Systems Microbiology Approach to Meat-Borne Pathogen-Phage Interactions: Decoding Evolutionary Arms Race, Antibiotic Resistance Modulation, and Precision Biocontrol Potential
Mudassir Ahmad1*, Waseem Sajjad2*, Mujaddad UR Rehman3*, Saman Hasan Abdullah1, Hafiz Muhammad Tariq Saeed4, Ayreen Sonia Chowdhury1
1School of Science and Technology, Nottingham Trent University, Nottingham, UK
2Department of Microbiology, Abbottabad University of Science and Technology, Pakistan
3Dean Faculty of Health and Biomedical Sciences, Abbottabad University of Science and Technology, Pakistan
4Veterinary Sciences, University of Veterinary & Animal Sciences Lahore, Pakistan
*Corresponding Authors:
Mujaddad UR Rehman, Dean Faculty of Health and Biomedical Sciences, Abbottabad University of Science and Technology, Pakistan
Waseem Sajjad, Department of Microbiology, Abbottabad University of Science and Technology, Pakistan
Mudassir Ahmad, School of Science and Technology, Nottingham Trent University, Nottingham, UK
Received: 12 July 2025; Accepted: 18 July 2025; Published: 25 July 2025
Article Information
Citation: Mudassir Ahmad, Waseem Sajjad, Mujaddad UR Rehman, Saman Hasan Abdullah, Hafiz Muhammad Tariq Saeed, Ayreen Sonia Chowdhury. Systems Microbiology Approach to Meat-Borne Pathogen-Phage Interactions: Decoding Evolutionary Arms Race, Antibiotic Resistance Modulation, and Precision Biocontrol Potential. International Journal of Plant, Animal and Environmental Sciences 15 (2025): 60-75.
View / Download Pdf Share at FacebookAbstract
The Antibiotic-resistant Bacillus spizizenii poses a double hazard to meat products as it can act as a spoiling agent and a reservoir for antibiotic resistance genes (ARGs). The current study investigates the tripartite interaction between B. spizizenii, its bacteriophages, and antibiotic resistance using a systems microbiology approach. In the current study total of 2 bacterial strains of Bacillus spizizenii strains were isolated from meat samples. The 2 isolates were then characterized on the basis of their morphology and microscopic analysis. Biochemical characterization was conducted to characterize isolates of pathogens. All two strains are classified and characterized as Bacillus spizizenii by giving creamy color on LB agar. Biochemical characterization was conducted to characterize isolates of pathogens. The biochemical test results show that isolates don’t react with indole test red test and did react with catalase and motility test. The disk diffusion technique was utilized for testing for antimicrobial sensitivity to a variety of drugs. Oxytetracycline, Kinamycin, Azithromycin, antibiotics disc was used in current study. Because of the emergence of antibiotic resistance, treating Bacillus spizizenii infections with antibiotics is becoming more difficult, necessitating the development of a novel alternative approach. A kind of virus called a bacteriophage was once employed to cure human illnesses caused on by a variety of bacteria. Bacteriophages may be viable options for treating Bacillus spizizenii, given their superior bacterial growth reduction, thermal stability, and host range. Future genetic, physiological, and clinical studies are required to fully characterize bacteriophages against Bacillus spizizenii.
Keywords
<p>Bacillus spizizenii bacteriophages; Phage-antibiotic synergy, Meat spoilage control, CRISPR-Cas dynamics, horizontal gene transfer; Food safety omics; Precision biocontrol; Antimicrobial resistance evolution</p>
Article Details
1. Introduction
Ensuring product safety and quality is becoming increasingly difficult for the global meat business, especially in light of microbial contamination that causes spoiling and possible health hazards to the general population. Bacillus species, which were formerly thought to be harmless, have lately been linked to meat rotting through proteolytic activity and biofilm development, which is one of the new concerns [1]. Although its ecological relevance is still unclear, Bacillus spizizenii, a recently identified species within the subtilis group, has been isolated from fresh meat more often [2]. This organism is a possible persistent contamination in meat processing settings due to its resilience, which is generated from endospore production and genes that respond to cold stress [3]. Adding to this problem, early research indicates that some strains of B. spizizenii may have extended-spectrum β-lactamases (ESBLs) and other antibiotic resistance genes (ARGs), which raises questions about their potential to function as reservoirs for the spread of resistance [4].
