Optimizing Neuromuscular Strategies of Ankle Muscles During Static and Proactive Postural Control in Senior Individuals with Sarcopenia and Obesity: A Multicenter, Randomized, Controlled Trial Study Protocol
Elmoetez Magtouf1, Hamza Ferhi1, Sabri Gaied Chortane1, Oussama Gaied Chortane1, Bruno Beaune2, Sébastien Boyas2, Sylvain Durand2, and Wael Maktouf3*
1Research Laboratory (LR23JS01), Sport Performance, Health and Society, Higher Institute of Sport and Physical Education of Ksar Saîd, University of “La Manouba”, Tunis 2010, Tunisia
2Laboratory “Movement, Interactions, Performance” (UR 4334), Department of Sport Sciences, Faculty of Sciences and Technologies, Le Mans University, 72000 Le Mans, France
3Bioengineering, Tissues and Neuroplasticity, UR 7377, University of Paris-Est Creteil, Faculty of Health / EPISEN, Creteil, France
*Corresponding Author: Wael Maktouf, Bioengineering, Tissues and Neuroplasticity, UR 7377, University of Paris-Est Creteil, Faculty of Health / EPISEN, Creteil, France.
Received: 10 January 2024; Accepted: 01 February 2024; Published: 22 February 2024
Article Information
Citation: Elmoetez Magtouf,Hamza Ferhi, Sabri Gaied Chortane, Oussama Gaied Chortane, Bruno Beaune, Sébastien Boyas, Sylvain Durand, and Wael Maktouf. Optimizing Neuromuscular Strategies in Ankle Muscles During Static and Proactive Postural Control in Senior Individuals with Sarcopenia and Obesity: A Multicenter, Randomized, Controlled Trial Study Protocol. Journal of Orthopedics and Sports Medicine. 6 (2024): 19-28.
View / Download Pdf Share at FacebookAbstract
The rising prevalence of sarcopenic obesity (SO) among older adults— characterized by an increase in obesity rates coupled with a decline in muscle mass—presents a significant health challenge. This condition adversely affects postural control and neuromuscular functions, which are crucial for performing daily activities. Despite the critical impact of SO, there has been scant research on the benefits of customized physical activity programs aimed to improve postural stability and neuromuscular performance in this demographic. This study protocol outlines a comprehensive evaluation of a physical activity program designed to enhance these capacities in older adults with SO. Employing a single-blinded, prospective, randomized, and controlled multicenter trial design, participants will be divided into a trained group (TG) and a control group (CG). While the CG will receive no intervention, the TG will engage in a targeted 4-month physical activity program, consisting of tri-weekly sessions. Evaluation metrics include anthropometric data, clinical assessments, ankle muscle activities during postural control testing, and maximal voluntary contraction tests of the plantar and dorsal flexor muscles. The anticipated outcomes of this study promise to shed light on the potential for improving postural control and neuromuscular functions in older adults with SO, offering critical insights into the effectiveness of tailored physical activity interventions.
Keywords
<p>Balance; Neuromuscular system; Clinical trial, Rehabilitation, Trainability</p>
Article Details
1. Introduction
The escalating prevalence of obesity among older adults, compounded by a concomitant reduction in muscle mass and strength, recognized as sarcopenic obesity (SO), has evolved into a critical global health issue [1–3]. The intricate relationship between obesity and muscle decline markedly amplifies the risk of unfavorable health outcomes, encompassing limitations in functionality and an augmented vulnerability to injuries [1,4]. Among the various functional limitations exacerbated by muscle weakness, impaired postural control, stands out, as they play pivotal roles in daily life activities [5,6]. Therefore, it is imperative to forge physical activity programs explicitly tailored for older adults with SO, addressing their distinct requirements, enhancing postural control, and fostering robust aging within this demographic.
Sarcopenia is closely linked to the decline of an array of neural, hormonal, and environmental signals that support muscle health [7,8]. This degradation of muscle mass and strength is influenced by various factors, including physical inactivity [9], hormonal fluctuations [10], pro-inflammatory conditions [11], malnutrition [12], and the reduction of alpha-motor units in the central nervous system [13]. These factors have[14–16] a detrimental impact on functional abilities, ultimately affecting independence and heightening the risk of falls [14,15].
Existing research suggests that obese individuals may face challenges in executing motor tasks and display heightened instability. [17–19]. These modifications result in detrimental effects on skeletal muscle, such as inflammation [20], oxidative stress [21], and insulin resistance [22]. The fatty infiltration of skeletal muscle correlates with a decrease in strength, functional status, muscle functionality, contractility, as well as interference with normal cellular signaling and motor unit recruitment [23].
Recent research has underscored the significant impact of both sarcopenia and obesity on the delicate postural control among older adults [24–26]. Strikingly, obesity not only exacerbates age-related alterations in postural control [17,27,28] but also heightens the risk of potentially injurious falls [29]. In this context, Maktouf et al. [17] illuminated a crucial aspect of this phenomenon by revealing that obesity-related changes in postural control involve heightened activation of the plantar flexor muscles. Consequently, this neuromuscular mechanism may well contribute to postural control alterations often observed in older adults grappling with obesity. These findings shed light on the intricate interplay between obesity and postural control regulation, opening avenues for further exploration and intervention in this critical area of geriatric health.
