Phytochemical Profile, Biological Activities and Potential Involvement of Opioid Receptors in Antinociceptive Effects of a Canarium Schweinfurthii Engl. Stem Bark Aqueous Extract
Ericka Lorleil Mayindza Ekaghba1,2, Noreen Orianna Koumba Madingou1, Manon Grenet3, Pierrick Gandolfo3, Olivier Perruchon2, Corinne Loutelier-Bourhis4, Isabelle Schmitz4, Carlos Afonso4, Patrice Lerouge2*, Line Edwige Mengome1*
1Institute of Pharmacopoeia and Traditional Medicine (IPHAMETRA), National Center for Scientific and Technical Research (CENAREST), BP: 12 141 Libreville, Gabon
2University of Rouen Normandy (UNIROUEN), Normandy Univ, GlycoMEV UR 4358, SFR Normandy Plant FED 4277, Carnot Chemistry Innovation, IRIB, GDR CNRS Chemobiology F-76000 Rouen, France
3University of Rouen Normandy (UNIROUEN), Normandy Univ, Inserm U1245, F-76000 Rouen, France
4Université de Rouen Normandie, INSA Rouen Normandie, Univ Caen Normandie, ENSICAEN, CNRS, Institut CARMeN UMR 6064, F-76000 Rouen, France
*Corresponding Author:
Line Edwige Mengome, Institute of Pharmacopoeia and Traditional Medicine (IPHAMETRA), National Center for Scientific and Technical Research (CENAREST), BP: 12 141 Libreville, Gabon
Patrice Lerouge,University of Rouen Normandy (UNIROUEN), Normandy Univ, GlycoMEV UR 4358, SFR Normandy Plant FED 4277, Carnot Chemistry Innovation, IRIB, GDR CNRS Chemobiology F-76000 Rouen, France
Received: 03 July 2025; Accepted: 10 July 2025; Published: 18 July 2025
Article Information
Citation: Ericka Lorleil Mayindza Ekaghba, Noreen Orianna Koumba Madingou, Manon Grenet, Pierrick Gandolfo, Olivier Perruchon, Corinne Loutelier- Bourhis, Isabelle Schmitz, Carlos Afonso, Patrice Lerouge, Line Edwige Mengome. Phytochemical Profile, Biological Activities and Potential Involvement of Opioid Receptors in Antinociceptive Effects of a Canarium Schweinfurthii Engl. Stem Bark Aqueous Extract. International Journal of Plant, Animal and Environmental Sciences. 15 (2025): 44-59.
View / Download Pdf Share at FacebookAbstract
The decoction of barks of Canarium schweinfurthii (DCS) is used by the Gabonese population against roundworms, colic, stomach and intestinal pain. However, compounds and biological mechanisms involved in its effect on pain are not known. The aim of the present study is to investigate the safety, the phytochemical profile and the analgesic activities of DCS, as well as the involvement of opioid receptors in its antinociceptive effects. The phytochemical composition was determined by spectrophotometry, gas chromatography-electron impact mass spectrometry (GC-MS) and by ultra-high performance liquid chromatography-tandem mass spectrometry (UHPLC-ESI-MS/MS). Phenolic compounds were revealed by spectrophotometry and many secondary metabolites were identified by UHPLC-ESI-MS/MS, including quinic acid and its derivatives. DCS did not exhibit any cytotoxicity in vitro on two human cell lines. Antinociceptive activity were investigated using the tests of acetic acid-induced torsion and of formaldehyde-induced leg licking. This decoction was demonstrated to exhibit a dose-dependent antinociceptive property in the two tests, while quinic acid has a central effect only in the formaldehyde test. Moreover, these effects were inhibited by naloxone. Together, these results show that DCS exhibits antinociceptive opioid-like activities which attenuate effects of pro-inflammatory and neurogenic mediators. This supports its potential medicinal for the treatment of pain in the traditional medicine of Gabon.
Keywords
<p><em>Canarium schweinfurthii</em>; Phytochemical; Quinic acid; Cytotoxicity; Antinociceptive</p>
Article Details
Abbreviations: DCS: Decoction of Canarium schweinfurthii Engl. barks; NSAID: Non-Steroidal Anti-Inflammatory Drug; HEK-293: Human Embryonic Kidney Cells; hCMEC/D3: human Cerebral Microvascular Endothelial Cells; GAE: Gallic Acid Equivalent; QE: Quercetin Equivalent; TAE: Tannic Acid Equivalent; GC-EI-MS: Gas Chromatography Coupled to Electron Ionization Mass Spectrometry; UHPLC-ESI-MS/MS: Ultra-High Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry; IPHAMETRA: Institute of Pharmacopoeia and Traditional Medicines; TA: Tannic Acid; Ac: Absorbance of Blank; As: Absorbance of Extracts; PBS: Phosphate Buffered Saline; D0: Average Writhing Response of the Control Group; Dt: Average Writhing Response of the Drug-Treated (indomethacin, quinic acid or plant extract); m/z: Mass-to-Charge Ratio; Ara: Arabinose; Rha: Rhamnose; Fuc: Fucose; Xyl: Xylose; GalA: Galacturonic Acid; Man: Mannose; Gal: Galactose; Glc: Glucose; nd: not determined; FA: Formic Acid; Exp: Experimental; Cal: Calculated; MSD: Mass Selective Detector; SD: Standard Error; COX: Cyclooxygenases; TNF-α: Tumor Necrosis Factor Alpha; VCAM-1: Vascular Cell Adhesion Molecule-1; VSMCs: vascular Smooth Muscle Cells; NF-κB: Nuclear Factor-Kappa B; QA: Quinic Acid; NHG: National Herbarium of Gabon; TRPV1: Transient Receptor Potential Cation Channel Subfamily V Member 1; TRPA1: Transient Receptor Potential Ankyrin 1; RT: Retention Time; ANOVA: Analysis of Variance
1. Introduction
Burseraceae family gathers 18 genera and about 640 species of trees and shrubs, mainly found in the tropical regions. Among Burseraceae, Canarium schweinfurthii Engl. is a large tree of the tropical forest belt of Central and West Africa. The decoction of its barks is traditionally used by the African population to treat chest pain, lung conditions, dysentery, hypertension, gonorrhea, cough, stomach upset and food poisoning [1-2]. The crushed barks are also used in traditional medicine to treat leprosy and ulcers [1,3]. Pharmacological studies performed on Canarium schweinfurthii Engl. extracts revealed antimicrobial, anti-inflammatory, antidiabetic, antioxidant, antihypertensive, antihelminthic, anti-onchocercal and antinociceptive activities [4-11]. In Gabon, the decoction of Canarium schweinfurthii Engl. barks is used against roundworms and other intestinal parasite infections [12]. Moreover, this decoction was previously reported for the treatment of symptoms of the irritable bowel syndrome [13]. Indeed, decoctions of Canarium schweinfurthii Engl. barks are generally recommended in traditional medicine for the treatment of abdominal pains that are associated to diarrhea or constipation [14]. For this reason, the phytochemical profile and antinociceptive activities of a decoction of Canarium schweinfurthii Engl. barks, named DCS was investigated in the present study.
Pain is a major cause of consultations and results in expensive medical expenses and economic losses to the society. Drugs used to manage pain, such non-steroidal anti-inflammatory drugs (NSAIDs) or opiates, target the H1 receptor for histamine, prostaglandins 1 and 2, cyclooxygenases 1 and 2, tumour necrosis factor-α and cysteinyl leukotrienes C4 and D4 receptors [15]. Previous studies demonstrated that in the acute and chronic models of pain, the opening of K+ channels involved in antinociception is induced by NSAIDs [16] or by agonists of G-protein coupled receptors (5-HT1A for serotonin, A1 for adenosine, α2 for noradrenalin, M2 for acetylcholine, GABAB for gamma-aminobutyric acid and also opioidergic and cannabinoidergic receptors) [17]. However, the prolonged use of them is often accompanied with central and peripheral severe side effects [18]. In this context, many current researches are carried out to select herbal medicines exhibiting expected activities with lesser or no side effects. Our study aimed to evaluate the safety, as well as the activity on the central and peripheral antinociceptive systems of DCS. The first step of our study consisted in the annotation of the main metabolites of the active fraction. To this end, gas chromatography-electron impact mass spectrometry (GC-EI-MS) and ultra-high performance liquid chromatography-tandem mass spectrometry (UHPLC-ESI-MS/MS) analyses were carried out. The activity on the central and peripheral antinociceptive systems of DCS was then investigated on two models of nociception, i.e. the acetic acid-induced torsion and formaldehyde-induced leg licking tests. Finally, we investigated the mechanism of antinociception using naloxone, a non-selective opioid receptor antagonist.