There are advantages and disadvantages to the identification of bacteriophages that target B. spizizenii. With their effective use against Salmonella and Listeria in ready-to-eat meats, phages have emerged as precise instruments against foodborne pathogens. But little is known about how they interact with Bacillus species linked to spoiling, especially how phage predation pressure could unintentionally favor resistant phenotypes or promote the horizontal gene transfer (HGT) of virulence genes [5]. Zhang et al. [4] recent study from 2023 showed that Bacillus phages may transfer genes that encode heat-stable toxins from one strain to another in dairy systems, indicating that meat matrices may be susceptible to the same dangers. Because of this duality, phages can act as both biocontrol agents and possible genetic exchange mediators, therefore a systems-level analysis is required to weigh the risks and advantages [1].
Gram-negative pathogens have historically been the focus of antibiotic resistance in meat-associated bacteria, leaving important knowledge gaps about Gram-positive resistomes. ARG acquisition from therapeutically significant genera like as Enterococcus and Staphylococcus may be made possible by B. spizizenii's genomic flexibility, which is supported by natural competence and conjugative elements [6]. The discovered connection between stress response pathways (such as SOS activation after phage infection) and enhanced competence for genetic absorption is particularly concerning. This phenomenon has been extensively researched in B. subtilis but has not been examined in meat ecosystems [7]. As phage applications receive regulatory approval, this gap gets worse. For example, the European Food Safety Authority (EFSA) recently approved a Salmonella phage product setting a precedent for similar formulations that target Bacillus without fully understanding the potential consequences of resistance [8].
Bacillus-related processes of meat rotting are also poorly understood. Proteolytic and lipolytic activities are known spoilage determinants, but it is still unknown whose regulatory networks govern these characteristics in B. spizizenii, particularly when phage predation stress is present. Our lab's preliminary proteomic data shows that extracellular metalloproteases (like AprE) are upregulated in beef extracts during the late-log phase development, which corresponds with the commencement of sensory spoiling (unpublished observations). Interestingly, these enzymes have structural similarities to virulence proteins of Staphylococcus, indicating that spoiling and pathogenicity pathways may have evolved together [9]. Phage infection could disrupt such systems through transcriptional reprogramming or direct cleavage of regulatory mRNAs, presenting novel intervention points [10].
The dynamics between B. spizizenii and phages are further complicated by the CRISPR-Cas system. CRISPR arrays offer a molecular record of previous phage interactions and are a bacterial adaptive immune system. Meat isolates' spacer sequences may be analyzed to predict sensitivity to commercial phage cocktails and uncover patterns of past phage exposure [8]. The use of anti-CRISPR proteins by phages to get beyond host defenses, on the other hand, results in an evolutionary arms race that affects the stability of resistance genes as well as spoiling control. According to recent structural analyses, Bacillus anti-CRISPRs (such AcrIIA1) maintain phage genomes inside host cells, which may extend the time available for gene transmission [10].
This study employs an integrated systems microbiology approach to address these interrelated issues. By combining functional assays (microfluidics, conjugation monitoring) with advanced omics (genomics, transcriptomics, and proteomics), we clarify how B. spizizenii-phage interactions affect: (1) ARG mobilization frequencies; (2) expression of spoilage phenotypes; and (3) evolutionary trajectories under conditions relevant to the industry. Our research connects practical food safety and basic microbiology by identifying possible hazards that need to be mitigated and offering data-driven frameworks for phage deployment. The meat industry's pressing need for antibiotic substitutes, which is expected to generate a $1.2 billion market by 2026 and the requirement by regulatory bodies for thorough risk analyses of phage-based treatments highlight how vital this study is [11].
