sequence of Indian isolates and compared with those of corresponding Wuhan isolate as a reference sequence [4]. The outcomes findings of present work suggest that mutational analysis of this contagious virus might be useful to develop new antiviral therapeutics against COVID-19.
Methods
The protein sequences of SARS-CoV-2 ORF1ab polyprotein, 7096 amino acid long were downloaded from NCBI virus database. Those sequences which were submitted from India in the month of August, 2020 were selected and used further for analysis. A reference sequence of Wuhan SARS-CoV-2 (Accession number YP_009724389) [4]. ORF1ab polyprotein was also downloaded to be used as a reference for mutational analysis.
The sequences downloaded were aligned using CLUSTAL Omega online server which performs alignment with HMM profiling [5]. For the alignment, Wuhan type SARS-CoV-2 was used as a reference to detect the variation. The aligned files were viewed using Jalview and recorded along with accession number to detect the mutation occurring in the Indian SARS-CoV-2 protein sequence with respect to Wuhan type SARS-CoV-2.
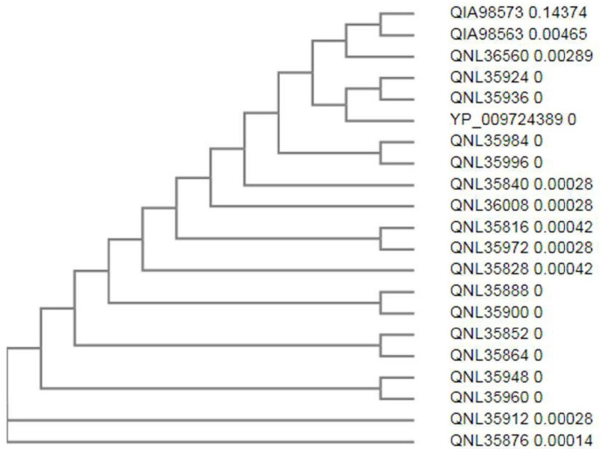
A phylogenetic tree was also constructed using CLUSTAL Omega which uses the aligned files to detect the relationship among different isolates as well as reference isolate.
Results
A total of 21 sequences were downloaded for ORF1ab polyprotein, which was submitted from India in the month of August, 2020 (including one reference). The accession number along with geographic location is as shown in table 1. The aligned files were viewed in Jalview to detect the mutational variants. A total of 64 point mutations were detected in the overall ORF1ab polyprotein of SARS-CoV-2 isolates of India. The position of mutation as well as the wild type and mutated amino acid sequence as shown in table 2.
Table 1: Details of SARS-CoV-2 sequences released from India in the month of August 2020.
|
Accession |
Release_Date |
Protein |
Geo_Location |
Collection_Date |
|
QNL35816 |
2020-08-29T00:00:00Z |
ORF1ab polyprotein |
India: Ahmedabad |
6/18/2020 |
|
QNL35828 |
2020-08-29T00:00:00Z |
ORF1ab polyprotein |
India: Ahmedabad |
6/18/2020 |
|
QNL35840 |
2020-08-29T00:00:00Z |
ORF1ab polyprotein |
India: Ahmedabad |
6/25/2020 |
|
QNL35852 |
2020-08-29T00:00:00Z |
ORF1ab polyprotein |
India: Ahmedabad |
6/25/2020 |
|
QNL35864 |
2020-08-29T00:00:00Z |
ORF1ab polyprotein |
India: Ahmedabad |
6/25/2020 |
|
QNL35876 |
2020-08-29T00:00:00Z |
ORF1ab polyprotein |
India: Ahmedabad |
7/27/2020 |
|
QNL35888 |
2020-08-29T00:00:00Z |
ORF1ab polyprotein |
India: Ahmedabad |
7/27/2020 |
|
QNL35900 |
2020-08-29T00:00:00Z |
ORF1ab polyprotein |
India: Ahmedabad |
7/27/2020 |
|
QNL35912 |
2020-08-29T00:00:00Z |
ORF1ab polyprotein |
India: Ahmedabad |
7/27/2020 |
|
QNL35924 |
2020-08-29T00:00:00Z |
ORF1ab polyprotein |
India: Adajan |
6/12/2020 |
|
QNL35936 |
2020-08-29T00:00:00Z |
ORF1ab polyprotein |
India: Adajan |
6/12/2020 |
|
QNL35948 |
2020-08-29T00:00:00Z |
ORF1ab polyprotein |
India: Mehsana |
7/6/2020 |
|
QNL35960 |
2020-08-29T00:00:00Z |
ORF1ab polyprotein |
India: Mehsana |
7/6/2020 |
|
QNL35972 |
2020-08-29T00:00:00Z |
ORF1ab polyprotein |
India: Mehsana |
7/6/2020 |
|
QNL35984 |
2020-08-29T00:00:00Z |
ORF1ab polyprotein |
India: Modasa |
7/9/2020 |
|
QNL35996 |
2020-08-29T00:00:00Z |
ORF1ab polyprotein |
India: Modasa |
7/9/2020 |
|
QNL36008 |
2020-08-29T00:00:00Z |
ORF1ab polyprotein |
India: Modasa |
7/9/2020 |
|
QNL36560 |
2020-08-29T00:00:00Z |
ORF1ab polyprotein |
India: Ahmedabad |
6/25/2020 |
|
QIA98563 |
2020-08-18T00:00:00Z |
ORF1ab polyprotein |
India: Kerala State |
1/30/2020 |
|
QIA98573 |
2020-08-18T00:00:00Z |
ORF1ab polyprotein |