2. Material and Methods
2.1 Materials
Instrumentation
Spectrophotometric analysis was performed on a spectrophotometer UV-VIS (Drawell). Ultra-high performance liquid chromatography coupled to tandem mass spectrometry (UHPLC-ESI-MS/MS) analyses were carried out using an UHPLC system (Vanquish, Thermo Scientific, San Jose, CA, USA) coupled to a quadrupole-Orbitrap mass spectrometer (Exploris 120, Thermo scientific, San Jose, CA, USA) equipped with an electrospray ionization source. Gas chromatography coupled to electron ionization mass spectrometry (GC-EI-MS) was performed on an Agilent 8860 GC instrument coupled to a 5977-mass selective detector (MSD) quadrupole MS instrument (Agilent Technologies, Palo Alto, CA, USA).
Chemical or reagents
Quercetin, dihydroxybenzoic acid, gallic acid, ascorbic acid, tannic acid, quinic acid, Folin-Ciocalteu reagent, disodium hydrogen phosphate (Na2HPO4), monobasic potassium phosphate (KH2PO4), dimethyl sulfoxide (DMSO) and indomethacin were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Organic solvents, acids and other chemicals, such as aluminium chloride, sodium carbonate, acetic acid, formaldehyde and hydrochloric acid were purchased from Merck (Darmstadt, Germany). Morphine was purchased in a local pharmacy. All other reagents and chemicals were of analytical grade and organic solvents were of HPLC grade. DCS was stored in a glass container at room temperature.
Plant material
The harvesting of stem barks of Canarium schweinfurthii Engl., was carried in 2020 in Libreville, Gabon. A sample of this plant was deposited at the National Herbarium of Gabon (NHG) where it was authenticated and preserved. The taxonomy of the plant has been validated on the World Flora Online (WFO) website (w.w.w. worlfloraonline.org, accessed on 4 April 2025) and on the International Plant Names Index (IPNI) website (w.w.w.ipni.org, accessed on 4 April 2025).
2.2 Procedures and analytical method
Plant extraction
The stem barks were dried out for two weeks at the Department of Traditional Medicine of IPHAMETRA, Libreville, Gabon and then reduced to a fine powder using a grinder. Five hundred grams of ground material were placed in 2 L of distilled water, brought to the boil at 100°C and then stirred for 1 h. The aqueous solution was filtered, frozen and freeze-dried to give the stem bark decoction of C. schweinfurthii Engl., named DCS. Extraction yield was determined as the ratio of the mass of DCS to the mass of the bark material. For biological assays, the DCS sample was then solubilized in 1 % DMSO in water.
2.3 Determination of total phenolic and flavonoid contents
Total phenolic and flavonoid contents of DCS were determined using the Folin-Ciocalteu and aluminium chloride methods, respectively, as previously reported [13].
2.4 Determination of total tannin contents
The total tannin contents were determined using the vanillin method described by Julkunen-Tito [19] with some modification. 0.5 mL of 1 mg/mL of DCS in distilled water or standard tannic acid solutions (10, 20, 30 et 40 µg/mL) was mixed with 1.5 mL of 4 % (w/v) vanillin in methanol and 1 mL of concentrated hydrochloric acid. After 20 minutes at room temperature in the dark, the absorbances were recorded at λ = 500 nm. The reaction mixture without any extract or tannic acid was considered as the blank. The reaction mixture without any extract or tannic acid was considered as the blank. The standard cuve is drawn and allows from the obtained equation to determine the total tannin concentrations of the tested extracts. The tannin contents are expressed in µg equivalent of tannic acid per mg dry extract (µg TAE/mg dry extract). All tests were performed in triplicate.
2.5 GC-EI-MS and UHPLC-ESI-MS/MS analyses
Phytochemical analyses of metabolites of DCS was performed by gas chromatography coupled to quadrupole mass spectrometer equipped with an electron ionization source (GC-EI-MS) as previously reported [13]. In addition to monosaccharide standards, gallic acid, dihydroxybenzoic acid and quinic acid were subjected to methanolysis and trimethylsilylation and analysed by GC-EI-MS for identification and quantification of these metabolites in the decoction of C. schweinfurthii stem barks.
UHPLC-ESI-MS/MS analyses were performed thanks to an UHPLC system (Vanquish, Thermo Scientific, San Jose, CA, USA) coupled to a quadrupole-Orbitrap mass spectrometer (Exploris 120, Thermo Scientific) equipped with an electrospray ionization source. The chromatographic separations were performed using a C18 column (Acquity UPLC HSS T3 1.8 µm × 2.1 mm × 100 mm, Waters Corporation, Milford, MA, USA) with a prefilter of 0.2 µm, kept at 50°C during the analysis. An autosampler kept the samples at 6°C. The injection volume was 3 µL. The solvents used for gradient separation were 0.1% (v/v) formic acid in water as mobile phase A and 0.1% (v/v) formic acid in acetonitrile as mobile phase B. The flow rate was 0.4 mL/min. The elution gradient was first 1 % B for 1 min, then increased linearly to 100 % B over 20 min and then maintained at 100 % B for 8 min. Samples were analyzed in both negative and positive modes. The ESI source parameters were as follows: spray voltage 3500 V and 3000 V for positive negative modes, respectively, sheath gas 35 (arbitrary unit), auxiliary gas 10 (arbitrary unit), sweep gas 2 (arbitrary unit), ion transfer tube 320°C and temperature of vaporizer 275°C. Data dependent acquisitions were carried out in both positive and negative modes. MS1 resolution was set at 60,000 with a standard AGC target, a maximum injection time set to auto, a microscan to 1, RF lens to 70%, and a scan range from m/z 80 to 1200. EASY-IC internal standard was used. For MS/MS, resolution was set at 15,000 with a maximum injection time of 50 ms. The isolation window was of 2 m/z, dynamic exclusion was set at 4s, mass tolerance was + 4 ppm and the precursor intensity threshold were set at 5.105 in positive mode and 1.105 in negative mode. The HCD collision energies were 15%, 40% and 60% in both positive and negative ion modes. Data processing was carried out using MZmine 4.5 (version 4.5.0) [20,21]. Annotation was performed based on accurate mass measurements and MS/MS spectra according to the literature and databases.
2.6 Cytotoxicity assay
Cellular toxicity assays were performed on human embryonic kidney (HEK-293) cells (ATCC®, CRL-1573™) and human brain endothelial cell line (hCMEC/D3method as previously [13].
2.7 Acetic acid-induced torsion assay
The acetic acid-induced torsion assay was performed according to Koster et al. [22] with some modifications. Seven groups of six Wistar rats were used in this study. Rats were administrated per os with 0.9 % NaCl (10 mL/kg body weight as control), indomethacin (10 mg/kg), DCS (250, 500 and 1000 mg/kg) or quinic acid (50 and 500 mg/kg). After 30 min, all rats received 10 mL/kg of acetic acid 1% and were placed in individual observation cages. The cumulative number of abdominal writhes exhibited by each animal was recorded over a period of 30 min. A decrease in the number of writes as compared to the control was considered as evidence of antinociceptive and the percentage inhibition of the writhing response was calculated using the formula: % Inhibition = (D0 - Dt /D0) × 100 where D0 was the average writhing response of the control group and Dt was the average writhing response of the drug-treated groups (indomethacin, quinic acid or plant extract).