2. Materials and Methods
2.1 Study area and isolation of samples
Bacillus spizizenii samples were gathered from the Science and Technology and Microbiology Labs at Abbottabad University. After streaking bacterial samples onto LB agar plates, the plates were incubated overnight at 37°C. The bacteria were identified using microscopy and Gram staining. Prior to each experiment, fresh bacterial samples were subcultured, and the cultures were utilized for eight hours. Regular subculturing preserved the culture's integrity and vitality.
2.2 Morphology basedCharacterization of Isolated Bacillus spizizenii
2.2.1 Gram staining
To perform the Gram staining, only a small amount of distilled water was put to a transparent slide. Using a sterile needle, a little amount of pure culture was added to the slide. The needle was used to evenly distribute the culture throughout the surface. Using sterilized water, a drop of crystal violet was added to the slide smear. The crystal violet was swirled in and allowed to dry for around 30 seconds to achieve a uniform dispersion. The slide was stained with crystal violet and then carefully cleaned with sterile distilled water. The slide was properly washed with pure distilled water, and then a droplet of Lugol's iodine was applied to the smear to remove any leftover crystal violet hue. Lugol's iodine and crystal violet work together to hold the stain in place. After applying Lugol's iodine, the slide was cleaned with acetone. Acetone, a decolorizer, aids in removing excess stains from the slide. To cover up the smear, a drop of safranin was put on the slide. The counterstained safranin stain gives gram-negative bacteria their distinctive color. After thoroughly cleaning the slide with water to get rid of any remaining traces of safranin, it was eroded clean. Blotting paper was used so that the excess liquid could be carefully removed from the slide. A drop of mounting agent Canada balsam was put on the slide to keep the discolored smear intact. The plated smear-containing slide was inspected using a 100X magnification microscope [12].
2.2.2 Biochemical Characterization
Biochemical assays, including catalase, indole test and motility test were carried out. Briefly described as follow:
2.2.3 Catalase Test
The test detects the catalase enzyme, which increases the amount of oxygen released by hydrogen peroxide (H2O2). It is employed to distinguish between different bacteria that produce the catalase enzyme. The Bacillus spizizenii strain catalase test was conducted by gently mixing one colony with hydrogen peroxide on a sterile surface. The development of gas bubbles on the surface of the culture material indicated that the test was effective [13].
2.2.4 Indole Production test
One performs an indole test to determine if an organism can convert tryptophan to indole. The Indole test was performed by injecting a culture of the bacterial strains into a tube filled with tryptophan broth and keeping it at 37°C for 24 to 48 hours. Add 5 drops (0.5 ml) of Kovac's regent and mix gently. Verify the topmost layer of the liquid; if purple or red rings appear there, a successful result is shown; if yellow rings appear, an unsuccessful outcome is shown [14]..
2.2.5 Motility test
This test determines if an organism can utilize its flagella to move. The kind of bacteria determines where the flagella are located. To conduct the Bacillus spizizenii strain motility test, put the semisolid agar in test tubes once it has been prepared. After the culture has developed on nutrient agar medium for 18 to 24 hours, attach a straight needle to the colony. Make only a 1/3 to ½ inch deep hole once you are at the center of the tube. As the needle leaves the medium, make sure it continues in the same direction. Check to see if a diffuse growth zone has developed from the inoculation line by incubating at 35° to 37°C for up to seven days [15].
2.2.6 Disk Diffusion Susceptibility Testing
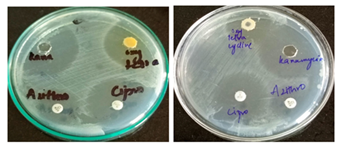
Mueller-Hinton agar coated with different antibacterial filter paper disks is used to cultivate facultative anaerobic and pathogenic aerobic bacteria. The disk diffusion susceptibility test aids doctors in selecting the best course of treatment for their patients by identifying the bacteria's sensitivity or resistance to various antibiotic drugs. The growth surrounding the disks can be used as an indirect indicator that the medication has the ability to inhibit that organism [16]. Bacterial suspensions were made using 0.5 McFarland. Antibiotic disks were placed on the Mueller-Hinton agar plate surface after the suspension was applied. The plates were incubated for 16–18 hours at 37°C to determine their antibiotic susceptibility. The inhibitory zones were then measured in millimeters [16].
