Phytochemicals and HIV Suppression: A Systematic Review
Kai Chi1, Yaya Guo2, Haiping Gu1, Qiuling Zhang1, Tabatabaeipozveh Meisam3, Yafeng Yang1, Xiangmeng Chen4, Su Shiung Lam3, Liran Xu2*, Christian Sonne5, Wanxi Peng1*
1Henan Province Engineering Research Center for Biomass Value-added Products, School of Forestry, Henan Agricultural University, Zhengzhou, China
2The First Clinical College of Medicine, Henan University of Chinese Medicine, Zhengzhou, China
3Higher Institution Centre of Excellence (HICoE), Institute of Tropical Aquaculture and Fisheries (AKUATROP), Universiti Malaysia Terengganu, 21030, Kuala Nerus, Terengganu, Malaysia
4School of Science, Henan Agricultural University, Zhengzhou, China
5Department of Ecoscience, Arctic Research Centre (ARC), Aarhus University, Frederiksborgvej, Roskilde, Denmark
*Corresponding Author: Wanxi Peng, Henan Province Engineering Research Center for Biomass Value-added Products, School of Forestry, Henan Agricultural University, Zhengzhou, China
Liran Xu, The First Clinical College of Medicine, Henan University of Chinese Medicine, Zhengzhou, China
Received: 19 August 2023; Accepted: 25 August 2023; Published: 07 September 2023
Article Information
Citation: Kai Chi, Yaya Guo, Haiping Gu, Qiuling Zhang, Tabatabaeipozveh Meisam, Yafeng Yang, Xiangmeng Chen, Su Shiung Lam, Liran Xu, Christian Sonne, Wanxi Peng. Phytochemicals and HIV Suppression: A Systematic Review. International Journal of Plant, Animal and Environmental Sciences 13 (2023): 44-55.
View / Download Pdf Share at FacebookAbstract
The human immunodeficiency virus (HIV) causes immune suppression known as acquired immunodeficiency syndrome (AIDS) leading to various opportunistic infections and malignancies having high mortality rates. Here we provide a systematic review and discussion of current knowledge on photochemical activities against HIV and AIDS. After several years of research, efficient antiretroviral therapy helps controlling the progression of AIDS. However, due to the overuse of antiretroviral drugs, viral resistance in patients and side effects from long-term use of drug therapy have emerged, which shorten life expectancy of patients. To improve HIV treatment, substances in plants may inhibit the life cycle of HIV through inhibition of the activity of reverse transcriptase, integrase or protease required for processes such as HIV transcription and replication. In addition, phytochemicals regulate the human immune system and thereby suppression of HIV and AIDS development in clinical treatments. Therefore, more experiments are needed to demonstrate the effectiveness and safety of plants for therapeutic AIDS treatment, which may bring forward new HIV and AIDS treatment options.
Keywords
AIDS; Human immune system; Safety, Antiretroviral therapy; Phytostatic therapy
AIDS articles; Human immune system articles; Safety articles, Antiretroviral therapy articles; Phytostatic therapy articles
Article Details
1. Background
AIDS is a highly harmful epidemic infectious disease caused by human immunodeficiency virus. Since 1981, when the U.S. released reports of five cases of AIDS, the speed of transmission has been accelerating, and the number of AIDS patients continues to increase. Over the past 30 years, AIDS has become one of the most serious and complex public health problems in the world. In 1985, a foreigner died after falling ill in China, kicking off the country's long battle against AIDS. The Global AIDS progress report released by UNAIDS on July 6, 2020, pointed out that currently 38 million are HIV-positive worldwide. At the same time, the report mentioned that if countries do not take action, the gains may be lost and the anti-AIDS work further delayed. The previous goal of global elimination of AIDS by 2020 so far not achieved. The following data are obtained from the relevant notices issued by the Chinese Health Commission, China launched the Human Immunodeficiency Virus Prevention Project in 2003 [1], as of the end of October 2020, the Chinese population with AIDS numbered 1.045 million. HIV infect human immune cells including T helper cells (CD4+T) and macrophages, and by attacking CD4+T cells, the virus accelerates the apoptosis of immune cells and causes the human immunity is gradually compromised by this condition. CD4+T lymphocytes are important subsets of immune cells in the body, HIV persists in a large variety of CD4+T cells [2]. After HIV infection, a large number of copies in CD4+T lymphocytes will cause damage to CD4+T lymphocytes, leading to immune function defects. Kaposi Sarcoma, non-Hodgkin's lymphoma, and invasive squamous cell carcinoma of the cervix are malignancies considered to be directly related to immunodeficiency in people with HIV [3,4], and eventually cause the death of the body.
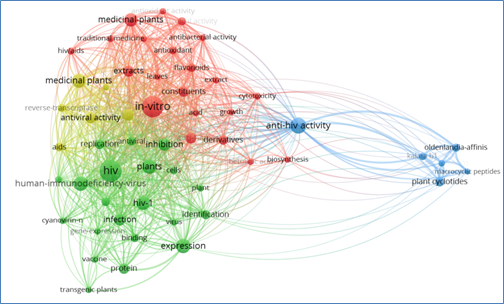
Antiretroviral Therapy, commonly known as "cocktail therapy", antiretroviral therapy with high antiviral activity (HAART), which is currently used as efficient AIDS treatment. In order to achieve a powerful antiviral effect, this therapy requires the combination of at least three antiviral drugs to inhibit HIV-RNA in plasma at low or undetectable levels, HAART therapy can reduce drug resistance caused by a single drug, effectively inhibit HIV replication in the body, and restore the body's damaged immune system, thus greatly reducing the morbidity and mortality of AIDS [5]. HIV patients can live longer with HAART by boosting their CD4+T lymphocyte count, thereby enhancing the body's immunity and reducing infectious complications [6]. However, due to the long-term use of antiretroviral drugs, it will cause serious side effects in the human body; in addition, it will lead to drug resistance of the virus. The toxic side effects of long-term use of antiretroviral drugs will also reduce the patient's compliance, resulting in poor treatment effect. Good compliance is the key factor for successful treatment. Compliance closely related to the degree and duration of viral suppression. Poor patient compliance can reduce the inhibition intensity of drugs on the virus, and then increase the morbidity and mortality of patients [7]. Based on the limitations of antiretroviral therapy in the actual treatment of AIDS, more than ten years ago, the study of Plant-based medicine in the treatment of AIDS, a large number of basic research found that a variety of Plant-based medicine has the effect of inhibiting HIV. Clinical trials have shown that Plant-based medicine has good effects on stabilizing and improving immune function, eliminating and relieving symptoms, and improving quality of life, but its effect on reducing viral load is limited. Plant-based medicine has the characteristics of slow onset, gentle and lasting action, small side effects, good compliance [8], which has great potential in the treatment of AIDS. In addition, some plants in nature contain new anti-AIDS chemical components [9]. By searching the keywords of AIDS suppression in plants, we waited for the hot keywords in the related research fields in Figure 1. Plants contain a variety of phytochemical components, including alkaloids, flavonoids, phenolic compounds, glycosides, tannins, saponins and other substances, so plants may have the effect of enhancing human immunity and preventing HIV replication [10].