2.8 Formaldehyde-induced leg licking test
The formaldehyde-induced paw lick test was performed as described by Soro et al. [23] with some modification. Rats received through intraperitoneal administration a saline solution (10 mL/kg of 0.9 % NaCl as control), morphine (5 mg/kg), indomethacin (10 mg/kg), DCS (100, 250 and 500 mg/kg) or quinic acid (50, 100, 200 and 400 mg/kg). After 30 min, all rats received 50 µL/kg of 2 % formaldehyde under the paw into the right hind foot pad and immediately placed in the cage where they can be observed easily. The painful response is manifested by licking or folding of the injected leg. The antinociceptive effects were recorded in two phases using a stopwatch. The first phase occurs between 0–5 min and the second phase between 15–30 min. The classification of the painful response is based on the following scale: 0: rats walk or lean firmly on treated paw and seem to feel no pain; 1: the treated paw is partially lifted; 2: the treated paw is frankly raised and 3: the rat licks, chews or shakes the treated leg and seems to have pain. The percentage inhibition of the paw lick response was calculated using the formula: % Inhibition = (D0 - Dt /D0) × 100 where D0 was the average writhing response of the control group and Dt was the average paw lick response of the drug-treated groups (morphine, indomethacin, quinic acid and plant extract). A significant reduction in the number of licks in the treated animals compared to the control group was considered as an antinociceptive response.
2.9 Investigation of opioid receptor involvement in the nociceptive activity
To investigate the mechanisms involved in the antinociceptive effects of DCS and quinic acid, 0.4 mg/kg of naloxone was administered to each animal. After 15 min, the animals received a solution of morphine (5 mg/kg), quinic acid (400 mg/kg) or DCS (500 mg/kg). After 30 min, 50 μL of 2 % formaldehyde was injected into the plantar pad of each rat’s right posterior leg. Pain score was observed and recorded as described above.
2.10 Data analysis
Statistical analysis was performed on Graph Pad Prism version 8.4.3. Data were presented as mean ± standard error (SD) with n = 3 or 6 replicates. A nonlinear regression was used to calculate IC50 values. One-way analysis of variance (ANOVA) followed by Dunnett's comparison test was used to assess differences between groups. A p- value <0.05 was considered statistically significant.
3. Results
3.1 Quantification of phenol content of a decoction of C. schweinfurthii Engl. stem barks
Phytochemical and pharmacological analyses of DCS were performed on a sample collected in Gabon. The extraction yield of DCS sample from total stem bark material was 2.7 %. The DCS fraction was then solubilized in 1 % of DMSO in water to ensure complete solubilization of all metabolites. A phytochemical analysis of DCS was first performed through spectroscopic approaches to estimate the total phenol content using the Folin-Ciocalteu method, the total flavonoid contents by the method of aluminium chloride [13] and the total tannin content by the sulfuric vanillin method [19]. The reference molecules were gallic acid, quercetin and tannic acid, respectively. These analyses showed that DCS is rich in phenolic compounds as reported in Table 1.
|
Assays/sample |
DCS |
|
Polyphenol in µg GAE/mg DCS |
217.6 ± 33a |
|
Flavonoid in µg QE/mg DCS |
218.7 ± 24a |
|
Tannins in µg TAE/mg DCS |
58.4 ± 8.6b |
|
Values are expressed as mean ± SD (n = 3). GAE: gallic acid equivalent; QE: quercetin equivalent; TAE: tannic acid equivalent. a: p < 0.0001; b: p < 0.01 according to One-Way ANOVA followed by Dunnett's multiple comparison tests. |
|
Table 1: Quantification of phenols in DCS sample.
3.2 Phytochemical analysis of the C. schweinfurthii Engl. stem bark decoction
The Phytochemical investigation of DCS was first performed by methanolysis and trimethylsilylation to identify and quantify constitutive monosaccharides, acids and phenolic compounds by gas chromatography coupled to electron ionization mass spectrometry (GC-EI-MS) (Table S1). Together with glucose and less abundant monosaccharides, quinic acid was identified in large amounts (98 µg/mg of DCS) although representing only about 10% of DCS. Gallic acid was also detected in GC-EI-MS as one of the main metabolites.
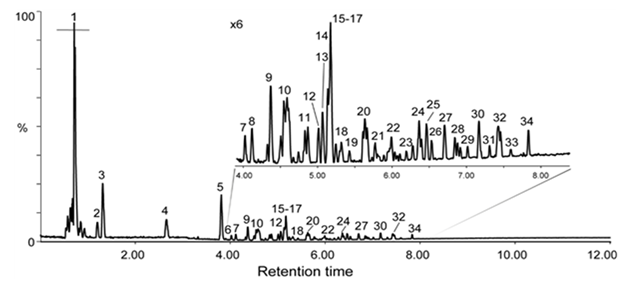
The analysis of metabolites contained in DCS was then performed by ultra-high performance liquid chromatography coupled to quadrupole-Orbitrap mass spectrometry in data dependant scan mode (UHPLC-ESI-MS/MS) in both negative and positive ion modes. The metabolites were mainly detected in negative ion mode as [M−H]- ions, except for di- and trimethoxyphenyl rhamnosylglucosides (metabolites 11b and 17) that were detected as formate adduct [M+FA−H]-(Table 2). In positive ion mode, [M+H]+, [M+NH4]+ and [M+Na]+ ions could be observed (Table 2). Figure 1 shows the base peak chromatogram (BPC) recorded in negative ion mode. The main metabolites were investigated and numbered according to their LC retention times. The annotation of metabolites contained in DCS was based on accurate mass measurements in negative and positive ion modes, analysis of the MS/MS fragmentation patterns and comparison with data of the literature and databases (Massbank, PubChem, HMDB, GNPS Databases). The first co-eluted compounds, between 0.5 and 1 min correspond to sugars, amino acids and organic acids, in particular quinic acid and its closely related metabolites shikimic acid and dehydroshikimic acid (Table 2).
Table 2: Putative main metabolites after UHPLC-ESI-MS/MS analysis of the decoction of C. schweinfurthii Engl. barks. *: Numbering of metabolites in negative ion mode (Figure 1). #: Percentage of each metabolite was determined on the basis of their relative ratio of the corresponding peak area in negative ion mode without considering specific ion response factors of each metabolite in ESI negative ion mode.
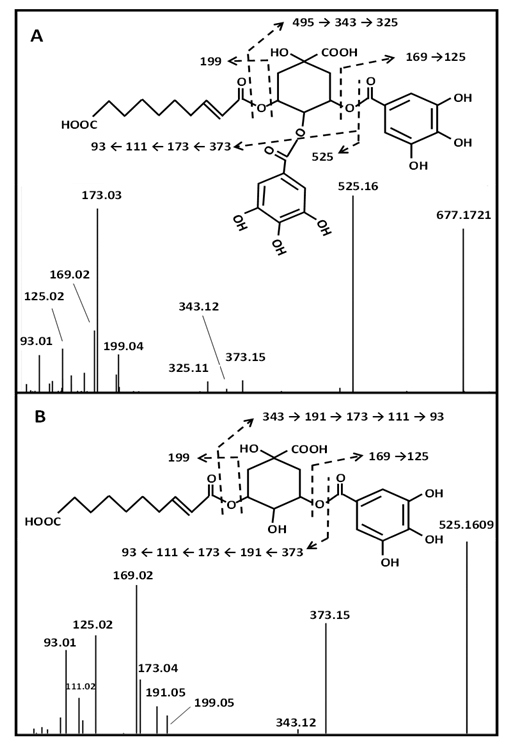
The LC-ESI-MS/MS analysis revealed that the major metabolites of DCS are quinic, gallic acids and their derivatives (Table 2). That is in accordance with GC-EI-MS data, as both analyses showed that quinic and gallic acids appear to be the main metabolites in DCS (Table S1 and Table 2). In a lesser extent, shikimic acid and its derivatives, as well as sugars, catechin derivatives or decenedioic acid derivatives, are also major metabolites. Thereby, quinic acid-containing metabolites accounts for about the half of metabolites of DCS. Quinic acid derivatives were mainly observed as esters of gallic acid but also as esters of C9 and C10 diacids. This gave rise to a complex mixture of isomers of mono, di and triesters of quinic acid. Only few of them are reported in the Table 2. Putative identification of quinic acid derivatives was deduced from their negative fragmentation patterns and by comparison to literature data [24-26]. Negative MS/MS spectra of these metabolites are characterized by quinate fragment ion at m/z 191 and/or consecutive fragment ions at m/z 173 (loss of H2O from m/z 191), as well as m/z 111 and m/z 93 arising from successive losses of CO2 and H2O molecules. For instance, Figures 2A and 2B shows the MS/MS spectra of m/z 677.1721 and m/z 525.1609 which correspond to [M−H]- ions of proposed decenedioyl digalloyl quinate and decenedioyl galloyl quinate, respectively. In addition to quinate diagnostic fragment ions at m/z 191, m/z 173, m/z 111 and m/z 93, fragment ions at m/z 169 (gallate) and m/z 199 (decenedioate) certifies that these three acidic molecules constitute the two metabolites detected at m/z 677.1721 and m/z 525.1609 (Figures 2A and 2B). This is confirmed by neutral losses of either gallic and/or decenedioic moieties from [M−H]- ions leading to fragment ions m/z 525.16 (from m/z 677.1721), m/z 373.15 (from m/z 525.1609) or m/z 343.12 (from both m/z 525.1609 and m/z 677.1721). Such characteristic fragmentation pattern was observed for the quinic derivatives. LC-ESI-MS/MS analysis in positive ion mode confirmed the annotation of esters of quinic acid of DCS with fragment ions at m/z 153 and m/z 183 assigned to galloyl and decenedioyl substituents, respectively (Table 2).