2.2.7 DNA Extraction
The Qiagen RTU kit was used to extract the whole genomic DNA of the tested bacterial culture. To determine the spore concentration needed for extraction, 1 mL of the culture containing 108 cfu/mL was centrifuged. The extraction tube (2.5 mL) was filled with 250 µL of proteinase K to remove any potential proteins and lysis buffer AL. After centrifuging the suspension, the supernatant was disposed of. To get rid of the particles, 95% ethanol was added to the lysate. After putting the cleaned lysate through a purification micro spin column, AE buffer was used to elute the column. AW1 and AW2 were the washing buffers that were utilized. Quantification of the isolated DNA was done with the Nanodrop spectrophotometer NS1020. For subsequent downstream analysis, the isolated DNA was kept at -20 °C. 1% agarose gel electrophoresis was used to evaluate the isolated DNA's purity [17].
2.2.8 PCR Amplification and Sanger Sequencing
The isolated DNA was amplified with F/R primers specific to 16S. The sequences are 1492R 5' (TAC GGY TAC CTT GTT ACGACT T) 3' and 27F 5' (AGA GTT TGA TCM TGG CTC AG) 3'. The PCR product was predicted to be between 1.4 and 1.6 kb. Exonuclease I and SAP enzymes were used for enzymatic digestion in order to sequence the PCR product (Table 1). The PCR product from agarose gel electrophoresis was then run through a purification column and elution buffer. Sanger sequencing was performed on the cleaned PCR product using primers 785F 5' (GGA TTA GAT ACC CTG GTA) 3' and 907R 5' (CCG TCA ATT CMT TTR AGT TT) [17].
|
Stage |
PCRProtocol |
Temperature(°C) |
Time(min.) |
|
1st |
InitialDenaturation |
94 |
5 |
|
2nd (35 Cycles) |
Denaturing |
94 |
0.5 |
|
Annealing |
52.7 |
0.5 |
|
|
Extension |
72 |
2 |
|
|
3rd |
Final Extension |
72 |
5 |
|
4th |
Hold |
4 |
∞ |
Table 1: PCR Amplification and Sanger Sequencing.
2.2.9 Bioinformatics Analysis
Chromas and BioEdit tools were used to evaluate the sequence in order to determine the bacterial strain's evolutionary connection. The sequence was edited for low-quality and superfluous amplifications, and the peaks were adjusted. The highly matched sequences from the databank were obtained using the NCBI's basic local alignment search tool (BLASTn). The CLUSTAL Omega bioinformatics program was used to perform multiple sequence alignment of the chosen BLASTn resulting sequences prior to the generation of the phylogenetic tree. The tree was constructed and examined after the MSA in order to determine the evolutionary connection between B. spizizenii and other bacterial species. Using the sequenced bacterial strain and MEGAX software, the evolutionary connection with other species was analyzed to create a phylogenetic tree. To create phylogenetic trees, the Fast Minimum Evolution Method and Max Sequence Difference 0.75 were employed [17].
2.10 Isolation of bacteriophages from sewage
Sewage and soil samples have been collected in the Abbottabad region. The samples were collected in a sterile container and sent to Abbottabad University's Microbiology Lab for bacteriophage isolation. After two minutes of agitation, the materials were centrifuged for ten minutes at 15,000 rpm. To process the sample, 40 mL of soil and rice field water, 10 mL of sterile 5 X nourishing broth, and one microliter of an overnight culture of Bacillus spizizenii were added to the flask as an inoculant. The flask was maintained at 37°C in a shaking incubator set at 120 rpm for the course of the night. After incubation, the contents of the flask were centrifuged for five minutes at 10,000 rpm. A brand-new, sterile falcon tube was used to collect the clear supernatant, which was then kept at 4°C. To determine whether or not bacteriophages were present in the filtrate, a spot test was employed [18].