2. The HIV life cycle and modes of transmission
2.1 The HIV life cycle
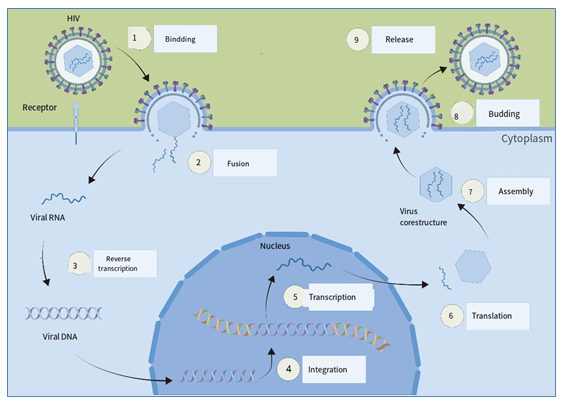
Immunodeficiency caused by AIDS infection is the main reason for the occurrence and development of AIDS-related diseases. HIV mainly uses CD4+T lymphocytes as host cells for reproduction, causes CD4+ T lymphopenia and immune dysfunction in the body, making the infected individuals prone to opportunistic infections or other diseases such as tumors, and eventually leading to the death of patients [11]. Studies show that CD4 molecules on mature T helper lymphocytes being the main HIV receptors [12]. In addition, HIV can also infect macrophages and dendritic cells, which in turn causes the human immune system to lose the ability to resist the virus [13]. Figure 2 shows the process of HIV replication in cells roughly divided into 9 steps. These include adsorption, penetration, decapsidation, early protein synthesis, viral genome nucleic acid replication, late protein synthesis, nuclear shell assembly, viral particle maturation and release [14]. HIV has two glycoproteins on the outer membrane, gp120 and gp41. The gpl20 subunit on the surface of HIV envelope glycoprotein and the transmembrane subunit gp41 contribute to HIV infection in host cells [14]. After HIV enters the human body, gp120 binds to CD4, the main receptor of the human cell membrane, and then binds to co-receptors such as CCR5 and CXCR4 after conformational changes. When an infection occurs, CCR5 and CXCR4 work in conjunction as co-receptors [15], and their conformational changes further expose gp41. After fusion with the human cell membrane, the viral nucleocapsid penetrates the envelope and then releases viral nucleic acid and reverse transcriptase, integrase, and protease required for viral replication. After entering the human host cell, HIV releases nucleotides into the cytoplasm of patient cells, and nucleotides are reverse-transcribed into viral DNA under the action of reverse transcriptase (RT). Viral DNA fuses with host chromosomal DNA under integrase (IN) action. The integrated viral DNA transcribed into viral gene RNA and messenger RNA, and further viral protein synthesized. The synthesis of viral proteins into mature virus particles and released from host cells under the action of protease (PR) [16]. After a series of repeated processes, the released HIV virus continues to replicate and reproduce through the above life cycle, so that the number of HIV in the human body surges.
2.2 Mode of transmission
Among the newly reported AIDS cases in China, 90% transmitted through sexual transmission, and nearly 9% transmitted through drug use. There are three main routes of HIV transmission: sexual transmission, blood transmission and mother-to-child transmission [17,18]. Unprotected sex with AIDS patients can lead to the transmission of AIDS, whether it is homosexual, heterosexual or between the two sexes and male homosexual behavior is more harmful. HIV infection transmitted through blood, semen, vaginal secretions, breast milk, the use of contaminated needles or syringes, and pregnancy or childbirth. It is also possible to spread HIV through incised or damaged mucous membranes [10], giving a person AIDS. Understanding how HIV transmit help to reduce the risk of HIV infection.
3. Pathogenesis of AIDS
During HIV infection, T cells react and proliferate slowly leading to chronic infection [19]. AIDS causes severe immunodeficiency in humans due to malfunctioning of CD4+ T cells during the late stages of HIV infection [20]. These important immune cells express receptors for antigen recognition (TCR) that bind to MHC class II molecules and participate in the antigen recognition process. HIV causes immunodeficiency by directly killing CD4+T lymphocytes and that hinders the renewal of CD4+T lymphocytes [21]. Because CD4+ T cells are pivotal in cellular immunity and regulation, their destruction inevitably lead to immune deficiency. CD4 is also the main receptor of HIV, and their assessment is therefore a convenient method for clinical evaluation of AIDS progress providing a reference for patient treatment. The human immune system is a complex entity consisting of many cell types interwoven in a structural framework, and the detection of CD4+T lymphocyte levels is only one of the ways to assess the progression of AIDS [22]. The pathogenesis of CD4+ T-cell depletion and AIDS is controversial and is the result of direct cytolytic effects of HIV, nonspecific activation of T-cell apoptosis, dysregulated cytokine production, or autoimmunity. After comparison of hypothesis experiments, it is concluded that HIV may cause immunosuppression, but not through the dreary cytolytic effect, but through the traditional virus-specific cytotoxic T cell-mediated immunopathology [19].
HIV integrates through the genome, the former viral form is present in CD4 cells, and in the early stage of infection, cytokines such as IL-2 are secreted by helper T cell 1, Under the stimulation of IL-2, CD8+T lymphocytes exert a strong immunosuppressive effect on CD4+T cells, thus keeping the virus in a latent state of suppression. In the late stage of infection, the secretion of helper T cell 2 is dominant. By secreting cytokines such as IL-10, CD8+ T cells lose their inhibition on CD4+T cells, the virus proliferates and releases new viral particles to infect more CD4+ T cells, resulting in the death of large amount of CD4+ T cells and eventual depletion and loss of immune function. In this way, the body eventually loses its immune function.
4. Types of HIV
Two types of HIV are identified (HIV-1 and HIV-2), which have similar viral structure and transmission route, the main difference is the difference in the envelope glycoprotein. HIV-1 is highly prevalent and aggressive, and HIV-2 discovered in West Africa in 1986 [23]. HIV-1 is the pathogen causing the AIDS epidemic worldwide. At present, the international research on AIDS based on HIV-1. Currently, HIV-2 mainly confined to various regions of Africa [24] and some countries and regions.