While existing studies emphasize the effectiveness of physical activity as a non-pharmacological strategy for managing older adults with SO [6,29–32], there remains a gap in research investigating the impact of a comprehensive combined physical activity program on neuromuscular capacities and postural control regulation. Furthermore, previous studies have demonstrated limitations, such as insufficient detail regarding the methodology for implementing the physical activity protocol, program progression, exercise quality and intensity, as well as personalized feedback-driven session quantification. These deficiencies underscore the critical necessity for thorough investigations aimed at developing effective physical activity interventions tailored to the unique requirements and capabilities of older adults with SO. To our current understanding, a limited number of studies have delved into the effects of combined physical activity programs tailored to older adults SO. Notably, Maktouf et al. [18] stands out, centering on the impact of their intervention on balance parameters. However, their investigation was primarily confined to outcomes related to balance and lacked a comprehensive evaluation of the crucial neuromuscular parameters around the ankle joint pivotal for enhancing balance – such as muscle activation, rate of force development (RFD), maximal and submaximal force production of ankle muscles. Based on the literature, it becomes increasingly evident that a comprehensive investigation encompassing postural control regulation, coupled with a detailed exploration of the potential underlying factors catalyzing improvements, is imperative. By concurrently scrutinizing the intricate mechanisms underpinning balance enhancement and gait parameter improvements, a more holistic understanding can be achieved. This multifaceted analysis sheds light on the intricate interplay between various neuromuscular parameters and functional outcomes, thereby providing a more profound insight into the mechanisms of improvement. The implementation of the Total Mobility Plus Program (TMP), designed to encompass a diverse spectrum of exercises targeting strength, power, endurance, and mobility, is poised to address these intricacies comprehensively. This multifunctional approach holds the potential to offer deeper insights into the intricate mechanisms of improvement, ultimately paving the way for more effective interventions.
This study protocol seeks to comprehensively assess the efficacy of the TMP program on postural control parameters and neuromuscular capacity of ankle muscles in older adults with SO. Furthermore, this investigation aims to explore the intricate relationship between the enhancements in postural parameters and the concurrent modifications in neuromuscular parameters.
2. Materials and Methods
2.1. Ethical approval and trial registration
The study protocol is meticulously developed and implemented in unwavering alignment with the principles delineated in the Helsinki Declaration. Furthermore, the study protocol, alongside the patient information letter and informed consent documents, has received approved from the Ethics Committee of the South Ethics Committee for the Protection of Persons in Tunisia, denoted by the reference number C.P.P. SOUTH /No. 0477/2022, on 22 February 2022. The study has also been formally registered with the Pan African Clinical Trials Registry, where it has been assigned the unique registration identifier PACTR202306912191110.
2.2. Research design
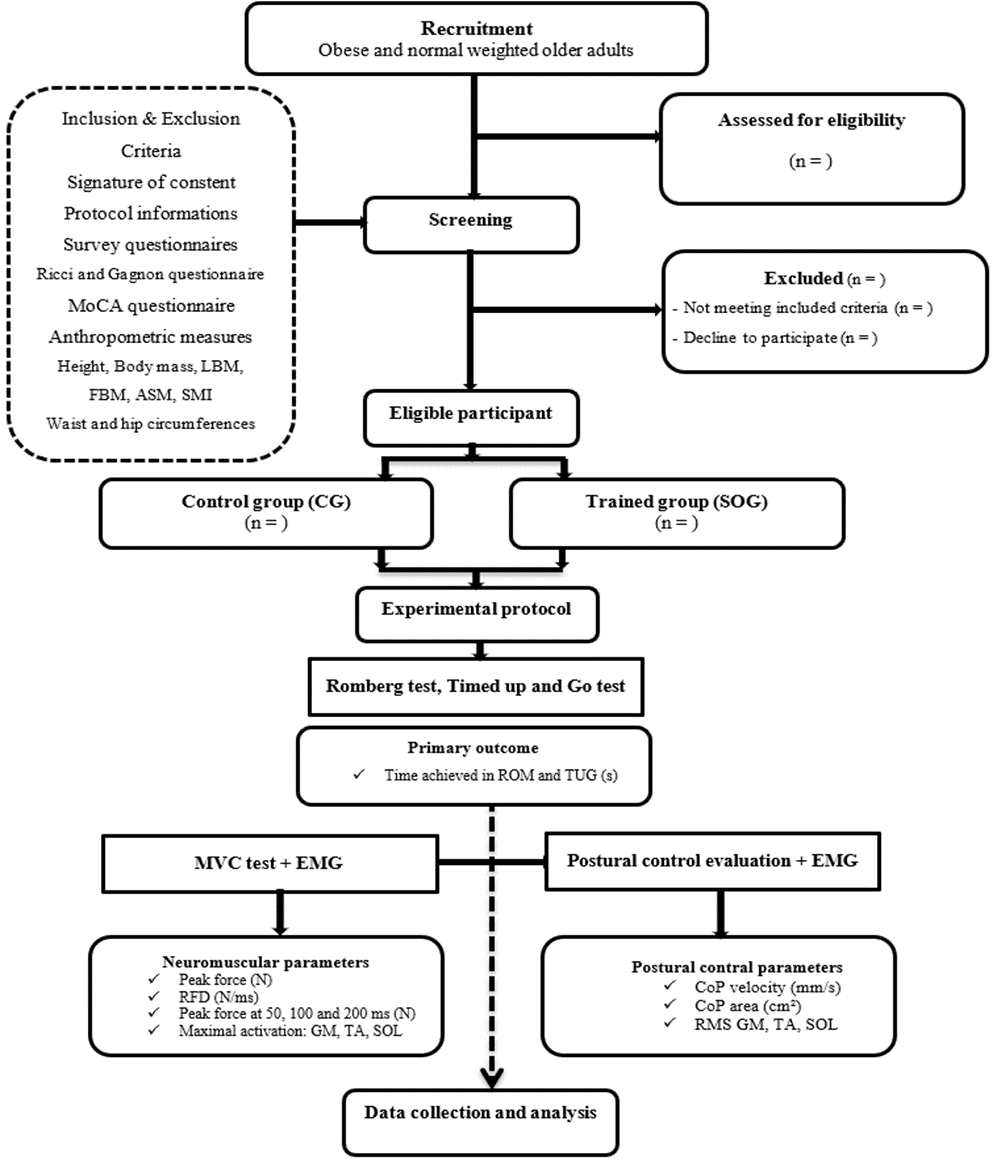
This study employs a single-blinded, prospective, controlled, randomized multicenter design. Participants are being randomly assigned to either the trained group (TG) or the control group (CG) (Figure 1). The CG, although not exposed to any intervention, undergoes both pre- and post-evaluation tests. On the other hand, the TG engages in a rigorous 4-month program involving tri-weekly sessions. The recruitment phase adheres to a standardized procedure, spanning 3 weeks for participant recruitment, 1 week for screening, and an additional 3 weeks for pre- and post-intervention experimental testing. Throughout the pre- and post-evaluation assessments, participants undergo a comprehensive standardized assessment protocol. This encompasses anthropometric measurements and clinical evaluations to gauge health status, balance tests coupled with electromyography evaluation of ankle muscle activation, and maximal voluntary contraction tests of plantar and dorsal flexor muscle.
2.3. Intervention: TMP program