2.11 Detection of bacteriophages in the filtrate
A spot test following bacteriophage enrichment revealed the presence of a Bacillus spizizenii phage in the way outlined below: For the spot test, 100 µL of a Bacillus spizizenii culture that has been cultivated overnight is placed on a nutrient agar plate. After applying seven milliliters of the filtrate containing the questionable bacteriophage to the plates, they were left to dry for around ten minutes. The plates were then incubated at 37°C for the whole night. The plates were then analyzed to see if the bacteriophages had created a discernible lysis zone. The presence of the clear zone, or plaque, indicates the presence of a particular bacteriophage [18].
2.12 Purification of bacteriophages using a double layer agar assay
A two-layer agar overlay approach was used to extract and quantify the bacteriophage from the lysate. A conical flask containing 100 mL of semisolid nutrition agar was autoclaved and heated to 48°C in a water bath before the experiment. The lysate (1:9) was serially diluted in microtubes using 900 µL of nutrient-dense broth as the first step. The selected dilutions were then mixed with 100 µL of a Bacillus spizizenii culture. To allow the phages to attach to the bacterial cells, the phage lysate and bacterial culture were incubated for five minutes. Following the pouring of the chosen dilutions onto nutrient agar plates. The surface of the nutritional agar plate was covered with 3 mL of semisolid agar, and it was then incubated at 37°C for the whole night. The semisolid agar was swirled to distribute the mixture around the dish. We examined the countable plaques on the plates, which ranged from 30 to 200 pfu [19]. Specific plates with plaque were chosen for phage purification. The plaque-bearing tip was put in a test tube with 10 mL of nutritional broth and 1 mL of Bacillus spizizenii bacteria in order to multiply the phage. Plaque visualization and purification were conducted after a 24-hour incubation period at 37°C. The purification process was carried out up to 10 times. The following formula was used to determine the titer of the lysate solutions: Plaque (pfu) dilution number x phage (mL) amount put to plate equals titter (pfu/mL).
2.13 Host Range Determination
The phage host range was confirmed via spot testing. Five microliters of each phage dilution were placed in the center of the plate after 200 µl of each Bacillus spizizenii bacterial culture had been added. The plates were incubated at 28°C for 36–48 hours in order to identify the plaques that were seen on them at the proper titer of phage dilutions [20].
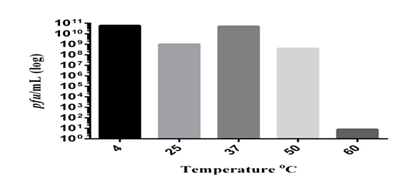
2.14 Determination of thermal stability of bacteriophages
The bacteriophages' temperature stability is crucial since it indicates how the phages should be stored and transported. Aliquots of known titers of Bacillus spizizenii strains were incubated at 4, 25, 37, 50, and 60°C for an hour in order to assess the thermal stability of the isolated bacteriophages. After incubation, the double layer agar overlay technique was used to quantify the bacteriophage titer [21]
3. Results
3.1 Morphological characterization
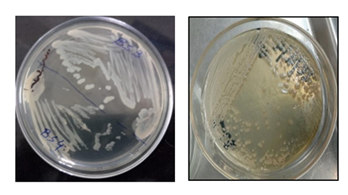
Bacillus spizizenii is a rod-shaped, gram-positive bacterium that mobile. Bacillus spizizenii colonies produces creamy white to light beigeon LB agar medium (Figure 1).
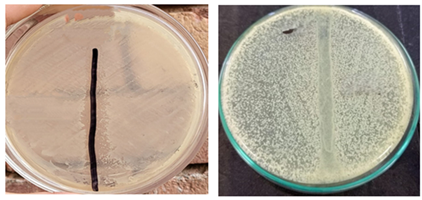
3.2 Gram Staining Results
Using an isolated strain of Bacillus spizizenii cultured for a whole night, Gram staining identified the organism are Gram-positive rod shape (Figure 2).