HIV belongs to the genus Lentivirus in the family Retroviridae in viral taxonomy. HIV RNA contains Gag, Env and Pol genes, as well as tat, vif, vpr, vpx (vpu), nef and rev six regulatory genes. gag gene encodes the core protein of the virus. env gene encodes the viral envelope protein, which is the main antigen for immunodiagnostic of HIV. Replication of viruses requires reverse transcriptase, protease, and integrase encoded by pol. Up to six regulatory genes encode accessory proteins that regulate viral protein synthesis and replication. HIV-1 originated from SIVcpz in chimpanzee [25] and HIV-2 originated from SIVsmm in mangabesii [26]. Multiple and different introductions of simian immunodeficiency virus (SIV) into the human population have led to global epidemics of HIV-1 and HIV-2 [27]. SIV only cause immune deficiency when the virus spreads across species [25]. Although the origins of them are different, they are interrelated retroviruses, showing about 55% similarity in Gag and Pol genes, more than 35% similarity in translated proteins, and about 55% overall similarity in nucleotides [28]. In terms of pathogenicity, according to relevant reports, HIV-2 has less pathogenicity [29], HIV-1 is much more virulent and transmissible than HIV-2, and the course of AIDS caused by HIV-2 is slow and mild. HIV-2 patients' CD4+T cell counts decline slowly [30]. In terms of viral load in the patient's blood, HIV-1 infected people have a higher viral load than HIV-2 infected people [31]. According to relevant studies, HIV-1 and HIV-2 infected patients with similar levels of untreated CD4+T cells have similar transcription levels of Gag mRNA, this study shows that despite the generally low viral load of HIV-2 patients, significant viral transcription occurs in such infected patients [32].
5. Antiretroviral Therapy
As early as in the early 1990s, anti-AIDS drugs as single drug therapy have made great achievements in the treatment of AIDS [10] to cope with the global pandemic of fatal HIV. At present, six categories of anti-HIV drugs improve the pathogenesis of HIV. Non-nucleoside and nucleoside reverse transcriptase inhibitors (NNRTIs and NRTIs), integrase inhibitors (INSTIs), protease inhibitors (PIs), viral maturation inhibitors (MIs), coreceptor CCR5 inhibitors. Zidovudine (AZT), a nucleoside reverse transcriptase inhibitor, was the first antiretroviral drug approved for use in 1987 and has shown a significant survival advantage compared with placebo in patients with advanced AIDS [33]. These drugs are the first to demonstrate that HIV infection is completely controllable and treatable, which provides new ideas for treating a range of viral targets. Antiretroviral therapy (ART) has reduced the extremely high mortality caused by infection, transforming HIV from a rapidly fatal disease into a chronic disease that can be treated [34]. Although the number of viruses reduce, disease progression delays, which prolong the survival period of patients in the process of NRTI single drug treatment, the use of single drug cannot continuously inhibit virus replication, and too long medication will lead to drug resistance and other problems. In addition, a single drug rarely reverses the immune function, which is not conducive to the recovery of the patient's autoimmune function [35]. In the mid-1990s, three protease inhibitor-based HIV drugs helped to slow down the global AIDS epidemic. Since then, antiretroviral therapy (cART) combining PI and NRTI drugs was developed, has significantly reduced the viral load of patients and improved their own immune function in clinical settings [36]. Combination antiretroviral therapy has resolved many opportunistic infections, such as Kaposi's sarcoma [37] and progressive multifocal leukoencephalopathy [38]; substantially reduced HIV-related mortality and extended the life expectancy of AIDS patients who underwent early intervention [39], which is now close to the life span of the general population. Since then, ART has become the mainstay of clinical HIV treatment. Thanks to antiretroviral therapy, the average life expectancy of people living with HIV-1 has been extended by about 14 years; If this is measured in life years, it is equivalent to the clinical use of antiretroviral drugs, which has saved millions of life years [40,41]. For example, an individual starting combination ART at age 20 expected to live into their 60s, a significant increase since the mid-1990s. Even so, life expectancy remains below that of the general population, calling for improvements. In addition, some antiretroviral drugs have significant side effects, including the risk of coronary heart disease and insulin resistance syndrome, which poses a challenge to the future promotion of antiretroviral drugs [42]. The main challenges of current retroviral therapy are as follows: 1. Drug resistance and increased genetic diversity of HIV-1. Due to the use of retrotherapy drugs, resistance too many drugs has emerged. In addition, the current direction of ART relies heavily on the targets encoded by the viral pol gene. 2. Cardiac and metabolic complications; Infectious disease specialists need to deal with dyslipidemia, insulin resistance, and other preventable causes of heart disease that may be unfamiliar. 3. The cost of investment in AIDS treatment is a heavy burden on the health systems of many countries in the world.
Antiretroviral therapy is efficient for clinical treatment of patients with AIDS, characterized by fulminant immunodeficiency and severe infection, and AIDS patients are prone to Kaposi's sarcoma, or other types of tumors that cause rapid death, which also leads to a low survival rate for AIDS patients. The application of antiretroviral therapy and rapid development of medicine increase the survival rate of patients and improve life quality. During the treatment of chronic antiretroviral drugs, patients may experience a number of side effects, including dyslipidemia, insulin abnormalities, abnormal redistribution of body fat, and related diseases, which in turn lead to a significant increase in the risk of heart disease and type 2 diabetes [43-45]. The development and clinical use of antiretroviral drugs have largely changed the face of HIV infection worldwide, transforming AIDS into a chronic disease controlled by drugs [46].
6. Plant Suppression AIDS Therapy
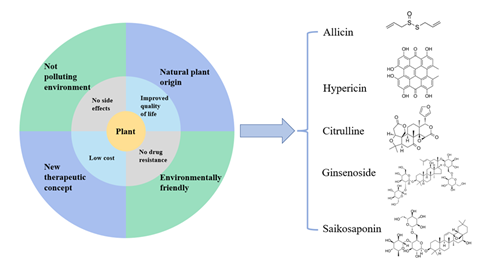
Due to the advantages of safety, no dependence and low cost of natural resources such as plants in the treatment of diseases, some achievements have been made in the research of using plants as drugs to treat various diseases in recent years including HIV, advantages of plant inhibiting AIDS (Figure 3).