It is worth noting that MS/MS spectra do not inform about the location of gallic acid or decenedioic acid substituents on the quinic acid backbone as this cyclic polyol contains four hydroxyl groups that may be esterified by acidic substituents at various positions. This variability of ester substitutions (gallic acid or diacids) is responsible for the multiplicity of mono- di- and tri-esterified metabolites as well as the large number of possible isomers observed in the LC profile (Figure 1). For instance, three isomers of decenedioyl galloyl-quinic acid (m/z 525.1609) were observed by LC MS/MS analysis, only the two more abundant (metabolites 18c and 19 eluted at 5.32 and 5.49 min) are reported in Table 2.
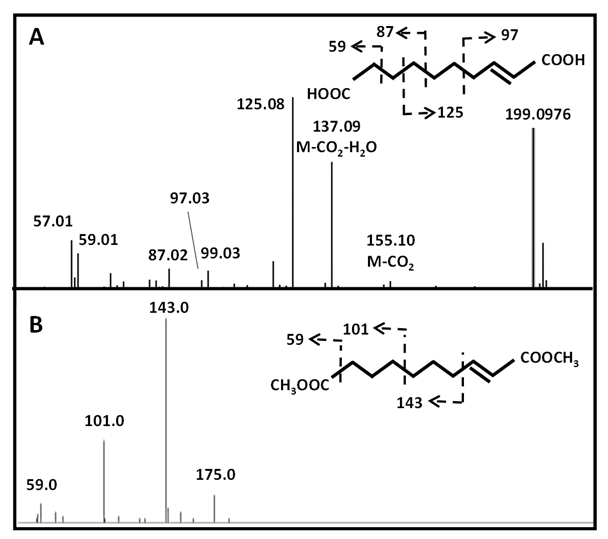
In contrast to other acyl quinic acids that are secondary metabolites prevalent in plants [27], the substitution of quinic acid by decenedioic acid has not been reported in the literature to date. This diacid was identified by GC-EI-MS (Table S1) and also detected in the LC-ESI-MS/MS analysis as a free diacid (metabolite 20a). Fragmentation pattern of its [M−H]- ion at m/z 199.0976 in negative electrospray ionization, as well the fragmentation of its methyl diester derivative in electron ionization suggested that this diacid is unsaturated on C-2 although the location of the double bond is still questionable (Figures S1A and B). Moreover, decenedioic acid was previously reported as a constituent of royal jelly and honey [28 - 29]. Other less abundant acyl quinic derivatives such as esters of nonane- (azelaic acid C9:0; metabolite 28a) and decane- (sebacic acid C10:0; metabolite 21) dioic acids were also identified (Table 2).
In addition to quinic acid derivatives, gallic acid and gallic acid-related metabolites, such as gallocatechin, epigallocatechin-catechin, brevifolin-carboxylic acid, cherubic acid and corilagin were also detected in the DCS bark decoction (Table 2). These metabolites have been previously reported in plant extracts rich in gallic acid [24 – 26, 30]. UHPLC-ESI-MS/MS analysis of metabolites of DCS also revealed the presence of deoxyhexosylhexosides of methoxyphenols that are proposed to be rhamnosylglucosides on the basis of the sugar composition of DCS (Table S1) and in accordance with the fragmentation patterns reported in the literature [13]. These metabolites exhibited reported glycosidic fragment ions at m/z 163 (deprotonated dHex) and 307 ([M-H-H2O]- : dHex→Hex) (Table 2). We postulate that this diglycoside is 6-O-α-L-rhamnosyl-D-glucose, named rutinose, that was previously reported in many plant flavonoid glycosides. It is worth noting the detection in ESI positive mode of scopoletin, an hydroxycoumarin and stachydrine, an alkaloid [31] also known as proline betaine. This alkaloid has been reported to have several biological activities, such as anti-inflammatory, antioxidant, anti-apoptotic, antithrombotic and cardioprotective effects [32]. Pharmacological activities against different chronic and inflammatory diseases have also been reported for scopoletin [33].
3.3 Cytotoxicity of DCS sample against human cell lines
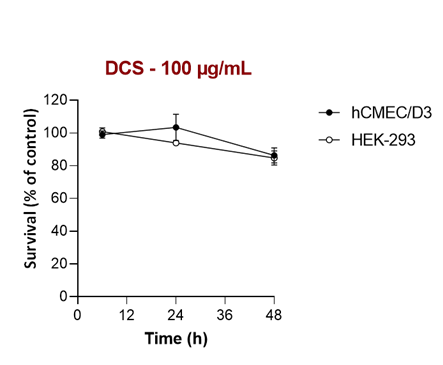
The putative cytotoxicity of DCS sample was evaluated by investigation of their effects on the cell viability of two human cell lines, HEK-293 and hCMEC/D3, obtained from embryonic kidney and adult cerebral vessels, respectively. We found that incubation of both cell lines with different concentrations of DCS sample had a very low impact on cell growth over 48 h, even at high concentration (100 µg/mL) (Figure S2).
3.4 Analgesic assays
Acetic acid-induced torsion and formaldehyde-induced leg licking tests were performed to assess peripheral and central-acting analgesic activity of DCS to corroborate the ethnobotanical information stating that DCS are used by the Gabonese population against stomach and intestinal pain. With regards to peripheral effects, DCS at doses ranging from 250 to 1000 mg/mL significantly (p < 0.001) reduced the number of writhes induced by acetic acid in rats as compared to the negative control, while quinic acid was inactive (Table 3). Indomethacin, a non-steroidal anti-inflammatory drug used to treat mild to moderate pain, was used as positive control. Although DCS reduced rat acetic acid-induced writhing, its effect remains weak compared to indomethacin (Table 3) and is not related to quinic acid that did not show any detectable activity in this test.
|
Treatment |
Doses (mg/kg) |
Number of writhes |
Antinociception (%) |
|
Control |
- |
67.2 ± 8.7b |
- |
|
Indomethacin |
10 |
5.2 ± 0.5a |
92.3 |
|
250 |
61.3 ± 6.6b |
8.8 |
|
|
DCS |
500 |
41.8 ± 5.7ab |
37.8 |
|
1000 |
3.2 ± 0.3a |
95.2 |
|
|
Quinic acid |
50 |
70.0 ± 7.1b |
0 |
|
500 |
121.3 ± 11.7ab |
0 |
|
|
Data are presented as means ± SD, n = 6. Analysis was performed with One-Way ANOVA followed by Dunnett's multiple comparison tests. a p < 0.0001 by comparison to the control group, b p < 0.001 by comparison to the indomethacin-received group. |
|||
Table 3: Analgesic effect of decoction of C. schweinfurthii Engl. stem barks and quinic acid on the Wistar rat’s response to acetic acid-induced writhing.