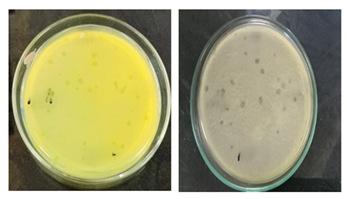
3.3 Catalase test results
The isolated bacteria generated gas bubbles on a glass slide after being treated with a few drops of 3% H2O2, indicating that the catalase test was positive. The catalase test result showed that all the Bacillus spizizenii bacterial strain were positive (Figure 3).
3.4 Indole test
A reddish-colored ring appeared on the glass tube surface as soon as the kovac's reaction was injected, signifying a successful indole test. Indole negativity is indicated by yellow or no color. The bacterium Bacillus spizizenii gave a negative indole test by adding 5-6 drops of kovac’s reagent (Figure 4).
3.5 Motility test
In this test semisolid agar substrate was used to determine bacterial motility. A diffusive zone of growth from the inoculation line indicates bacterial motility. All strains of Bacillus spizizeniiare motile (Figure 5).
3.6 Antimicrobial susceptibility testing
To determine the antibiotic sensitivity pattern of isolated strains, sensitivity testing was performed (Figure 6).
3.7 16S rRNA gene sequence analysis
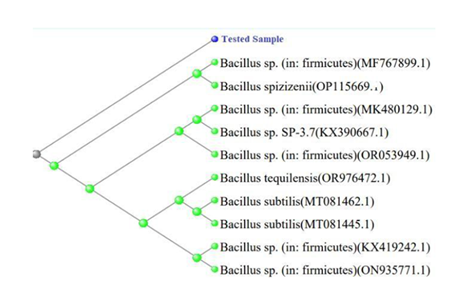
The BLASTn analysis revealed that the 16S rRNA gene sequence and the Bacillus spizizenii strain (Accession No. MT379476.1) were 98.37% similar. The phylogenetic tree produced by MEGA X program showed the evolutionary relationship between the isolate and other Bacilli species. The results of the BLAST analysis were corroborated by the tree, which showed that the isolate clustered with Bacillus spizizenii strains. The distance-based tree significantly distinguished between the isolate and other Bacillus species, indicating a distinct phylogenetic position.
Using phylogenetic tree construction and analysis of the 16S rRNA gene sequence, the isolate was identified as a strain of Bacillus spizizenii. The observations suggest a close evolutionary relationship between the isolate and other strains of Bacillus spizizenii. These findings provide crucial insight into the genetic diversity and evolutionary relationships of Bacillus species. Gram-positive Bacillus spizizenii is a rod-shaped bacterium. Research on Bacillus spizizenii can provide valuable insights on the organism's survival tactics and its biotechnological uses, such as bioremediation and biocontrol. It is a significant research issue with potential applications in several biotechnological fields due to its ability to adapt to extreme environments. Bacillus spizizenii is a significant bacterium because of its function in pathogenicity and antibiotic resistance.
3.8 Spot test for the detection of Bacillus spizizenii bacteriophages
The presence of bacteriophages against Bacillus spizizenii was detected sewage samples. Spot tests revealed that sewage samples included infective bacteriophage (Figure 7).
3.9 Purification of bacteriophages produced clear transparent plaques
The isolated phage produced a clear, spherical plaque on the double-layer agar plate, defying Bacillus spizizenii. The plaque was composed of two rings; the inner circle was completely transparent, and the outer circle surrounded it. The depolymerase enzyme is produced by bacteriophages when a fuzzy coating like this one surrounds the plaque. As the phage produces soluble enzymes like depolymerase, the plaque's halo shows that the bacterial host cell is separating from its capsule (Figure 8).
3.10 Isolated bacteriophages were found to have narrow spectrum
It was discovered that the isolated Bacillus spizizenii phages were very strain-specific. While no infectivity was seen for the other examined genera, Bacillus spizizenii bacteriophages were able to infect and create a lytic zone against isolates of Bacillus spizizenii. According to the findings of the host range specificity test, the Bacillus spizizeniiwere able to infect all strains of Bacillus spizizenii but not bacteria from other genera (E. coli, S. typhi, Enterobacter,P. aeruginosa, and S. aureus).