India: Kerala State |
1/31/2020 |
The phylogenetic analysis showed the occurrence of all the Indian isolates on different subtrees with respect to Wuhan type (Figure 1). This deviation is the result of the point mutation occurring in the sequences and hence providing a multifaceted nature to the virus. This also helps in evading the host immune response and is a barrier in vaccine as well as antiviral therapeutics development.
Table 2: Mutational locations after multiple sequence alignment of SARS-CoV-2 full length protein sequences with position and sequence.
Discussion
RNA viruses such as SARS-CoV-2 have very high potential to exhibit point mutation which is utmost beneficial for these viruses to adapt and evolve in rapidly changing environmental conditions which is responsible for its transmission worldwide [2,3]. These viruses can survive in dynamic environment of hosts because mutation brings natural selection and selecting those traits of viruses which are essential for their survival [9].
The results of present study revealed the presence of total 64 point mutations in 20 isolates of SARS-CoV-2 from India submitted in the month of August. These Coronavirus variants clustered in the different subtree to that of Wuhan SARS-CoV-2 and therefore, expressed their variability and rapid evolution with lapse of time [10]. Unstable protein structures are formed as results of mutagenic alterations caused by this RNA virus which helps in evasion of the host defense [11]. Recurrent mutations are big hurdle in the designing of antiviral drugs, moreover some drugs like chloroquine, remdesvir and favipiravir were found at some extent effective in treatment of COVID-19 infections. Our findings shown in this study, addresses a snap shot in time of a changing situation.
Conclusion
Occurrence of new mutations at different locations supports viral evolvability and provides a crucial insight into the development of effective vaccine and drug against COVID-19.
Financial support:
Nil
Declaration of conflicting interests:
No conflict of interest.
References
- Bogoch II, Watts A, Thomas-Bachli A, et al. Pneumonia of unknown aetiology in Wuhan, China: potential for international spread via commercial air travel. Journal of Travel Medicine 27 (2020): taaa008.
- McKeegan KS, Borges-Walmsley MI, Walmsley AR. Microbial and viral drug resistance mechanisms. Trends in Microbiology 10 (2002): s8-s14.
- Gordon CJ, Tchesnokov EP, Woolner E, et al. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. Journal of Biological Chemistry 295 (2020): 6785-6797.
- Domingo EJ, Holland JJ. RNA virus mutations and fitness for survival. Annual Review of Microbiology 51 (1997): 151-178.
- Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 579 (2020): 265-269.
- Madeira F, Park YM, Lee J, et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Research 47 (2019): W636-W641.
- Mercatelli D, Giorgi FM. Geographic and Genomic Distribution of SARS-CoV-2 Mutations.
- Duffy S. Why are RNA virus mutation rates so damn high?. PLoS Biology 16 (2018): e3000003.
- Irwin KK, Renzette N, Kowalik TF, et al. Antiviral drug resistance as an adaptive process. Virus Evolution 2 (2016).
- Yashvardhini N and Jha DK. Genome Organization and Pathogenesis of SARS-CoV-2. International Journal of Current Microbiology and Applied Sciences 9 (2020): 2153-2156.
- Yashvardhini N and Jha DK. Insilico Analysis of Mutational Variants of SARS-CoV-2 RDRP Protein. Research Journal of Life science, Bioinformatics, Pharmaceutical and Chemical sciences 6 (2020): 68-76.


 Impact Factor: * 3.3
Impact Factor: * 3.3 Acceptance Rate: 74.39%
Acceptance Rate: 74.39%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 