Afterwards, formaldehyde (2 %) was administered subcutaneously in the right posterior leg of rats to induce central and peripheral nociception. This biphasic test was composed of a neurogenic phase 1 (0 to 5 min) and an inflammatory phase 2 (15 to 30 min). Two drugs were used as control: morphine, an opioid analgesic acting on both phases 1 and 2 and indomethacin, a non-steroidal anti-inflammatory, specifically inhibiting phase 2 (Table 4). It was found that, in phase 1, DCS inhibited nociceptive action only at the highest concentration whereas quinic acid acted in a dose-dependent manner with a better sensitivity (Table 4). In addition, DCS completely inhibited the nociceptive action at concentrations (250 and 500 mg/kg). However, quinic acid acted only at its highest concentration. These results had a statistically significant difference (p < 0.0001) compared to control, morphine and indomethacin.
We then investigated the involvement of naloxone opioid receptors in the antinociceptive effects of DCS, quinic acid and morphine (Table 4). Antagonist effects of naloxone were investigated with doses of DCS and quinic acid giving the best analgesic response in the previous experiment. As expected, the action of morphine was completely antagonized in both phases. A strong effect was also observed for DCS with a decrease from 100 % to 11.1 % (Table 4). However, the quinic acid concentration only decreased from 100 % to 66 % during phase 1, and from 88.9 % to 33.3 % during phase 2. The antinociceptive activity induced by naloxone on DCS and quinic acid suggests that these components use the opioid pathway and highlight the potential of plant decoction as an analgesic agent.
|
Treatment |
Nalo-xone |
Dose mg/kg |
Neurogenic phase |
Inflammatory phase |
||
|
Pain threshold score |
Inhibition (%) |
Pain threshold score |
Inhibition (%) |
|||
|
Control |
- |
- |
3 ± 0.0 |
0 ± 0.0b |
3 ± 0.0 |
0 ± 0.0bc |
|
Morphine |
- |
5 |
0 ± 0.0 |
100 ± 0.0ac |
0 ± 0.0 |
100 ± 0.0a |
|
Indomethacin |
- |
10 |
3 ± 0.0 |
0 ± 0.0b |
0.3 ± 0.0 |
88.9 ± 7.0a |
|
DCS |
- |
100 |
2.2 ± 0.2 |
27.8 ± 5. 6abc |
2 ± 0.0 |
33.3 ± 0.0abc |
|
- |
250 |
2 ± 0.0 |
33.3 ± 0.0abc |
0 ± 0.0 |
100 ± 0.0a |
|
|
- |
500 |
0 ± 0.0 |
100 ± 0.0ac |
0 ± 0.0 |
100 ± 0.0a |
|
|
Quinic acid |
- |
50 |
3 ± 0.0 |
0 ± 0.0b |
3 ± 0.0 |
0 ± 0.0bc |
|
- |
100 |
1.5 ± 0.2 |
50 ± 7.5abc |
2 ± 0.0 |
33.3 ± 0.0abc |
|
|
- |
200 |
0.5 ± 0.0 |
83.3 ± 7.5abc |
1.7 ± 0.2 |
44.4 ± 7.0abc |
|
|
- |
400 |
0 ± 0.0 |
100 ± 0.0 ac |
0.3 ± 0.0 |
88.9 ± 7.0a |
|
|
Morphine |
+ |
5 |
3 ± 0.0 |
0 ± 0.0d |
3 ± 0.0 |
0 ± 0.0d |
|
DCS |
+ |
500 |
2.7 ± 0.2 |
11.1 ± 7.0d |
2.67 ± 0.2 |
11.1 ± 7.0d |
|
Quinic acid |
+ |
400 |
1± 0.0 |
66.7 ± 0.0d |
2 ± 0.0 |
33.3 ± 0.0 d |
|
Data are presented as means ± SD, n = 6. Analysis was performed with One-Way ANOVA followed by Dunnett's multiple comparison tests. In the neurogenic phase, a,b,c p < 0.0001, a by comparison to the control group, b by comparison to the morphine group and c by comparison to indomethacin. In the inflammatory phase, a,b,c p < 0.0001, a by comparison to the control group, b by comparison to the group that received morphine and c by comparison to the indomethacin-received group. In presence of naloxone, d by comparison to the morphine without naloxone. |
||||||
Table 4: Antinociceptive activity of stem barks decoction of C. schweinfurthii Engl. and quinic acid on formaldehyde-induced leg licking response in Wistar rats.
4. Discussion
Many metabolites, including fatty acids, phenolic compounds, coumarins, saponins, mono- and triterpenes, have been previously identified in alcohol or organic extracts from resin, fruits and leaves of C. schweinfurthii Engl. [8, 34-37]. With regards to stem barks, triterpenoids, coumarins and prostaglandins have been characterized in an ethanolic fraction [7,38]. In the present study, phytochemical, toxicological and pharmaceutical analyses were focused on hot water-soluble metabolites of stem barks of C. schweinfurthii Engl. (DCS) in accordance with the use of decoctions in traditional medicine in Gabon. Spectrophotometric quantification revealed the presence of high levels of polyphenols in the decoction of C. schweinfurthii Engl. stem barks. In contrast to a recent publication on the same plant extract [11], phytochemical analysis of DCS by and GC-EI-MS and quadrupole-Orbitrap mass spectrometry (UHPLC-ESI-MS/MS) allowed the identification of quinic acid (30 %) and its esterified derivatives (20 %). It is worth noting that some of the gallic acid-related metabolites identified in the present study have been reported in previously phytochemical analyses on C. schweinfurthii Engl., such as brevifolin-carboxylic acid, corilagin, galloyl quinic acid and gallic acid [36]. Occurrence in plants of quinic acid has been widely reported in the literature [27]. However, to our knowledge, substitution of quinic acid with C9 and C10 diacids has not been reported to date.
With regards to pharmaceutical assays, DCS did not show any toxicity on two human cell lines, even at high concentration (100 µg/L) and over a 2-day incubation period. Our results corroborate the results of studies on the ethanol extract of C. schweinfurthii Engl. bark that showed no toxicity on rats [39] and the absence of behavioural changes or signs of toxicity or deaths in an acute toxicity performed with a bark aqueous extract administered at a dose of up to 2 g/kg [11]. This indicated that DCS is safe and can therefore be used for medicinal purposes.
Pain and inflammation treatments usually involve the administration of different classes of analgesics and anti-inflammatory drugs that are often accompanied with side effects [40-41]. Although herbal medicine is inexpensive, of easy access and biologically effective with few side effects [42], many of them are not pharmacologically tested yet. In this context, the antinociceptive activity of DCS was investigated using two in vivo assays and by analysing the involvement of the opioid pathway in this activity. Commercially available quinic acid was also tested to determine whether the antinociceptive effects of DCS are due to this phytochemical. This compound represents about 50 % of detected metabolites by ESI-MS/MS if we also consider its ester derivatives (Table 2) but only 10 % of DCS on the basis of the quantification by GC (Table S1). Acetic acid-induced abdominal constriction test is widely used for detecting peripheral analgesic activity of drugs [43,44]. During this nociception test, acetic acid causes pain by stimulating chemoreceptors that lead to the release of many chemical mediators involved in pain, such as histamine, prostaglandins, serotonin and bradykinin [45,46]. Indomethacin is a non-steroidal anti-inflammatory used as control agent which inhibits the action of COX, a key enzyme in the formation of prostaglandins. COX is expressed in two different isoforms, COX-1 and COX-2, with COX-2 playing a major role in inflammation, analgesic effects and cell growth [47-49]. Our results show that in the acetic acid-induced torsion test specific for the investigation of the peripheral analgesic activity, DCS reduced abdominal constrictions in a dose-response manner. At the highest dose (1000 mg/kg), it significantly inhibited abdominal constrictions compared to indomethacin (10 mg/kg). However, quinic acid had no effect. This suggests that biomolecules other than quinic acid present in DCS could be responsible for its peripheral analgesic activity. Natural products or derivatives that induce antinociceptive activity in the test for abdominal constriction induced by acetic acid would act as NSAIDs by attenuating the synthesis and/or release of pro-mediator inflammatory drugs that would play a key role in inflammation. They are often considered as a promising alternative to peripheral-acting drugs [50].