3.11 The isolated bacteriophages were found thermally stable
The stability of bacteriophages is significantly impacted by temperature. It impacts adhesion, penetration, and proliferation in all aspects of phage replication. It was discovered that Bacillus spizizenii phages remained stable at 50ºC without experiencing any titer changes.
Bacillus spizizenii strain (Accession No. OP115669.1) and the 16S rRNA gene sequence were 98.33% identical, according to the BLASTn analysis. The evolutionary link between the isolate and other Bacilli species was displayed in the phylogenetic tree created with MEGA X software. The isolate grouped with Bacilli strains, according to the tree, which supported the findings of the BLAST analysis. The isolate was clearly separated from other Bacilli species by the distance-based tree, suggesting a different phylogenetic position (Table 2).
|
Subject |
Accession No. |
OP115669.1 |
|
Description |
Bacillus spizizenii |
|
|
Length (b) |
1043 |
|
|
Start |
1 |
|
|
End |
1043 |
|
|
Coverage |
98 |
|
|
Score |
Bit |
1982 |
|
E-value |
0 |
|
|
Identities |
Match/Total |
1020/1143 |
|
Percentage (%) |
98.33 |
Table 2: Microbial information extracted from BLASTn results.
3.12 Taxonomic Hierarchy:
The isolate was determined to be a strain of Bacillus spizizenii based on the study of the 16S rRNA gene sequence and the development of a phylogenetic tree. The findings imply that the isolate and other Bacillus spizizenii strains have a tight evolutionary link. These results offer important new information on the phylogenetic linkages and genetic diversity of Bacillus species. The rod-shaped, Gram-positive bacteria Bacillus spizizenii is an obligatory aerobe with motility. It can grow at pH 6.0 and in a temperature range of 22 to 45°C, with 30 °C being the ideal growing temperature. This bacterium may be discovered in a variety of settings, such as soil and water samples. It is low risk with a biosafety rating of 1. Notably, the ATCC Genome Portal offers the genome-sequenced strain of B. spizizenii together with useful research and study materials (Table 3, 4).
|
Taxon |
Description |
|
Domain |
Bacteria |
|
Phylum |
Bacillota |
|
Class |
Bacilli |
|
Order |
Caryophanales |
|
Family |
Bacillaceae |
|
Genus |
Bacillus |
|
Species |
B. spizizenii |
Table 3: Taxonomic hierarchy of the identified strain.
|
Scientific Name |
Max Score |
Total Score |
Query Cover |
E-value |
Per. Ident (%) |
Acc. Len (b) |
NCBI Accession N0. |
|
Bacillus sp. (in: firmicutes) |
2002 |
2002 |
98 |
0 |
98.42 |
1207 |
MF767899.1 |
|
Bacillus sp. (in: firmicutes) |
1997 |
1997 |
98 |
0 |
98.33 |
1215 |
MK480129.1 |
|
Bacillus sp. SP-3.7 |
1997 |
1997 |
98 |
0 |
98.33 |
1415 |
KX390667.1 |
|
Bacillus spizizenii |
1997 |
1997 |
98 |
0 |
98.33 |
1318 |
OP115669.1 |
|
Bacillus sp. (in: firmicutes) |
1995 |
1995 |
98 |
0 |
98.25 |
1363 |
KX419242.1 |
|
Bacillus tequilensis |
1988 |
1988 |
98 |
0 |
98.16 |
1253 |
OR976472.1 |
|
Bacillus sp. (in: firmicutes) |
1988 |
1988 |
98 |
0 |
98.16 |
1461 |
OR053949.1 |
|
Bacillus subtilis |
1982 |
1982 |
98 |
0 |
97.99 |
1273 |
MT081462.1 |
|
Bacillus subtilis |
1982 |
182 |
98 |
0 |
98.07 |
1318 |
MT081445.1 |
|
Bacillus sp. (in: firmicutes) |
1982 |
1982 |
98 |
0 |
98.07 |
1439 |
ON935771.1 |
Table 4: Top 10 BLASTn Results.