The TPM program is reported using the Template for Intervention Description and Replication Guidelines (TIDieR) and based on the methodology presented in the study of Ferhi et al. [33] as demonstrated in Table 1.
|
Intervention Description and Replication Guidelines (TIDieR) |
|
|
Name |
The TMP program. |
|
Why |
To improve static postural control in older adults with SO. |
|
What (materials) |
Diverse range of physical materials: chairs, balls, markers, slats, cups, hoops, elastic bands, and weighted bags, foam rollers, balance boards, resistance tubes and bands, exercise mats, medicine balls, step platforms, cones, kettlebells, and step platforms. |
|
What (procedures) |
The TMP program will span 16 weeks, with three 60-minute sessions per week, amounting to a total of 48 sessions throughout the intervention period. Each session will follow a structured format, commencing with a 10-minute warm-up. The core of the session will include motor skill exercises and strengthening/posture exercises, with the duration determined by the prescribed training volume. The session will conclude with a 5-minute cool down phase. |
|
Who |
By a kinesiologist specialized in adapted physical activity. |
|
How |
In collective, face-to-face sessions. |
|
Where |
In the fitness hall of each respective structure. |
|
When and how much |
The TMP program will commence and is expected to be completed over a 4-month duration. Participants will attend three sessions per week, accumulating a total of 48 sessions throughout the intervention period. Each session will have a duration of 60 minutes. The design of exercise types within the program will be tailored to the training load of each session and will be based on predetermined training intensity and volume for individual sessions. Each exercise type regimen will consist of 1 to 5 sets, with repetitions ranging from a minimum of 3 to a maximum of 15 per set. |
|
Tailoring |
The adjustment in training load will be determined following each session using the Rating of Perceived Exertion (RPE) scale. The RPE scale spans from 0 (no difficulty) to 10 (extremely difficult), and the group's training load will be calculated by multiplying the RPE score of the session by its duration. For instance, if a group has an average RPE score of 6 in a 60-minute session, the training load will total 360 arbitrary units (a.u.). This approach will ensure the effectiveness of the training, particularly concerning the progressive challenge it presents, and will facilitate the monitoring of potential overtraining syndrome. When the training load exceeds 300 a.u. (equivalent to 5 × 60 minutes), it will be maintained for the subsequent session. If the training load falls below 300, the number of sets and repetitions will be increased by 25% in the following session. |
|
Modifications |
Adjustments will be made to the exercises in each session, with an increase in the number of series or repetitions based on the training load. The difficulty will also be progressively enhanced by adding obstacles, setting a time limit for task completion, and other means to ensure gradual progression and increasing solicitation. |
|
How well (planned) |
The TMP program will include 3 micro-cycles, each comprising 16 sessions: the first micro-cycle will emphasize volume, the second will focus on intensity, and the third will aim to balance both volume and intensity. |
|
How well (actual) |
We anticipate full attendance from all participants in the SOG group. |
Table 1: Intervention description.
2.4. Outcome measures
2.4.1. Primary outcomes
Measures of steady-state balance will be evaluated using the Romberg Test (ROM). Participants will receive instructions to maintain an upright stance without shoes. They will need to keep their feet close together, arms fully extended in front of their bodies with palms facing upwards, and eyes closed for the duration of the test. If participants open their eyes, adjust their stance or arm position to regain balance, or seek assistance from the operator, the attempt will be terminated. Each participant will complete three attempts at the ROM, with a one-minute rest between each attempt. The longest time (s) a participant can stand will be recorded.
Proactive balance will be assessed through the Timed Up and Go test (TUG). Instructions will guide participants to begin in a seated position on a chair with a height of 46 cm, placing their arms on the armrests. Participants will be prompted to rise from the chair, walk 3 meters at their regular walking speed, turn around, return to the chair, and sit down. This test will be performed twice, and the fastest completion time in seconds will be noted (s).