According to relevant reports, at present clinical use of little or no side effects of medicinal plants to treat AIDS. Plants can not only inhibit the replication of HIV, but also some plants have the functions of antioxidant and enhancing human immunity, which can play the role of human immune regulator and immune stimulator [10]. The HIV-1 genome is composed of nine genes, encoding 19 proteins, including proteases, reverse transcriptase and integrase, structural proteins, accessory proteins, and envelope proteins that are cleaved into two glycoproteins, gp120 and gp41. Scientists have carried out a lot of work in plants to suppress AIDS. The discovery of suitable drug targets is limited, including protease, transcriptase, integrase, gp41 and host protein CCR5 [47], and plant studies with HIV reverse transcriptase (RT) inhibitory activity, HIV protease (PR) and viral integrase (IN) has become a hot topic, some of the plants with inhibitory effects on HIV are listed in Table 1. It was experimentally found that chitosan lactone (coumarin) ursolic acid and betulinic acid (pentyclic triterpene), baicalin (flavonoid), polylimonone A (alkaloid) and shikonin (phenolic compound) contained in plants are considered to have anti-HIV effects [48].
|
Plant species |
Active ingredients |
Mechanism of action |
Reference |
|
Aureobasidium Pilatus (Flammulina velutipes) |
Lectins, Ribosomal inactivating Protein, Fungal immunomodulatory protein |
Inhibition of HIV-RT and HIV activity |
Zhou et al. [49] |
|
Wild paint (Toxicodendron succedaneum) |
Bioflavonoids |
Inhibition of HIV-RT activity |
Lin et al. [50] |
|
Hypericum perforatum (Hypericum perforatum L.) |
St John's wort, Pseudohypericin |
Inhibition of HIV-IN activity |
Sanna et al. [51]; Kubin et al. [52]; Birt et al. [53] |
|
Ghost needle grass (Bidens pilosa L.) |
Flavonoids, Polyacetylene compounds |
Inhibition of HIV-PR activity |
Kim et al. [54]; Zeng et al. [55] |
|
Galangal (Alpinia officinarum Hance) |
Acetoxypiperol acetate |
Inhibition of HIV-IN activity |
Tamura et al. [56]; Zubair et al. [57] |
|
Mahogany (Swietenia mahagoni (L.) Jacq.) |
Limonin |
Inhibition of HIV-PR activity |
Parihar et al. [48]; Dong et al. [58] |
|
Garlic (Allium sativum L.) |
Allicin, Flavonoids, Polyphenols |
Inhibition of HIV-PR activity |
Sabde et al. [8]; Kim et al. [54]; Silprasit et al. [59] |
|
Red flower Manjusri orchid (Crinum amabile) |
Alkaloids such as lycorine and narchicine |
Inhibition of HIV-RT activity |
Ali et al. [60] |
|
Wild grapes (Ampelopsis brevipedunculata M. Trautv.) |
Phenolic compounds |
Inhibition of HIV activity |
Sigidi et al. [61] |
|
Ox-heart Annona (Annona reticulata Linn.) |
Alkaloids, Acetanilide |
Inhibition of HIV activity |
Hien et al. [62] |
|
Laura fu wood (Rauvolfia verticillata (Lour.) Baill.) |
Monoterpene indole alkaloids papaverine |
Inhibition of HIV-RT and HIV activity |
Sabde et al. [8]; Stöckigt et al. [63] |
|
Betel nut (Areca catechu L.) |
B1 arecoline, procyanidins |
Inhibition of HIV-PR activity |
Vermani and Garg [64] |
|
Fruit of Chinese magnoliavine (S. chinensis (Turcz.) Baill.) |
Fructus schisandrae, Triterpenoids, Nortriterpenoids, |
Inhibition of HIV-RT and HIV activity |
Szopa et al. [65]; Xiao et al. [66]; Xu et al. [67] |
|
Kelp (Laminaria japonica) |
Lectins, Sulfated polysaccharide |
Glycoprotein receptors on T lymphocytes, Inhibition of HIV activity |
Nakashima et al. [68]; Singh and Walia [69] |
|
Bitter melon (Momordica charantia L.) |
Ribosome inactivates proteins, Bitter gourd lectin, Bitter gourd anti-HIV protein |
Inhibition of HIV-RT activity, viral core protein p24, immune cells |
Fang et al. [70]; Lee-Huang et al. [71]; Meng et al. [72]; Puri et al. [73] |
|
Rhizoma coptidis (Coptis chinensis Franch.) |
Limonin, Quaternary ammonium alkaloid |
Inhibition of HIV-PR and HIV activity |
Dong et al. [58]; Gupta et al. [74]; Qian et al. [75] |
|
Scutellaria baicalensis georgi (Scutellaria baicalensis Georgi) |
Flavonoids |
Inhibition of HIV-PR, HIV-RT and HIV activity |
Li-Weber [76]; Zhao et al. [77] |
|
Herba violae (Viola phillipina) |
Cyclic peptide compounds |
Inhibition of HIV activity |
He et al. [78]; Wang et al. [79] |
|
Chinese wolfberry (Lycium chinense Miller) |
LBP, Quaternary ammonium alkaloid |
Inhibition of HIV activity |
Shah et al. [80] |
|
Ginseng (Panax ginseng C. A. Meyer) |
Ginseng saponin, Ginseng polysaccharide |
Inhibition of HIV-RT activity, improve immune cell function |
Cho et al. [81]; Kim et al. [82] |
|
The root of remembranous milk vetch (A. membranaceus (F. Bunge.) |
Astragalus saponin, Astragalus polysaccharides |
Human immunomodulators, Inhibition of HIV activity |
Chen and Huang [83]; Hirotani et al. [84]; Rios and Waterman [85] |
|
Artemisia capillaris (Artemisia capillaris Thunb.) |
Dicaffeoylquinic acid, Coumarin, Flavonoids |
Inhibition of HIV-PR activity |
Kim et al. [54]; Evers et al. [86]; McDougall et al. [87]; Tan et al. [88]; Zhu et al. [89] |
|
Creat (Andrographis paniculata (Burm. F.) Nees) |
Andrographolide |
Inhibition of HIV and HIV-PR activity |
Chang et al. [90]; Hossain et al. [91]; Reddy et al. [92]; Xu et al. [93] |
|
Fructus arctii (Arctium lappa L.) |
Wooden fat element, Dicaffeoylquinic acid, Flavonoids |
Inhibition of HIV-RT and HIV activity |
Kim et al. [54]; McDougall et al. [87]; Schröder et al. [94]; Wang et al. [95] |
|
Licorice (Glycyrrhiza uralensis Fisch.) |
Glycyrrhizin |
Inhibition of HIV-RT activity |
Afreen et al. [96]; Fomenko et al. [97] |
|
Honeysuckle (Lonicera japonica Thunb.) |
Ethyl caffeic acid, Caffeic acid |
Inhibition of HIV-PR activity |
Wang et al. [98] |
|
Radix bupleuri (Bupleurum chinense) |
Bupleurum saponins |
Inhibition of HIV activity |
Guo et al. [99]; Nyobe et al. [100] |
|
Radix arnebiae seu lithospermi (L. erythrorhizon Sieb. et Zucc.) |
Shikonin |
Inhibition of HIV replication |
Chen et al. [101] |
|
Golden retriever dog (Cibotium barometz (L.) J. Sm.) |
Anthraquinones, Flavonoids, Tannin |
Inhibition of HIV-RT activity, Inhibition of HIV activity |
Esposito et al. [102]; Heng et al. [103]; Xu et al. [104] |
|
Selfheal (Prunella vulgaris L) |
Tannin |
Inhibition of HIV activity |
Liu et al. [105] |
Table 1: Plants with HIV inhibitory activity and with clinical promise for the treatment of AIDS.