Although sensitive, the response of the acetic acid-induced abdominal constriction test is known to be attenuated by antihistamines, adrenergic blockers and muscle relaxants [50,51]. Thus, the formaldehyde test was performed to investigate the antinociceptive activity of DCS. The formaldehyde assay is usually used for predictive nociception of acute and specific tonic pain in the evaluation of peripheral and central-acting analgesic drugs [52,53]. This assay is a biphasic test in which phase 1 (neurogenic), occurring from 0 to 5 min, is due to the direct stimulation of the transient receptor potential cation channel subfamily V member 1 (TRPV1) and the transient receptor potential ankyrin 1 (TRPA1) by formaldehyde or by the sensitisation of nociceptors by pain mediators such as substance P and bradykinin [54]. On the other hand, the so-called inflammatory phase 2 from 15 to 30 min is investigated to predict anti-hyperalgesia activity of drugs in neuropathic pain models. Phase 2 is the combination of an inflammatory reaction in peripheral tissues and changes in central treatment [55-58]. It is recognized that the centrally acting drugs, such as morphine, inhibit both phases of pain, while peripheral drugs such as acetylsalicylic acid or indomethacin only inhibit phase 2 [59]. Morphine induces a feeling of euphoria by releasing dopamine and spinal or supra-spinal analgesia by reducing neurantransmitter release via opiodergic receptor activation [60]. In our study, morphine completely inhibited the action of formaldehyde in both phases, while indomethacin had an effect only in phase 2. Regarding DCS, the antinociceptive action was significantly mediated in phases 1 at the dose of 500 mg/kg and in phase 2 at doses of 250 and 500 mg/kg. As for quinic acid, the antinociceptive action was mediated in a dose-dependent manner in phase 1. Indeed, the antinociceptive activity of DCS and quinic acid could be mediated by neurogenic and/or inflammatory mediators such as prostaglandins, serotonin, histamine, bradykinin, and substance P [46]. However, the differences observed between the two samples suggest that quinic acid is not solely responsible for the observed antinociceptive activities of DCS.
Taken together, acetic acid-induced torsion and formaldehyde-induced leg licking tests suggest that DCS provides both central and peripheral nociception. Considering that DCS contains a moderate proportion of quinic acid (» 10%), only a part of these actions may be related to this phytochemical because it exhibits antinociceptive effects on formaldehyde-induced paw licking response in rats only at high doses. Indeed, quinic acid is significantly active in the neurogenic phase and previous studies have already reported an analgesic activity of quinic acid [61]. However, other phenolic compounds of DCS may also contribute to the observed antinociceptive effects. Indeed, phytochemicals such as corilagin is known to significantly reduce capsaicin-induced nociception, suggesting that this tannin may be involved in the antagonism of the neurogenic receptor TRPV1 [62]. Moreover, it has been reported that gallic acid also acts as an antagonist of TRPA1 [63]. This channel plays a critical role in neurogenic pain and inflammation by activating sensory nerves, both at the central and peripheral levels [64]. It is worth noting that stachydrine, also named proline betaine, was detected by UHPLC-ESI-MS/MS analysis in positive mode. Many studies have demonstrated that stachydrine is able to decrease inflammatory and oxidative stress and have cardioprotective and vasoprotective activities [32].
It should be noted that the two antinociceptive tests were performed by different routes of administration for quinic acid which showed no effect in the test with acetic acid when administrated per os. However, the formaldehyde test revealed an effect of intra-peritoneal administration of quinic acid, suggesting that quinic acid acts by injection. Our data also indicate that the activation of the naloxone-opioid-sensitive pathway is involved in the analgesic effect of DCS and quinic acid, as demonstrated by the effects of naloxone, an antagonist of μ, δ and k opioid receptors [65,66] in formaldehyde-induced leg licking tests. Recently, antinociceptive and anti-inflammatory effects of a hot water extract of C. schweinfurthii Engl. barks have been reported [11]. Our present study confirms most of the biological activities observed in this publication. However, we investigated more in details the antinociceptive activity of DCS, allowing the demonstration of naloxone opioid receptors mobilization. On the other hand, the phytochemical analysis of the hot water extract of C. schweinfurthii Engl. barks reported by Umeh et al. [11] is questionable because mostly hydrophobic terpenes were identified by GC-EIMS in their study, while the analysis by LC-ESI MS/MS of water-soluble metabolites in the present study revealed quinic acid and gallic acid derivatives as major metabolites.
5. Conclusion
The decoction of C. schweinfurthii Engl. stem barks (DCS) is non-toxic and exhibits anti-inflammatory and antinociceptive opioid-like activities in rats that corroborate the ethnobotanical information on its use by the Gabonese population against stomach and intestinal pain. This decoction could be used as an alternative source of anti-inflammatory and antinociceptive compounds. However, further studies should be undertaken to elucidate the specific mechanism of antinociceptive activities of this decoction, for instance through the hot plate test which is specific to central analgesics. Active biomolecules other than quinic acid should also be isolated and studied individually to investigate the relative involvement of each metabolite on the antinociceptive activity of DCS.
Author Contributions: E.L.M.E.: Methodology, Investigation. Writing – original draft. N.O.K.M.: Methodology, Investigation. O.P.: Methodology, Investigation. M.G.: Methodology, Investigation. P.G.: Methodology, Investigation. C.L.-B.: Methodology, Investigation. I.S.: Methodology, Investigation. C.A.: Writing – review & editing. P.L.: Writing – review & editing, Supervision, Funding acquisition. L.E.M.: Writing – review & editing, Supervision, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Acknowledgments: The authors would like to express their sincere thanks to the administrative and technical staff of IPHAMETRA in Libreville, Gabon and also those of the GlycoMEV laboratory in University of Rouen, France for all the technical and financial support provided for this work (BQI 2022).
Supplementary Materials: The following supporting information can be downloaded at
Table S1: Metabolites annotation by GC-EI-MS in the decoction of C. schweinfurthii Engl. barks. Percentage of each metabolite was determined on the basis of the relative ratio in GC of the corresponding peak area. Metabolites were analysed as their methylester or methylglycoside trimethylsilyl derivatives; Figure S1: (A) A) ESI-MS/MS spectrum of [M−H]- ion of decenedioic acid at m/z 199.0976 and (B) EI-MS spectrum of decenedioic acid dimethylester at m/z 228.136. Figure S2: Percentage of cell viability over 48 h of human cell lines HEK-293 and hCMEC/D3 incubated with 100 µg/mL of DCS sample over non-treated control cells;
Ethical considerations
The experimental protocol was carried out in accordance with international standardized protocols [guidelines established by the European Union on the protection of the environment]. International [Directives ARRIVE, (NC3Rs)] and adopted by the Laboratoire de Pharmacologie et Toxicologie of IPHAMETRA, Gabon. All efforts were made to minimize animal suffering and ensure humane treatment during the experiments. The use of animals was essential for the investigation of the pharmacological effects, and the study was designed to minimize the number of animals used, adhering to the principles of the 3Rs (Replacement, Reduction, and Refinement). Ethical approval for this study was obtained from the scientific ethical committee of our institution.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Raponda-Walker A, Sillans R. Les Plantes utiles du Gabon, Lechevalier, P., Ed, Paris, France (1961): ISBN: 978-2-907888-72-1.
- Kuete V. Canarium schweinfurthii. In: Kuete V Ed. Medicinal Spices and Vegetables from Africa. Academic Press (2017): 379-384. ISBN 9780128092866.
- Owolabi MS, Ogundajo A, Solomon BO, et al. Essential oil compositions, antibacterial and antifungal activities of Nigerian members of the Burseraceae: Boswellia dalzielii and Canarium schweinfurthii. Natural Product Communications 8 (2020): 1-9.
- Adesina SK, Johnny II, Olayiwola G. Plants in respiratory disorders II- Antitussives, a review. Journal of Pharmaceutical Research International 16 (2017): 1-21.
- Seukep JA, Ngadjui B, Kuete V. Antibacterial activities of Fagara macrophylla, Canarium schweinfurthii, Myrianthus arboreus, Dischistocalyx grandifolius and Tragia benthamii against multi-drug resistant Gram-negative bacteria. Springerplus 4 (2015): 567.