2.4.2. Secondary outcomes:
Anthropometric parameters: participants' body mass (BM) and height (H) will be measured using a digital floor scale and a wall-mounted stadiometer, respectively. This data will be then used to compute the Body Mass Index (BMI) as:
BMI (kg/m²) = BM (kg) / H² (m) (1)
To ascertain muscle constitution, a validated formula will be used to estimate the appendicular skeletal muscle mass (ASM) [34]:
ASM (kg) = 0.193 × BM (kg) + 0.107 × H (cm) - 4.157 × Sex - 0.037 × Age (years) - 2.631 (2)
In this formula, males will be assigned a value of 1 and females a value of 2. Using the derived ASM, the Skeletal Muscle Mass Index (SMI) will be then calculated as [34]:
SMI (kg/m²) = ASM (kg) / H² (m) (3)
Postural control parameters: postural parameters will be measured using a force platform (Zebris; FDM S; sampling rate: 100 Hz; Zebris Medical GmbH, Isny, Germany). Participants will be instructed to stand barefoot on the platform; their feet together with their arms alongside their body and performed postural trials through two conditions: with eyes opened (EO), with eyes closed (EC). Each trial lasted 30 s and will be separated to the following by a 30 s rest period. Displacements of the CoP will be used to extract: the mean sway area of the 95% confidence ellipse (area, cm²) and the mean velocity of the CoP displacements (velocity, mm.s-1).
Neuromuscular parameters: Neuromuscular parameters of the ankle plantar flexors (PF) and dorsiflexors (DF) of the dominant leg will be assessed using a dynamometer (K-Force, Kinvent, Montpellier, France) during maximal isometric voluntary contractions (MVC). This assessment will include both plantar and dorsiflexion movements. Throughout each contraction attempt, participants will be instructed to apply their maximum effort. To ensure reliability, each participant will perform two attempts for both plantar flexion and dorsiflexion, with a rest period of one minute between attempts. For the analysis, the average of the highest recorded values of maximal force from the two attempts will be calculated for both PF (Absolute PF, N) and DF (Absolute DF, N). The relative peak force will be calculated by normalizing the maximal force to the participant's body mass for PF (Relative PF, N/kg) and DF (Relative DF, N/kg). The dynamometer signals will be stored offline for subsequent analysis. Absolute force will be recorded at 50 ms (F50_PF, F50_DF, N), 100 ms (F100-PF, F100-DF, N) and 200 ms (F200-PF, F200-PF, N). Absolute forces will be normalized to BM (Relative F50-PF, Relative-F100_PF, Relative F200-PF, Relative F50-DF, Relative F100-DF, Relative F200-DF, respectively, N/kg). Early RFD will also be obtained from each MVC contraction from onset to 50 ms (PF-RFD(50-100), DF-RFD(50-100), N/ms). Late RFD was acquired from 100 to 200 ms (PF-RFD(100-200), DF-RFD(100-200), N/ms). All RFD will be calculated from the linear slop of the force – time curve (Δ force/Δ time).
Electromygraphy (EMG) data: EMG data from the ankle joint muscles will be captured during MVC of PF and DF and during postural control tests. The collection process will use the Trigno® Wireless Biofeedback System (Delsys Inc., Natick, MA). The sensors, consisting of two pairs of silver bar contacts with 10 mm interelectrode spacing, are positioned on the gastrocnemius medialis (GM), soleus (SOL), and tibialis anterior (TA) of the dominant leg, in accordance with the recommendations of SENIAM. The raw EMG signals are then post-processed using Matlab software (Matlab R2013a, MathWorks, Natick, USA). Data collected over a 10-second period, beginning at the 10th second of each postural control trial. The data will be band-pass filtered at 15–500 Hz via a second-order Butterworth digital filter to remove any noise or movement interference [35]. Subsequently, the data will be rectified and smoothed through root mean square analysis (RMS) with a 20-ms window, using the following equation [36]:
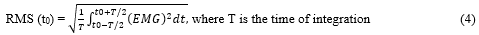
For the MVC tests, a moving window with a width of 20 ms is employed to identify the peak RMS EMG activity resulting from the three MVC efforts for each type of contraction. Subsequently, all RMS EMG data collected during postural control tests will be normalized using the following equation for each muscle:
EMG RMS% = [(RMS EMG trial/RMS EMG MVC) × 100%] (5)
The study utilizes the normalized RMS of the gastrocnemius medialis (RMS GM), soleus (RMS SOL), and tibialis anterior (EMG TA) from each postural control test.
2.5. Recruitment and randomization
This multicenter trial involves four distinguished Tunisian centers, each specializing in diverse obesity care within the designated region. The study is planned to be completed within a six-month timeframe, with each center commencing its participation once the necessary number of participants has been secured. As a result, the objective is to simultaneously implement the study protocol for all eligible participants within each center. This study protocol aims to initially recruit a cohort of 100 volunteers for future investigations. The process of inclusion and randomization will be carried out electronically through an online system at each investigator center. Clinical assessments, postural control testing, and EMG procedures will be conducted at a single investigating center. The initial screening visits will coincide with regular clinic appointments at each center. Participants who meet the selection criteria (Table 2) will receive an invitation to participate in the study. They will be provided with written information describing the study and given a week to make an informed decision about their participation. Once they decide to join, informed consent will be obtained and documented.