7. Plants with Potential Value for HIV Suppression
Hypericum perforatum, also known as St John's wort, is a perennial herb of the Hypericum genus in the Garcyaceae family. It is considered as an important medicinal plant and is often used to improve depression and other related symptoms in clinical practice. Hypericin isolated from Hypericum perforatum and pseudohypericin confirmed to inhibit HIV reverse transcriptase and viral integrase, which has great potential in the clinical treatment of AIDS [51-53]. Garlic is an underground bulb of Allium species in the Liliaceae family. Garlic is a famous edible and medicinal plant with biological activities such as prevention and treatment of cardiovascular diseases and anti-tumor. Garlic contains many active components, among which allicin has broad-spectrum antibacterial, sterilization and anti-inflammatory effects, and has strong antiviral ability. In studies, allicin, flavonoids and polyphenolic compounds extracted from garlic have /been found to have strong effects on inhibiting HIV reverse transcriptase and protease [8,54,59,104]. Schisandrin is a genus of Schisandrin in the Magnolia family. Its fruit contains Schisandrin, vitamin C, resin, tannin and a small amount of sugars. Schisandra chinensis extract confirmed to inhibit HIV reverse transcriptase activity [67]. The triterpenoids isolated from the leaves and stems of Schisandra chinensis chinensis show anti-HIV-1 activity [66]. As a famous Plant-based medicine, the anti-HIV effect of Schisandra chinensis is worth further exploration. Bitter gourd is a bitter gourd plant in Cucurbitaceae. Bitter gourd is rich in protein, sugar, minerals and vitamins. In addition, the ribosome inactivation protein, bitter gourd lectin and bitter gourd anti-HIV protein 30 kD extracted from bitter gourd, etc. It has been confirmed that bitter gourd can selectively kill HIV-infected lymphocytes and macrophages, inhibit HIV-RT activity and inhibit viral core protein p24 [70-73]. Bitter gourd shows significant anti-HIV efficacy. Coptis is a genus of buttercups, and as a commonly used medicinal plant, it has antibacterial, anti-inflammatory and antiviral effects, used in clinical practice to clear heat and dry dampness, and to relieve fire and detoxify the body. The lemon bitter extract from Coptis has the effect of inhibiting HIV-PR activity [58,75], quaternary ammonium alkaloids isolated from Coptis, with significant inhibitory effect on HIV activity [74], Coptis has HIV inhibiting properties. Scutellaria Baicalensis is a plant of the genus Scutellaria in the Labiaceae family. The root of Scutellaria Baicalensis used as a medicine. Plant-based medicine used to treat upper respiratory tract infection including cough with lung heat, yellow gallbladder with damp heat and pneumonia. Baicalein, baicalein, baicalein and other flavonoids found in the roots of Baicalein [77]. Flavonoids extracted from Scutellaria baicalensis can inhibit HIV-PR and HIV-RT activity [76]. The low drug resistance and high efficiency of inhibiting HIV in Scutellaria Baicalensis are the natural plants for us to explore anti-AIDS. The whole plant used as a medicine, which has the functions of clearing heat and detoxing, cooling blood and reducing swelling. The cyclic peptide isolated from Zi-hua, shown to have anti-HIV effect (He et al., 2011). A positive correlation between the hydrophobicity and anti-HIV activity of cyclic peptides isolated from Zi-hua was also found, and the anti-HIV activity. Moreover, this trend related to their ability to destroy cell membranes [78]. In the future, Zihua has great research value in the treatment of AIDS. Arctium burdock is a burdock plant in Asteraceae family. Arctium contains lignans such as arctiin and arctigenin, and arctiin strongly inhibit the efficacy of HIV replication [94]. Dicaffeoylquinic acid and flavonoids isolated from arctium burdock have been proved to have the effect of inhibiting HIV [95]. Arctium burdock has great potential in inhibiting HIV and need to be explored.
8. Conclusion
Drug resistance and side effects that reduce life expectancy of patients hamper AIDS treatment. A variety of phytochemicals not only inhibit the replication of HIV, but also repair the immune system damaged by the virus. A number of plants may therefore be used as new efficient drugs for the treatment of AIDS. For this purpose, it is necessary to understand the mechanism of effective chemical components of plants and conduct cytotoxicity tests on corresponding extracts to ensure the safety and effectiveness in clinical application. This requires a number of chemicals analyses, preclinical and clinical trials before that could be in place 10-20 years from now.
References
- Dong, Y, Guo W, Gui X, et al. Preventing mother to child transmission of HIV: lessons learned from China. Bmc Infectious Diseases 20 (2020): 792.
- Fromentin, R, Chomont N. HIV persistence in subsets of CD4+T cells: 50 shades of reservoirs. Seminars in Immunology 51 (2021): 101438.
- Reed M, Cosgrove JM, Cindrich R, et al. Ten Years Later: A Single Hospital Experience with Malignancy in HIV/AIDS. Journal of Surgical Oncology 102 (2010): 282-286.
- Orem J, Otieno MW, Banura C, et al. Capacity building for the clinical investigation of AIDS malignancy in East Africa. Cancer Detection and Prevention 29 (2005): 133-145.
- Lao DH, Liu R, Liang J. Study on plasma metabolomics for HIV/AIDS patients treated by HAART based on LC/MS-MS. Front. Pharmacol 13 (2022): 1-10.
- Vandhuick O, Guias B, De Saint Martin L, et al. Traitement antirétroviral et risque cardio-vasculaire. Journal des Maladies Vasculaires 29 (2004): 192-199.
- Paterson DL, Swindells S, Mohr J, et al. Adherence to Protease Inhibitor Therapy and Outcomes in Patients with HIV Infection. Annals of Internal Medicine 133 (2002): 21-30.
- Sabde S, Bodiwala HS, Karmase A, et al. Anti-HIV activity of Indian medicinal plants. Journal of Natural Medicines 65 (2011): 662-669.
- Kayombo EJ, Uiso FC, Mbwambo ZH, et al. Experience of initiating collaboration of traditional healers in managing HIV and AIDS in Tanzania. Journal of Ethnobiology and Ethnomedicine 3 (2007).
- Laila U, Akram M, Ali Shariati M, et al. Role of medicinal plants in HIV/AIDS therapy. Clinical and Experimental Pharmacology and Physiology 46 (2019): 1063-1073.
- Hupfeld J, Efferth T. Drug Resistance of Human Immunodeficiency Virus and overcoming it by Natural Products. In Vivo. 23 (2009).
- Dalgleish AG, Beverley PCL, Clapham PR, et al. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature 312 (1984): 763-767.