- Sokoudjou JB, Atolani O, Njateng GSS, et al. Isolation, characterization and in vitro anti-salmonellal activity of compounds from stem bark extract of Canarium schweinfurthii. BMC Complementary Medicine Therapies 20 (2020): 316.
- Mbosso TJE, Fokou NT, Nguemfo EL, et al. Screening phytochimique et étude de la toxicité aigùe et subaigùe de l’extrait ethanolique des écorces du tronc de Canarium schweinfurthii Engl. (Burseraceae) chez le rat. Journal of Applied Biosciences 174 (2022): 18043-18055.
- Okoli BJ, Ndukwe GI, Ayo RG, et al. Inhibition of the developmental stages of Ascaris suum and antimicrobial activity of 3-β Hydroxylolean-12, 18-diene isolated from the aerial parts of Canarium schweinfurthii (Engl). American Chemical Science Journal 11 (2016): 1-11.
- Koga MD, Nveikoueing F, Bambe R, et al. Anti-onchocercal activity and in vivo toxicity of methanolic extract of Canarium schweinfurthii (Burseraceae) from the equatorial region (Cameroun). Cameroon Journal of Biological and Biochemical Sciences 28 (2020): 14-25.
- Mayindza Ekaghba EL, Itoudi Bignoumba PE, Bourobou Bourobou JA, et al. Etude épidémiologique des troubles fonctionnels intestinaux dans les structures sanitaires à Libreville (Gabon). Journal of Applied Biosciences 155 (2020): 15986-15993.
- Umeh NK, Onuorah RT, Ekweogu CN, et al. Chemical profiling, toxicity assessment, anti-diarrhoeal, anti-inflammatory and antinociceptive activities of Canarium schweinfurthii Engl. (Burseraceae) bark in rats. Journal of Ethnopharmacology 333 (2024): 118460.
- Adjanohoune EJ, Aké Assi L, Chibon P, et al. Contribution aux études ethnobotaniques et floristiques au Gabon. Agence de Coopération Culturelle et Techique Paris, France (1984): 294.
- Mayindza Ekaghba EL, Grenet M, Gandolfo P, et al. Phytochemical analysis and antidiarrheal activity of stem bark decoctions of Pentadesma butyracea sabine (Clusiaceae). Molecules 29 (2024): 5789.
- Kellow JE, Phillips SF. L'altération de la motilité de l'intestin grêle dans le syndrome du côlon irritable est corrélée aux symptômes. Gastroenterology 92 (1987): 1885-1893.
- Simmons DL. What makes a good anti-inflammatory drug target?. Drug Discoverv Today 11 (2006): 210-219.
- Tsantoulas C, McMahon SB. Opening paths to novel analgesics: the role of potassium channels in chronic pain. Trends Neurosciences 37 (2014): 146-158.
- North RA. Twelfth Gaddum memorial lecture. Drug receptors and the inhibition of nerve cells. British Journal of Pharmacology 98 (1989): 13-28.
- Deghrigue M, Festa C, Ghribi L, et al. Anti-inflammatory and analgesic activities with gastroprotective effect of semi–purified fractions and isolation of pure compounds from Mediterranean gorgonian Eunicella singularis. Asian Pacific Journal of Tropical Medicine 8 (2015): 606-611.
- Julkunen-Tito R, Phenolic constituents in the leaves of northern willows: methods for the analysis of certain phenolics. Journal of Agriculture and Food Chemistry 3 (1985): 213-217.
- Pluskal T, Korf A, Smirnovc A, et al. Metabolomics data analysis using MZmine. New Developments in Mass Spectrometry. No. 8 Processing Metabolomics and Proteomics Data with Open Software: A Practical Guide, Edited by Robert Winkler. The Royal Society of Chemistry 7 (2020): 232.
- Heuckeroth S, Damiani T, Smirnov A, et al. Reproducible mass spectrometry data processing and compound annotation in MZmine 3. Nature Protocols 19 (2024): 2597-2641.
- Koster R, Anderson M, De Beer EJ. Acetic acid-induced analgesic screening. Federation Proceedings 18 (1959): 412-417.
- Soro TY, Traore F, Sakande J. Activité analgésique de l'extrait aqueux de Ximenia americana (Linné) (Olacaceae). Comptes Rendus Biologiques 332 (2009): 371-377.
- Mekam PN, Martini S, Nguefack J, et al. Phenolic compounds profile of water and ethanol extracts of Euphorbia hirta L. leaves showing antioxidant and antifungal properties. South African Journal of Botany 127 (2019): 319-332.
- Luo S, Guo L, Sheng C, et al. Rapid identification and isolation of neuraminidase inhibitors from mockstrawberry (Duchesnea indica Andr.) based on ligand fishing combined with HR-ESI-Q-TOF-MS. Acta pharmaceutica Sinica B 10 (2020): 1846-1855.
- Drioiche A, Ailli A, Remok F. Analysis of the chemical composition and evaluation of the antioxidant, antimicrobial, anticoagulant, and antidiabetic properties of Pistacia lentiscus from Boulemane as a natural nutraceutical preservative. Biomedicines 11 (2023): 2372.
- Clifford MN, Jaganath IB, Ludwig IA, et al. Chlorogenic acids and the acyl-quinic acids: discovery, biosynthesis, bioavailability and bioactivity. Natural Product Report 34 (2017): 1391-1421.
- Kokotou MG, Mantzourani C, Babaiti R, et al. Study of the royal jelly free fatty acids by liquid chromatography-high resolution mass spectrometry (LC-HRMS). Metabolites 10 (2020): 40.
- Kim SG, Kim HY, Kim S, et al. Validation of analytical method for (E)-2-decenedioic acid quantification in honey samples. Journal of Asia-Pacific Entomology 24 (2021): 1153-1157.
- Cerulli A, Napolitano A, Hošek J, et al. Antioxidant and In Vitro Preliminary Anti-Inflammatory Activity of Castanea sativa (Italian Cultivar "Marrone di Roccadaspide" PGI) Burs, Leaves, and Chestnuts Extracts and Their Metabolite Profiles by LC-ESI/LTQOrbitrap/MS/MS. Antioxidants (Basel) 10 (2021): 278.
- Kuchta K, Volk RB, Rauwald HW. Stachydrine in Leonurus cardiaca, Leonurus japonicus, Leonotis leonurus: detection and quantification by instrumental HPTLC and 1H-qNMR analyses. Die Pharmazie-An International Journal of Pharmaceutical Sciences. 68 (2013): 534-540.
- Liao L, Tang Y, Li B, Stachydrine, a potential drug for the treatment of cardiovascular system and central nervous system diseases. Biomedicine Pharmacotherapy 161 (2023): 114489.
- Parama D, Girisa S, Khatoon E, et al. An overview of the pharmacological activities of scopoletin against different chronic diseases. Pharmacologycal Research, 179 (2022): 106202.
- Koudou J, Abena AA, Ngaissona P, et al. Chemical composition and pharmacological activity of essential oil of Canarium schweinfurthii. Fitoterapia, 76 (2005): 700-703.
- Atawodi SE. Polyphenol composition and in vitro antioxidant potential of Nigerian C. schweinfurthii Engl. oil. Advances in Biological Research 4 (2010): 314-322.
- Mogana R, Teng-Jin K, Wiart C. In Vitro Antimicrobial, Antioxidant Activities and Phytochemical Analysis of Canarium patentinervium Miq. from Malaysia. Biotechnology Research International 2011 (2011): 768673.
- Uzama D, Bwai MD, Olajide OO, et al. Antioxidant and phytochemicals of hexane and ethanolic extracts of Canarium schweinfurthii Burseraceae. Asian Journal Pharmaceutical & Biological Research 2 (2012): 188-190.
- Sokoudjou JB, Tagousop CN, Khan A, et al. Canarimoic acid: new tirucallane triterpene with antisalmonellal activity from the stem bark of Canarium schweinfurthii Engl. Natural Product Research 36 (2022): 2363-2369.
- Nzodjou FT, Assob NJC, Ngoupayo J, et al. Quantitative screening and study of the in vivo subchronic toxicity of ethanolic extract from the stem bark of Canarium schweinfurthii Engl. (Burseraceae) in wistar rats. Saudi Journal of Medicine and Pharmaceutical Sciences 92 (2023): 74-93.