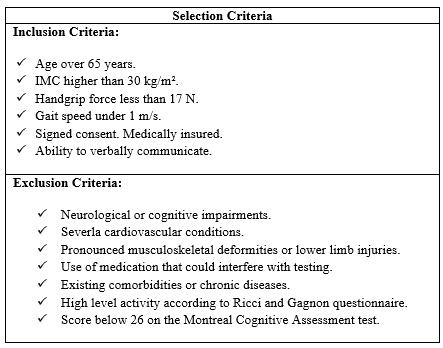
Table 2: Inclusion and exclusion criteria.
Randomization will occur on the day of inclusion, overseen by the chief investigator at each center. Each participant will be randomly assigned to one of the two groups: the CG and the SOG. To minimize any potential negative effects in the trained group, the study coordinator will not disclose the specifics of the TMP program to the participants. In practice, a blinded assessor will visit participants twice before and after the program, while unblinded kinesiologist will conduct separate visits during the program to provide treatment and exercise sessions. The randomization list will be generated by an independent statistician from the Clinical Research Unit of our laboratory, with no direct involvement in the study. This list will be computer-generated and subsequently uploaded into an online case report form (eCRF). To perform randomization, the chief investigator at each center will access the eCRF through a web browser. They will input participant characteristics and assign individuals to their respective study groups directly via the online platform. Each study participant will receive a unique allocation study number in a sequential format (TMP00X). Randomization will occur after participant enrollment, and individuals will be assigned to either the SOG or the CG. A confirmation email will be sent to the unblinded investigator at the coordinating center to acknowledge the randomization process. Blinding will be maintained until the database is finalized.
2.6. Data management and monitoring
All the necessary data stipulated by the study protocol will be diligently entered into the Electronic Data Capture (EDC) system. Data will be logged in real-time as they are acquired, ensuring a contemporaneous record. Access to the EDC system will be granted to all participating sites. To facilitate effective utilization of this tool, comprehensive guidance documents will be provided to investigators. Each investigator holds the responsibility for maintaining the accuracy, quality, and relevance of all recorded data. In instances where a study subject discontinues their participation or withdraws their consent, all data collected up to the point of cessation will be subject to analysis. In cases of subjects becoming lost to follow-up, investigators will exert every reasonable effort to reestablish contact with the patient. However, individuals who have prematurely terminated their involvement in the research will not be substituted with new participants. A Clinical Research Associate will oversee the meticulous handling of the study. Their duties encompass the collection, documentation, recording, and reporting of all handwritten data, in strict adherence to the principles of Good Clinical Practice. To maintain data integrity and accuracy, a computerized quality control system will be implemented, which includes mechanisms for detecting missing data and verifying data consistency. Should any discrepancies or inaccuracies arise during the completion of EDC forms, investigators will be promptly notified and requested to rectify the issues accordingly. This approach ensures the reliability and fidelity of the study data throughout the research process.
3. Statistical Methods
3.1. Sample size
The sample size is calculated using the freeware G*Power (version 3.1.9.4). The ANOVA test was predefined for power analysis. The estimation is based on predefined control of type I error (alpha = 0.05) and Type II error (beta = 0.60), with a moderate level of estimated effect size (r = 0.35). Under these settings, 40 participants were required as the minimum sample size.
3.2. Statistical analysis
Statistical analyses will be conducted using Statistica Software 13.0 (Software, Inc., Tulsa, OK). We will begin by verifying the normality of the data distribution using Kolmogorov-Smirnov tests. For data exhibiting a normal distribution, paired t-tests will be employed to compare results within the same group both before and after the implementation of the TMP program. Furthermore, an independent samples t-test will be used to draw comparisons between the TG and CG pre- and post-TMP program. We also aim to examine the relationships between postural and neuromuscular parameters using Pearson's correlation analysis. All data will be presented as means and standard deviations, with the threshold for significance set at p < 0.05 across all results.
4. Discussion
Our study focuses on older adults with SO and aims to evaluate the impact of a TMP program, which includes combined exercises for muscle strengthening, balance, and motricity, on improving postural control and neuromuscular capacities. Specifically, we aim to demonstrate the potential for trainability in this specific population and explore the reversibility of both postural control and neuromuscular capacities. Since postural control is a crucial indicator of autonomy, our study seeks to provide valuable insights into optimizing functional capacity, and enhancing overall mobility and quality of life among older adults with SO. Moreover, the results of our research hold the potential to inform the development of tailored interventions that promote healthy aging, prevent functional limitations, and have far-reaching implications for health outcomes and socio-economic factors. To our knowledge, this study represents the first attempt to comprehensively assess the impact of a combined physical activity program on neuromuscular capacities and their role in enhancing postural control among older adults with SO.
Previous research has highlighted the association between sarcopenia and obesity with increased postural sway, potentially compromising biomechanical aspects of daily activities and raising the risk of injury [4,37]. In obese adults, postural control alterations can be attributed to several factors. Firstly, the constant load bearing necessitated by excess weight often leads to diminished plantar sensitivity due to hyperactivation of plantar mechanoreceptors [38,39]. Secondly, managing the mechanical demands of an increased body mass, especially when it is distributed further from the rotational axis (as in the ankle joint, resembling an inverted pendulum model), results in an amplified gravitational torque [40]. To counteract this torque, acting along the anteroposterior axis, obese individuals must generate increased muscular torque to maintain an upright posture [41]. Additionally, cognitive challenges that require greater effort to maintain balance may exacerbate balance control issues in obese individuals [42]. These challenges are further compounded by the presence of sarcopenia, which is characterized by progressive alterations in neuromusculoskeletal, proprioceptive, and visual systems [43,44]. This condition also affects sensory integration, collectively impairing postural control [18,45]. Thus, it is evident that the interplay between obesity and sarcopenia in older adults significantly influences postural control [28,46,47].