- Veenhuis RT, Abreu CM, Shirk EN, et al. HIV replication and latency in monocytes and macrophages. Seminars in Immunology 51 (2021): 101472.
- Rawson JMO, Nikolaitchik AO, YOO JA, et al. Adaptation of HIV-1/HIV-2 Chimeras with Defects in Genome Packaging and Viral Replication MBIO 13 (2022).
- Zaitseva M, Blauvelt A, Lee S, et al. Expression and function of CCR5 and CXCR4 on human Langerhans cells and macrophages: implications for HIV primary infection. Nat Med 3 (1997): 1369-1375.
- Coffin JM, Hughes HS, Varmus HE. Retroviruses. Cold Spring Harbor Laboratory Press, Copyright © 1997, Cold Spring Harbor Laboratory Press, Cold Spring Harbor (NY) (1997).
- Qashqari FS, Alsafi RT, Kabrah SM, et al. Knowledge of HIV/AIDS transmission modes and attitudes toward HIV/AIDS infected people and the level of HIV/AIDS awareness among the general population in the kingdom of Saudi Arabia: A cross-sectional study. Front. Public Health 10 (2022).
- Zinkernagel RM, Hengartner H. T-cell-mediated immunopathology versus direct cytolysis by virus: implications for HIV and AIDS. Immunology Today 15 (1994): 262-268.
- Dash S, Balasubramaniam M, Villalta F, et al. Impact of cocaine abuse on HIV pathogenesis. Front. Microbiol 6 (2015).
- Fauci AS, Pantaleo G, Stanley S, et al. Immunopathogenic Mechanisms of HIV Infection. Annals of Internal Medicine 124 (1996): 654-663.
- Herndier BG, Kaplan LD, MCGrath MS. Pathogenesis of AIDS lymphomas. AIDS 8 (1994): 1025-1049.
- Clavel F, Guetard D, Brun-Vezient F, et al. Isolation of a New Human Retrovirus from West African Patients with AIDS. Science 233 (1986): 343-346.
- van der Loeff MFS, Awasana AA, Sarge-Njie R, et al. Sixteen years of HIV surveillance in a West African research clinic reveals divergent epidemic trends of HIV-1 and HIV-2. International Journal of Epidemiology 35 (2006): 1322-1328.
- Gao F, Bailes E, Robertson DL, et al. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature 397 (1999): 436-441.
- Santiago Mario L, Range F, Keele Brandon F, et al. Simian Immunodeficiency Virus Infection in Free-Ranging Sooty Mangabeys (Cercocebus atys atys) from the Taï Forest, Côte d'Ivoire: Implications for the Origin of Epidemic Human Immunodeficiency Virus Type 2. Journal of Virology 79 (2005): 12515-12527.
- Sauter D, Kirchhoff F. Key Viral Adaptations Preceding the AIDS Pandemic. Cell Host and Microbe 25 (2019): 27-38.
- Motomura, K., et al., 2008. Genetic Recombination between Human Immunodeficiency Virus Type 1 (HIV-1) and HIV-2, Two Distinct Human Lentiviruses. Journal of Virology. 82, 1923-1933.
- Esbjörnsson J, Jansson M, Jespersen S, et al. HIV-2 as a model to identify a functional HIV cure. AIDS Research and Therapy 16 (2019).
- Drylewicz J, Matheron S, Lazaro E, et al. Comparison of viro-immunological marker changes between HIV-1 and HIV-2-infected patients in France. AIDS 22 (2018).
- Popper SJ, Travers Sarr AD, Gueye-Ndiaye A, et al. Lower Human Immunodeficiency Virus (HIV) Type 2 Viral Load Reflects the Difference in Pathogenicity of HIV-1 and HIV-2. The Journal of Infectious Diseases 180 (1999): 1116-1121.
- Soares Rui S, Tendeiro R, Foxall Russell B, et al. Cell-Associated Viral Burden Provides Evidence of Ongoing Viral Replication in Aviremic HIV-2-Infected Patients. Journal of Virology 85 (2011): 2429-2438.
- Fischl MA, Richman DD, Grieco MH, et al. The Efficacy of Azidothymidine (AZT) in the Treatment of Patients with AIDS and AIDS-Related Complex. New England Journal of Medicine 317 (1987): 185-191.
- Broder S. The development of antiretroviral therapy and its impact on the HIV-1/AIDS pandemic. Antiviral Research. 85 (2010): 1-18.
- Pau AK, George JM. Antiretroviral Therapy: Current Drugs. Infectious Disease Clinics 28 (2014): 371-402.
- Lederman MM, Connik E, Landay A, et al. Immunologic Responses Associated with 12 Weeks of Combination Antiretroviral Therapy Consisting of Zidovudine, Lamivudine, and Ritonavir: Results of AIDS Clinical Trials Group Protocol 315. The Journal of Infectious Diseases 178 (1998): 70-79.
- Cattelan AM, Calabro ML, Gasperini P, et al. Acquired Immunodeficiency Syndrome-Related Kaposi's Sarcoma Regression After Highly Active Antiretroviral Therapy: Biologic Correlates of Clinical Outcome. J Natl Cancer Inst Monogr 28 (2000): 44-49.
- De Luca, A., et al., 2000. The Effect of Potent Antiretroviral Therapy and JC Virus Load in Cerebrospinal Fluid on Clinical Outcome of Patients with AIDS-Associated Progressive Multifocal Leukoencephalopathy. The Journal of Infectious Diseases. 182, 1077-1083.http://doi.org/10.1086/315817.
- Palella FJ, Delaney KM, Moorman AC, et al. Declining Morbidity and Mortality among Patients with Advanced Human Immunodeficiency Virus Infection. New England Journal of Medicine 338 (1998): 853-860.
- Walensky RP, David Paltiel A, Losina E, et al. The Survival Benefits of AIDS Treatment in the United States. The Journal of Infectious Diseases 194 (2006): 11-19.
- Vermund SH. Millions of Life-Years Saved with Potent Antiretroviral Drugs in the United States: A Celebration, with Challenges. The Journal of Infectious Diseases 194 (2006): 1-5.
- Hawkins T. Understanding and managing the adverse effects of antiretroviral therapy. Antiviral Research 85 (2010): 201-209.
- Williams PL, Wu JW, Cohn SE, et al. Improvement in lipid profiles over 6 years of follow-up in adults with AIDS and immune reconstitution. HIV Medicine 10 (2009): 290-301.
- Silverberg MJ, Leyden W, Hurley L, et al. Response to Newly Prescribed Lipid-Lowering Therapy in Patients With and Without HIV Infection. Annals of Internal Medicine 150 (2009): 301-313.
- Filardi PP, Paolillo S, Marciano C, et al. Cardiovascular effects of antiretroviral drugs: clinical review. Cardiovasc Hematol Disord Drug Targets 8 (2008): 238-244.