- Harirforoosh S, Asghar W, Jamali F. Adverse effects of nonsteroidal antiinflammatory drugs: an update of gastrointestinal, cardiovascular and renal complications. Journal of Pharmacy & Pharmaceutical Sciences 16 (2013): 821-847.
- Fokunang CN, Tembe E, Frederick K, et al. Overview of on-Steroidal Anti-Inflammatory Drugs (NSAIDs) in resource limited countries.MOJ Toxicol 4 (2018): 5-13.
- Olela B, Mbaria J, Wachira T, et al. Acute oral toxicity and anti-inflammatory and analgesic effects of aqueous and methanolic stem bark extracts of Piliostigma thonningii (Schumach.). Evidence-Based Complementary and Alternative Medicine 2020 (2020): 5651390.
- Gawade SP. Acetic acid induced painful endogenous infliction in writhing test on mice. Journal of Pharmacology & Pharmacotherapeutics 3 (2012): 348.
- Austin PJ, Moalem-Taylor G. The neuro-immune balance in neuropathic pain: involvement of inflammatory immune cells, immune-like glial cells and cytokines. Journal of Neuroimmunology 229 (2010): 26-50.
- Jang SA, Park DW, Kwon JE, et al. Quinic acid inhibits vascular inflammation in TNF-α-stimulated vascular smooth muscle cells. Biomedicine & Pharmacotherapy 96 (2017): 563-571.
- Tewari D, Gupta P, Bawari S, et al. Himalayan Ficus palmata L. Fruit Extract Showed In Vivo Central and Peripheral Analgesic Activity Involving COX-2 and Mu Opioid Receptors. Plants 10 (2021): 1685.
- Smith CJ, Zhang Y, Koboldt CM, et al. Pharmacological analysis of cyclooxygenase-1 in inflammation. Proceedings of the National Academy of Sciences 95 (1998): 13313-13318.
- Araico A, Terencio MC, Alcaraz MJ, et al. Evaluation of the anti-inflammatory and analgesic activity of Me-UCH9, a dual cyclooxygenase-2/5-lipoxygenase inhibitor. Life Sciences 80 (2007): 2108-2117.
- Slighoua M, Chebaibi M, Mahdi I, et al. The LC-MS/MS identification and analgesic and wound healing activities of Lavandula officinalis Chaix: In vivo and in silico approaches. Plants 11 (2022): 3222.
- Kamarudin N, Hisamuddin N, Ong HM, et al. Analgesic effect of 5-(3,4-Dihydroxyphenyl)-3-hydroxy-1-(2-hydroxyphenyl)penta-2,4-dien-1-one in experimental animal models of nociception. Molecules 23 (2018): 2099.
- Woode E, Ameyaw EO, Boakye-Gyasi E, et al. Analgesic effects of an ethanol extract of the fruits of Xylopia aethiopica (Dunal) A. Rich (Annonaceae) and the major constituent, xylopic acid in murine models. Journal of Pharmacy and Bioallied Sciences 4 (2012): 291-301.
- Hunskaar S, Hole K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain 30 (1987): 103-114.
- Godínez-Chaparro B, López-Santillán FJ, Argüelles CF, et al. Role of 5-HT1B/1D receptors in the reduction of formalin-induced nociception and secondary allodynia/hyperalgesia produced by antimigraine drugs in rats. Life Sciences 92 (2013): 1046-1054.
- Azi HL, Woode E. Evaluation of analgesic property of petroleum ether/ethyl acetate stem bark extract and fractions of Maerua angolensis in murine models of pain. Journal of Applied Pharmaceutical Sciences 5 (2015): 091-102.
- Fischer MJ, Btesh J, McNaughton PA. Disrupting sensitization of transient receptor potential vanilloid subtype 1 inhibits inflammatory hyperalgesia. Journal of Neuroscience 33, (2013): 7407-7414.
- Cobos JE, Portillo-Salido E. Bedside-to-Bench: Behavioral outcomes in animal models of pain: Beyond the evaluation of reflexes. Current neuropharmacology 11 (2013): 560-591.
- Taneja A, Troconiz IF, Danhof M, et al. Semi-mechanistic modelling of the analgesic effect of gabapentin in the formalin-induced rat model of experimental pain. Pharmaceutical Research 31 (2014): 593-606.
- Alonso-Castro AJ, Arana-Argáez V, Yáñez-Barrientos E, et al. Antinociceptive and anti-inflammatory effects of Cuphea aequipetala Cav (Lythraceae). Inflammopharmacology 29 (2021): 295-306.
- Bannon AW, Malmberg AB. Models of nociception: hot-plate, tail-flick, and formalin tests in rodents. Current protocols in neuroscience 41 (2007): 8-9.
- Listos J, Lupina M, Talarek S, et al. The mechanisms involved in morphine addiction: an overview. International Journal of Molecular Sciences 20 (2019): 4302.
- Khorasgani AT, Amini-Khoei H, Shadkhast M, et al. Quinic acid through mitigation of oxidative stress in the hippocampus exerts analgesic effect in male mice. Future Natural Products 7 (2021): 1-11.
- Taghi Mansouri M, Naghizadeh B, Ghorbanzadeh B, et al. Central and peripheral antinociceptive effects of ellagic acid in different animal models of pain. European Journal of Pharmacology 707 (2013): 46-53.
- Ghorbanzadeh B, Mansouri MT, Hemmati AA, et al. Involvement of L-arginine/NO/cGMP/K(ATP) channel pathway in the peripheral antinociceptive actions of ellagic acid in the rat formalin test. Pharmacology Biochemistry and Behavior 126 (2014): 116-121.
- Yao K, Dou B, Zhang Y, et al. Inflammation-the role of TRPA1 channel. Frontiers in Physiology 14 (2023): 1093925.
- Rosenblum A, Marsch LA, Joseph H, et al. Opioids and the treatment of chronic pain: controversies, current status, and future directions. Experimental and clinical psychopharmacology 16 (2008): 405-416.
- Scherrer G, Imamachi N, Cao YQ, et al. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell 137 (2009): 1148-1159.
Supplementary Materials:
|
Compound |
Fragment ions in GC-MS |
Relative abundance (% ± SD a) |
µg/mg of DCS ± SD a |
|
Arabinose (Ara) |
204/217/333* |
5 ± 0.8 |
15 ± 2 |
|
Rhamnose (Rha) |
204/217/305* |
3 ± 0.6 |
9 ± 1.5 |
|
Fucose (Fuc) |
204/217/305* |
< 1 |
< 1 |
|
Xylose (Xyl) |
204/217/333* |
1 ± 0.3 |
3 ± 1 |
|
Galacturonic acid (GalA) |
204/217/391* |
4 ± 0.7 |
11 ± 2 |
|
Mannose (Man) |
204/217/377* |
1 ± 0.3 |
3 ± 1 |
|
Galactose (Gal) |
204/217/377* |
3 ± 1 |
9 ± 3 |
|
Glucose (Glc) |
204/217/377* |
34 ± 5 |
99 ± 12 |
|
Decenedioic acid |
59/101/143/175 |
5 ± 0.5 |
n.d. |
|
Quinic acid |
147/191/255/276/345 |
31 ± 6 |
94 ± 10 |
|
Shikimic acid |
147/204/299 |
< 1 |
n.d. |
|
Dihydroxy benzoic acid |
193/312 |
4 ± 0.2 |
10 ± 2 |
|
Gallic acid |
281/400 |
8 ± 1 |
22 ± 3 |
a SD: Standard deviation of three independent experiments. * Monosaccharide diagnostic fragment ions. n.d.: not determined.
Table S1: Annotation by GC-EI-MS of monosaccharides and other metabolites after methanolysis and trimethylsilylation of DCS. Percentage of each metabolite was determined on the basis of the relative ratio in GC of the corresponding peak aera. Metabolites were analysed as their methylester or methylglycoside trimethylsilyl derivatives.
Article Views: 869
Journal Statistics




Discover More: Recent Articles
Grant Support Articles
© 2016-2026, Copyrights Fortune Journals. All Rights Reserved!