Recent findings by Maktouf et al. [17] highlight the cumulative effect of age and obesity on postural control, indicating that the mechanisms driving postural control alterations in obese older adults can be attributed to the combined influences of age and obesity. According to their results, two primary mechanisms contribute to these alterations. First, the additional force required to maintain body posture and correct imbalance in the presence of increased body fat mass may exceed the available force production capacity in obese older adults, contributing to postural control deficits. Second, the observed increase in postural control alterations in obese older adults, particularly the positive correlations between GM activity and postural parameters, suggests that obese older adults may need to augment PF muscle activity to counteract forward instability and compensate for reduced force production capacity. Moreover, it is conceivable that older individuals maintain their muscles in a state of high activation, leading to increased muscle co-activation to counteract the decline in relative force and maintain stability [43,48,49]. Consequently, obese older adults may need to increase PF muscle activity to compensate for reduced force production capacity by increasing muscle co-activation. However, heightened muscle activity comes at the cost of increased energy expenditure, leading to premature fatigue and a heightened risk of falls [50].
The implementation of the TMP Program signifies a critical step forward in our quest to unravel the complexities surrounding the interplay between obesity, sarcopenia, and postural control among older adults [51,52]. By offering a multifaceted regimen that spans various dimensions of physical fitness, including strength, power, endurance, and mobility, TMP transcends the limitations of previous interventions [33]. This multifunctional approach holds great promise as it delves into the nuanced intricacies of improvement within this population [33]. By engaging participants in a holistic exercise program that systematically progresses in intensity and complexity, we anticipate that TMP will provide a deeper understanding of the underlying mechanisms of improvement [31]. This, in turn, could usher in a new era of more precisely tailored and impactful interventions for older adults grappling with the challenges of sarcopenic obesity [53]. The holistic and data-driven approach of TMP stands as a testament to our commitment to improving the overall quality of life for this population, ultimately striving for a future where individuals can age gracefully with enhanced postural control and independence [54].
The successful implementation of the current physical activity program for older adults with SO hinges on a holistic approach that meticulously addresses both qualitative and quantitative dimensions of the intervention. Firstly, it is imperative to craft an exercise regimen that is meticulously tailored to address the characteristics and challenges inherent in this population, as previously delineated. This personalized approach serves as a cornerstone, ensuring that exercises are custom designed to meet the distinct needs of older adults grappling with SO, thus fostering a more effective intervention. Secondly, we place considerable emphasis on leveraging participants' perceived exertion as a guiding principle for modulating exercise intensity within the SO cohort. By factoring in each individual's perceived exertion, our aim is to calibrate exercise intensity to align with their specific capabilities, ultimately promoting optimal outcomes. This individualized strategy empowers us to mobilize older adults with SO at an intensity that strikes a harmonious balance between challenge and manageability, all while taking into account their distinctive physiological and functional attributes. Continuous assessments throughout the program's duration will further ensure that exercise intensity remains finely tuned, adapting to participants' evolving perceived exertion levels and progress. By embracing participants' perceived exertion as a guiding metric and tailoring exercise intensity accordingly, our overarching goal is to maximize the program's benefits, fostering sustained engagement in physical activity among older adults with SO.
5. Conclusions
Our study protocol focuses on older adults with SO and aims to evaluate the effectiveness of a combined physical activity program in improving postural control ability by targeting the neuromuscular system. Our findings will provide valuable insights into the potential for trainability in this population, exploring the reversibility of both postural control and neuromuscular capacities. Furthermore, our research holds great promise in optimizing functional capacity, reducing fall risk, and enhancing overall mobility and quality of life among older adults with SO.
Author Contributions: Conceptualization, H.F., E.M., W.M.; Methodology, H.F., E.M., W.M.; Validation, B.B., S.B., W.M.; Writing—original draft, H.F., E.M., W.M.; Writing—review & editing, S.D., O.G.C., O.G.C; Supervision, W.M., S.D., S.G.C; Project administration, S.G.C., B. B., All authors have read and agreed to the published version of the manuscript.
Funding:
This research received no external funding.
Acknowledgments:
We would like to express our gratitude to all the organizations that have provided their approval for the implementation and collaboration in this protocol.
Conflicts of Interest:
The authors declare no conflict of interest.
References
- Zamboni M, Rubele S, Rossi AP. Sarcopenia and Obesity. Curr Opin Clin Nutr Metab Care 22 (2019): 13-19.
- Colleluori G, Villareal DT. Aging, Obesity, Sarcopenia and the Effect of Diet and Exercise Intervention. Exp Gerontol 155 (2021): 111561.
- Zhang L, Liu S, Wang W, et al. Dynapenic Abdominal Obesity and the Effect on Long-Term Gait Speed and Falls in Older Adults. Clin. Nutr 41 (2022): 91-96.
- Handrigan GA, Maltais N, Gagné M, et al. Sex-Specific Association between Obesity and Self-Reported Falls and Injuries among Community-Dwelling Canadians Aged 65 Years and Older. Osteoporos Int 28 (2017): 483-494.