- D:A:D Study Group. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. The Lancet 371 (2008): 1417-1426.
- Arts EJ, Hazuda DJ. HIV-1 Antiretroviral Drug Therapy. Cold Spring Harbor Perspectives in Medicine 2 (2012): a007161.
- Parihar S, Sharma D, Telrandhe U. Phytochemistry and Pharmacological Activities of Swietenia macrophylla King (Meliaceae) 11 (2022): 6-12.
- Zhou R, Liu Zhao K, Zhang Ye Ni, et al. Research Progress of Bioactive Proteins from the Edible and Medicinal Mushrooms. Current Protein and Peptide Science 20 (2019): 196-219.
- Lin YM, Anderson H, Flavin MT, et al. In Vitro Anti-HIV Activity of Biflavonoids Isolated from Rhus succedanea and Garcinia multiflora. Journal of Natural Products 60 (1997): 884-888.
- Sanna C, Scognamiglio M, Fiorentino A, et al. Prenylated phloroglucinols from Hypericum scruglii, an endemic species of Sardinia (Italy), as new dual HIV-1 inhibitors effective on HIV-1 replication. PLOS ONE 13 (2018): e0195168.
- Kubin A, Wierrani F, Burner U, et al. Hypericin-The Facts About A Controversial Agent. Current Pharmaceutical Design 11 (2005): 233-253.
- Birt DF, Widrlechner Mark P, Hammer Kimberly DP, et al. Hypericum in infection: Identification of anti-viral and anti-inflammatory constituents. Pharmaceutical Biology 47 (2009): 774-782.
- Kim HJ, Woo ER, Shin CG, et al. A new flavonol glycoside gallate ester from Acer okamotoanum and its inhibitory activity against human immunodeficiency virus-1 (HIV-1) integrase. Journal of Natural Products 61 (1998): 145-148.
- Zeng X. Research progress on chemical constituents of medicinal plants of Stipa L. Chinese herbal medicine (2017).
- Tamura S, Shiomi A, Kimura T, et al. Halogenated analogs of 1 '-acetoxychavicol acetate, Rev-export inhibitor from Alpinia galanga, designed from mechanism of action. Bioorganic and Medicinal Chemistry Letters 20 (2010): 2082-2085.
- Zubair MS, Maulana S, Widodo A, et al. Docking Study on Anti-HIV-1 Activity of Secondary Metabolites from Zingiberaceae Plants. J Pharm Bioallied Sci 12 (2020): S763-S767.
- Dong X, Fu J, Yin X, et al. Emodin: A Review of its Pharmacology, Toxicity and Pharmacokinetics. Phytotherapy Research 30 (2016): 1207-1218.
- Silprasit, K, Seetaha S, Pongsanarakul P, et al. Anti-HIV-1 reverse transcriptase activities of hexane extracts from some Asian medicinal plants. Journal Of Medicinal Plants Research 5 (2011): 4899-4906.
- Ali H, König GM, Khalid SA, et al. Evaluation of selected Sudanese medicinal plants for their in vitro activity against hemoflagellates, selected bacteria, HIV-1-RT and tyrosine kinase inhibitory, and for cytotoxicity. Journal of Ethnopharmacology 83 (2022): 219-228.
- Sigidi MT, Traore AN, Boukandou MM, et al. Anti-HIV, pro-inflammatory and cytotoxicity properties of selected Venda plants. Indian Journal of Traditional Knowledge 16 (2017): 545-552
- Hien NTT, Nhiem NX, Yen DTH, et al. Chemical constituents of the Annona glabra fruit and their cytotoxic activity. Pharmaceutical Biology 53 (2015): 1602-1607.
- Stöckigt J, Obitz P, Falkenhagen H, et al. Natural products and enzymes from plant cell cultures. Plant Cell, Tissue and Organ Culture 43 (1995): 97-109.
- Vermani K, Garg S. Herbal medicines for sexually transmitted diseases and AIDS. Journal of Ethnopharmacology 80 (2002): 49-66.
- Szopa A, Barnas M, Ekiert H. Phytochemical studies and biological activity of three Chinese Schisandra species (Schisandra sphenanthera, Schisandra henryi and Schisandra rubriflora): current findings and future applications. Phytochemistry Reviews 18 (2019): 109-128.
- Xiao WL, Yang LM, Li LM, et al. Sphenalactones A-D, a new class of highly oxygenated trinortriterpenoids from Schisandra sphenanthera. Tetrahedron Letters 48 (2007): 5543-5546.
- Xu L, Grandi N, Vecchio Claudia D, et al. From the traditional Chinese medicine plant Schisandra chinensis new scaffolds effective on HIV-1 reverse transcriptase resistant to non-nucleoside inhibitors. Journal of Microbiology 53 (2015): 288-293.
- Nakashima H, Yoshida O, Tochikura TS, et al. Sulfation of Polysaccharides Generates Potent and Selective Inhibitors of Human Immunodeficiency Virus Infection and Replication In Vitro. Japanese Journal of Cancer Research GANN. 78 (1987): 1164-1168.
- Singh RS, Walia AK. Lectins from red algae and their biomedical potential. Journal of Applied Phycology 30 (2018): 1833-1858.
- Fang EF, et al., 2012. The MAP30 protein from bitter gourd (Momordica charantia) seeds promotes apoptosis in liver cancer cells in vitro and in vivo. Cancer Letters 324 (2012): 66-74.
- Lee-Huang S, Huang PL, Chen HC, et al. Anti-HIV and anti-tumor activities of recombinant MAP30 from bitter melon. Gene 161 (1995): 151-156.
- Meng Y, Liu S, Li J, et al. Preparation of an antitumor and antivirus agent: chemical modification of alpha-MMC and MAP30 from Momordica Charantia L. with covalent conjugation of polyethyelene glycol. International Journal of Nanomedicine 7 (2012): 3133-3142.
- Puri M, Kaur I, Kanwar RK, et al. Ribosome Inactivating Proteins (RIPs) from Momordica charantia for Anti Viral Therapy. Current Molecular Medicine 9 (2009): 1080-1094.
- Gupta M, Singh N, Gulati M, et al. Herbal bioactives in treatment of inflammation: An overview. South African Journal of Botany 143 (2021): 205-225.
- Qian P, Jin HW, Yang XW. New limonoids from Coptidis Rhizoma-Euodiae Fructus couple Journal of Asian Natural Products Research 16 (2014): 333-344.
- Li-Weber M. New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treatment Reviews 35 (2009): 57-68.
- Zhao Q, Chen XY, Martin C. Scutellaria baicalensis, the golden herb from the garden of Chinese medicinal plants. Science Bulletin 61 (2016): 1391-1398.