- Hsu KJ, Liao CDe, Tsai MW, et al. Effects of Exercise and Nutritional Intervention on Body Composition, Metabolic Health, and Physical Performance in Adults with Sarcopenic Obesity: A Meta-Analysis. Nutrients 11 (2019).
- Liao CDe, Tsauo JY, Lin LF, et al. Effects of Elastic Resistance Exercise on Body Composition and Physical Capacity in Older Women with Sarcopenic Obesity: A CONSORT-Compliant Prospective Randomized Controlled Trial. Medicine (Baltimore) 96 (2017).
- Frontera WR. Physiologic Changes of the Musculoskeletal System with Aging: A Brief Review. Phys Med Rehabil Clin N Am 28 (2017): 705-711.
- Kalinkovich A, Livshits G. Sarcopenic Obesity or Obese Sarcopenia: A Cross Talk between Age-Associated Adipose Tissue and Skeletal Muscle Inflammation as a Main Mechanism of the Pathogenesis. Ageing Res Rev 35 (2017): 200-221.
- Cunningham C, O’ Sullivan R, Caserotti P, et al. Consequences of Physical Inactivity in Older Adults: A Systematic Review of Reviews and Meta-Analyses. Scand J Med Sci Sport 30 (2020): 816-827.
- van den Beld AW, Kaufman JM, Zillikens MC, et al. The Physiology of Endocrine Systems with Ageing. Lancet Diabetes Endocrinol 6 (2018): 647-658.
- Campisi J, Kapahi P, Lithgow GJ, et al. From discoveries in ageing research to therapeutics for healthy ageing. Nature 571 (2020): 183-192.
- Norman K, Haß U, Pirlich M. Malnutrition in Older Adults-Recent Advances and Remaining Challenges. Nutrients 13 (2021): 2764.
- Tezuka T, Inoue A, Hoshi T. The MuSK Activator Agrin Has a Separate Role Essential for Postnatal Maintenance of Neuromuscular Synapses. Proc Natl Acad Sci USA 111 (2014): 16556-16561.
- Chen SH, Chou LS. Gait Balance Control after Fatigue: Effects of Age and Cognitive Demand. Gait Posture 95 (2022): 129-134.
- Nascimento MM, Gouveia ÉR, Gouveia BR, et al. Associations of Gait Speed, Cadence, Gait Stability Ratio, and Body Balance with Falls in Older Adults. Int J Environ Res Public Health 19 (2022): 13926.
- Macie A, Matson T, Schinkel-Ivy A. Age Affects the Relationships between Kinematics and Postural Stability during Gait. Gait Posture 102 (2023): 86-92.
- Maktouf W, Durand S, Boyas S, et al. Combined Effects of Aging and Obesity on Postural Control, Muscle Activity and Maximal Voluntary Force of Muscles Mobilizing Ankle Joint. J Biomech 79 (2018): 198-206.
- Maktouf W, Durand S, Beaune B, et al. Influence of Obesity and Impact of a Physical Activity Program on Postural Control and Functional and Physical Capacities in Institutionalized Older Adults: A Pilot Study. J Phys Act Heal 17 (2019): 169-176.
- Capodaglio P, Gobbi M, Donno L, et al. Effect of Obesity on Knee and Ankle Biomechanics during Walking. Sensors 21 (2021): 7114.
- Vankrunkelsven W, Derde S, Gunst J, et al. Obesity Attenuates Inflammation, Protein Catabolism, Dyslipidaemia, and Muscle Weakness during Sepsis, Independent of Leptin. J. Cachexia. Sarcopenia Muscle 13 (2022): 418-433.
- Pérez-Torres I, Castrejón-Téllez V, Soto ME, et al. Oxidative Stress, Plant Natural Antioxidants, and Obesity. Int J Mol Sci 22 (2021): 1-26.
- Al-Sulaiti H, Diboun I, Agha MV, et al. Metabolic Signature of Obesity-Associated Insulin Resistance and Type 2 Diabetes. J Transl Med 17 (2019): 348.
- Khan IM, Perrard XYD, Brunner G, et al. Intermuscular and Perimuscular Fat Expansion in Obesity Correlates with Skeletal Muscle T Cell and Macrophage Infiltration and Insulin Resistance. Int J Obes (Lond) 39 (2015): 1607-1618.
- Maktouf W, Durand S, Boyas S, et al. Interactions among Obesity and Age-Related Effects on the Gait Pattern and Muscle Activity across the Ankle Joint. Exp Gerontol 140 (2020): 111054.
- Meng H, Gorniak SL. Obesity Is Associated With Gait Alterations and Gait Asymmetry in Older Adults. Motor Control 27 (2022): 6-19.
- Liao CDe, Chen HC, Liou TH, et al. Impact of Sarcopenia and Obesity on Gait Speed After Total Knee Replacement. J Am Med Dir Assoc 23 (2022): 631-637.
- Maffiuletti NA, Agosti F, Proietti M, et al. Postural Instability of Extremely Obese Individuals Improves after a Body Weight Reduction Program Entailing Specific Balance Training. J Endocrinol Invest 28 (2005): 2-7.
- Melzer I, Oddsson LIE. Altered Characteristics of Balance Control in Obese Older Adults. Obes Res Clin Pract 10 (2016): 151-158.
- Theodorakopoulos C, Jones J, Bannerman E, et al. Effectiveness of Nutritional and Exercise Interventions to Improve Body Composition and Muscle Strength or Function in Sarcopenic Obese Older Adults: A Systematic Review. Nutr Res 43 (2017): 3-15.
- Kim B, Tsujimoto T, So R, et al. Changes in Muscle Strength after Diet-Induced Weight Reduction in Adult Men with Obesity: A Prospective Study. Diabetes Metab Syndr Obes Targets Ther 10 (2017): 187-194.
- Chiu SC, Yang RSRJ, Yang RSRJ, et al. Effects of Resistance Training on Body Composition and Functional Capacity among Sarcopenic Obese Residents in Long-Term Care Facilities: A Preliminary Study. BMC Geriatr 18 (2018): 1-11.
- Hita-Contreras F, Bueno-Notivol J, Martínez-Amat A, et al. Effect of Exercise Alone or Combined with Dietary Supplements on Anthropometric and Physical Performance Measures in Community-Dwelling Elderly People with Sarcopenic Obesity: A Meta-Analysis of Randomized Controlled Trials. Maturitas 116 (2018): 24-35.
- Ferhi H, Chortane SG, Durand S, et al. Effects of Physical Activity Program on Body Composition , Physical Performance , and Neuromuscular Strategies during Walking in Older Adults with Sarcopenic Obesity: Randomized Controlled Trial Healthcare (Basel) 11 (2023): 2294.
- Wang H, Hai S, Liu Y, et al. Skeletal Muscle Mass as a Mortality Predictor among Nonagenarians and Centenarians: A Prospective Cohort Study. Sci Rep 9 (2019): 1-8.
- Luca CJ De, Gilmore LD, Kuznetsov M, et al. Filtering the Surface EMG Signal: Movement Artifact and Baseline Noise Contamination. J Biomchanics 43 (2010): 1573-1579.
- Hermens HJ, Freriks B, Disstelhorst-Klug C, et al. Development of Recommendations for SEMG Sensors and Sensor. J Electromyogr Kinesiol 10 (2000): 361-374.
- Wearing SC, Hennig EM, Byrne NM, et al. The Biomechanics of Restricted Movement in Adult Obesity. Obes Rev 7 (2006): 13-24.
- Handrigan GA, Simoneau M, Teasdale N, et al. The Effects of Added Mass on Plantar Sole Sensitivity in Upright Standing. J Biomech 45 (2012): S233.
- Wu X, Madigan ML. Impaired Plantar Sensitivity among the Obese is Associated with Increased Postural Sway. Neurosci Lett 583 (2014): 49-54.
- Corbeil P, Simoneau M, Rancourt D, et al. Increased Risk for Falling Associated with Obesity: Mathematical Modeling of Postural Control. IEEE Trans. Neural Syst. Rehabil. Eng 9 (2001): 126-136.
- Simoneau M, Teasdale N. Balance Control Impairment in Obese Individuals is Caused by Larger Balance Motor Commands Variability. Gait Posture 41 (2015): 203-208.
- Mignardot JB, Olivier I, Promayon E, et al. Obesity Impact on the Attentional Cost for Controlling Posture. PLoS One 5 (2010): 1-6.
- Donath L, Kurz E, Roth R, et al. Different Ankle Muscle Coordination Patterns and Co-Activation during Quiet Stance between Young Adults and Seniors Do Not Change after a Bout of High Intensity Training. BMC Geriatr 15 (2015): 19.
- Nagai K, Yamada M, Mori S, et al. Effect of the Muscle Coactivation during Quiet Standing on Dynamic Postural Control in Older Adults. Arch. Gerontol. Geriatr 56 (2013): 129-133.
- Dutil M, Handrigan GA, Corbeil P, et al. The Impact of Obesity on Balance Control in Community-Dwelling Older Women. Age (Omaha) 35 (2013): 883-890.
- Rossi-Izquierdo M, Santos-Pérez S, Faraldo-García A, et al. Impact of Obesity in Elderly Patients with Postural Instability. Aging Clin Exp Res 28 (2016): 423-428.
- Mainenti MRM, Rodrigues ÉdeC, Oliveira JFde, et al. Adiposity and Postural Balance Control: Correlations between Bioelectrical Impedance and Stabilometric Signals in Elderly Brazilian Women. Clinics 66 (2011): 1513-1518.
- Maktouf W, Boyas S, Beaune B, et al. Differences in Lower Extremity Muscular Coactivation during Postural Control between Healthy and Obese Adults. Gait Posture 81 (2020): 197-204.
- Power GA, Dalton BH, Rice CL. Human Neuromuscular Structure and Function in Old Age: A Brief Review. J Sport Heal Sci 2 (2013): 215-226.
- Mian OS, Thom JM, Ardigo LP, et al. Metabolic Cost, Mechanical Work, and Efficiency during Walking in Young and Older Men. Acta Physiol 186 (2006): 127-139.
- Zembron-Lacny A, Dziubek W, Rogowski L, et al. Sarcopenia: Monitoring, Molecular Mechanisms, and Physical Intervention. Physiol Res 63 (2014): 683-691.
- Wiklund P. The Role of Physical Activity and Exercise in Obesity and Weight Management: Time for Critical Appraisal. J. Sport Heal Sci 5 (2016): 151-154.
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European Consensus on Definition and Diagnosis. Age Ageing 39 (2010): 412-423.
- Bilski J, Pierzchalski P, Szczepanik M, et al. Multifactorial Mechanism of Sarcopenia and Sarcopenic Obesity. Role of Physical Exercise, Microbiota and Myokines. Cells 11 (2022) 160.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 73.64%
Acceptance Rate: 73.64%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 