- He WJ, Chen LY, Zeng G, et al. Isolation and characterization of cytotoxic cyclotides from Viola philippica. Peptides 32 (2011): 1719-1723.
- Wang CKL, Colgrave ML, Gustafson KR, et al. Anti-HIV cyclotides from the Chinese medicinal herb Viola yedoensis. Journal of Natural Products 71 (2008): 47-52.
- Shah T, Bule M, Niaz K. Chapter 3.21 - Goji Berry (Lycium barbarum)— A Superfood. Nonvitamin and Nonmineral Nutritional Supplements. Academic Press (2019): 257-264.
- Cho YK, Sung H, kim TK, et al. Korean red ginseng significantly slows CD4 T cell depletion over 10 years in HIV-1 infected patients: association with HLA. Journal of Ginseng Research 28 (2004): 173-182.
- Kim HS, Kacew S, Lee BM. In vitro chemopreventive effects of plant polysaccharides (Aloe barbadensis Miller, Lentinus edodes, Ganoderma lucidum and Coriolus versicolor). Carcinogenesis 20 (1999): 1637-1640.
- Chen L, Huang GL. The antiviral activity of polysaccharides and their derivatives. International Journal of Biological Macromolecules 115 (2018): 77-82.
- Hirotani M, Zhou Y, Lui H, et al. Astragalosides from Hairy Root Cultures of Astragalus-Membranaceus .100. Studies On Plant-Tissue Cultures. Phytochemistry 36 (1994): 665-670.
- Rios JL, Waterman PG. A review of the pharmacology and toxicology of Astragalus. Phytotherapy Research. 11 (1997): 411-418.
- Evers DL,Chao CF, Wang X, et al. Human cytomegalovirus-inhibitory flavonoids: Studies on antiviral activity and mechanism of action. Antiviral Research 68 (2005): 124-134.
- McDougall B, King PJ, Hostomsky Z, et al. Dicaffeoylquinic and Dicaffeoyltartaric Acids Are Selective Inhibitors of Human Immunodeficiency Virus Type 1 Integrase. Antimicrobial Agents and Chemotherapy 42 (1998): 140-146.
- Tan XJ, Li Q, Chen XH, et al. Simultaneous determination of 13 bioactive compounds in Herba Artemisiae Scopariae (Yin Chen) from different harvest seasons by HPLC-DAD. Journal of Pharmaceutical and Biomedical Analysis 47 (2008): 847-853.
- Zhu M, Ma L, Wen J, et al. Rational design and Structure-Activity relationship of coumarin derivatives effective on HIV-1 protease and partially on HIV-1 reverse transcriptase. European Journal of Medicinal Chemistry 186 (2020).
- Chang RS, Ding L, Chen GQ, et al. Dehydroandrographolide Succinic Acid Monoester as an Inhibitor against the Human Immunodeficiency Virus. Proceedings of the Society for Experimental Biology and Medicine 197 (1991): 59-66.
- Hossain S, Urbi Z, Karuniawati H, et al. Andrographis paniculata (Burm. f.) Wall. ex Nees: An Updated Review of Phytochemistry, Antimicrobial Pharmacology, and Clinical Safety and Efficacy. Life 11 (2021): 348.
- Reddy VLN, Reddy SM, Ravikanth V, et al. A new bis-andrographolide ether from Andrographis paniculata nees and evaluation of anti-HIV activity. Natural Product Research 19 (2005): 223-230.
- Xu HX, Wan M, Loh BN, et al. Screening of Traditional Medicines for their Inhibitory Activity Against HIV-1 Protease. Phytotherapy Research 10 (1996): 207-210.
- Schröder HC, Merz H, Steffen R, et al. Differential in vitro Anti-HIV Activity of Natural Lignans 45 (1990): 1215-1221.
- Wang DD, Badarau AS, Swamy MK, et al. Arctium Species Secondary Metabolites Chemodiversity and Bioactivities. Frontiers in Plant Science 10 (2019a).
- Afreen F, Zobayed SMA, Kozai T. Spectral quality and UV-B stress stimulate glycyrrhizin concentration of Glycyrrhiza uralensis in hydroponic and pot system. Plant Physiology and Biochemistry 43 (2005): 1074-1081.
- Fomenko VV, Rudometova NB, Yarovaya OL, et al. Synthesis and In Vitro Study of Antiviral Activity of Glycyrrhizin Nicotinate Derivatives against HIV-1 Pseudoviruses and SARS-CoV-2 Viruses. Molecules 27 (2022).
- Wang X, Wei Y, Tian WY, et al. Characterization of Nine Compounds Isolated from the Acid Hydrolysate of Lonicera fulvotomentosa Hsu et S. C. Cheng and Evaluation of Their In Vitro Activity towards HIV Protease. Molecules 24 (2019b): 4526.
- Guo Q, Cao H, Qi H, et al. Research Progress in Reversal of Tumor Multi-drug Resistance via Natural Products. Anti-Cancer Agents in Medicinal Chemistry 17 (2017): 1466-1476.
- Nyobe L, Zhnag J, Huang ST. Saikosaponins a and d roots concentration in five Bupleurum species from four mountains in China. African Journal of Biotechnology 11 (2012): 1138-1150
- Chen X, Yang L, Zhnag N, et al. Shikonin, a component of Chinese herbal medicine, inhibits chemokine receptor function and suppresses human immunodeficiency virus type 1. Antimicrobial Agents and Chemotherapy 47 (2003): 2810-2816.
- Esposito F, Corona A, Zinzula L, et al. New Anthraquinone Derivatives as Inhibitors of the HIV-1 Reverse Transcriptase-Associated Ribonuclease H Function. Chemotherapy 58 (2012): 299-307.
- Heng YW, Ban JJ, Khoo KS, et al. Biological activities and phytochemical content of the rhizome hairs of Cibotium barometz (Cibotiaceae). Industrial Crops and Products 153 (2020): 112612.
- Xu HX, Wan M, Dong H, et al. Inhibitory Activity of Flavonoids and Tannins against HIV-1 Protease. Biological & Pharmaceutical Bulletin 23 (2000): 1072-1076.
- Liu SW, Jiang S, Wu Z, et al. Identification of inhibitors of the HIV-1 gp41 six-helix bundle formation from extracts of Chinese medicinal herbs Prunella vulgaris and Rhizoma cibotte. Life Sciences 71 (2002): 1779-1791.
- Berginc K, Trdan T, Trontelji J, et al. HIV Protease Inhibitors: Garlic Supplements and First-pass Intestinal Metabolism Impact on the Therapeutic Efficacy. Biopharmaceutics & Drug Disposition 31 (2010): 495-505.


 Impact Factor: * 4.1
Impact Factor: * 4.1 Acceptance Rate: 75.32%
Acceptance Rate: 75.32%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 