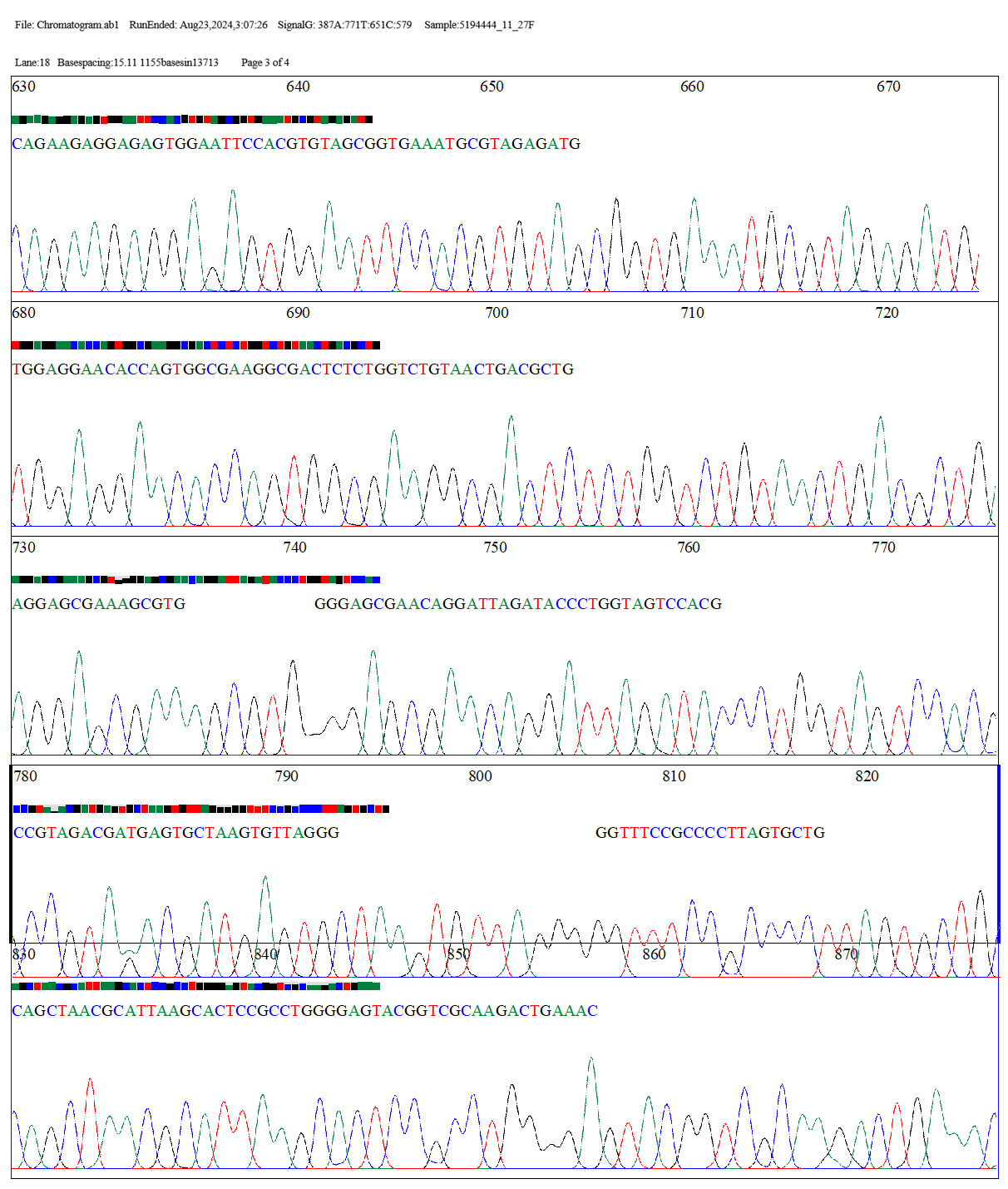
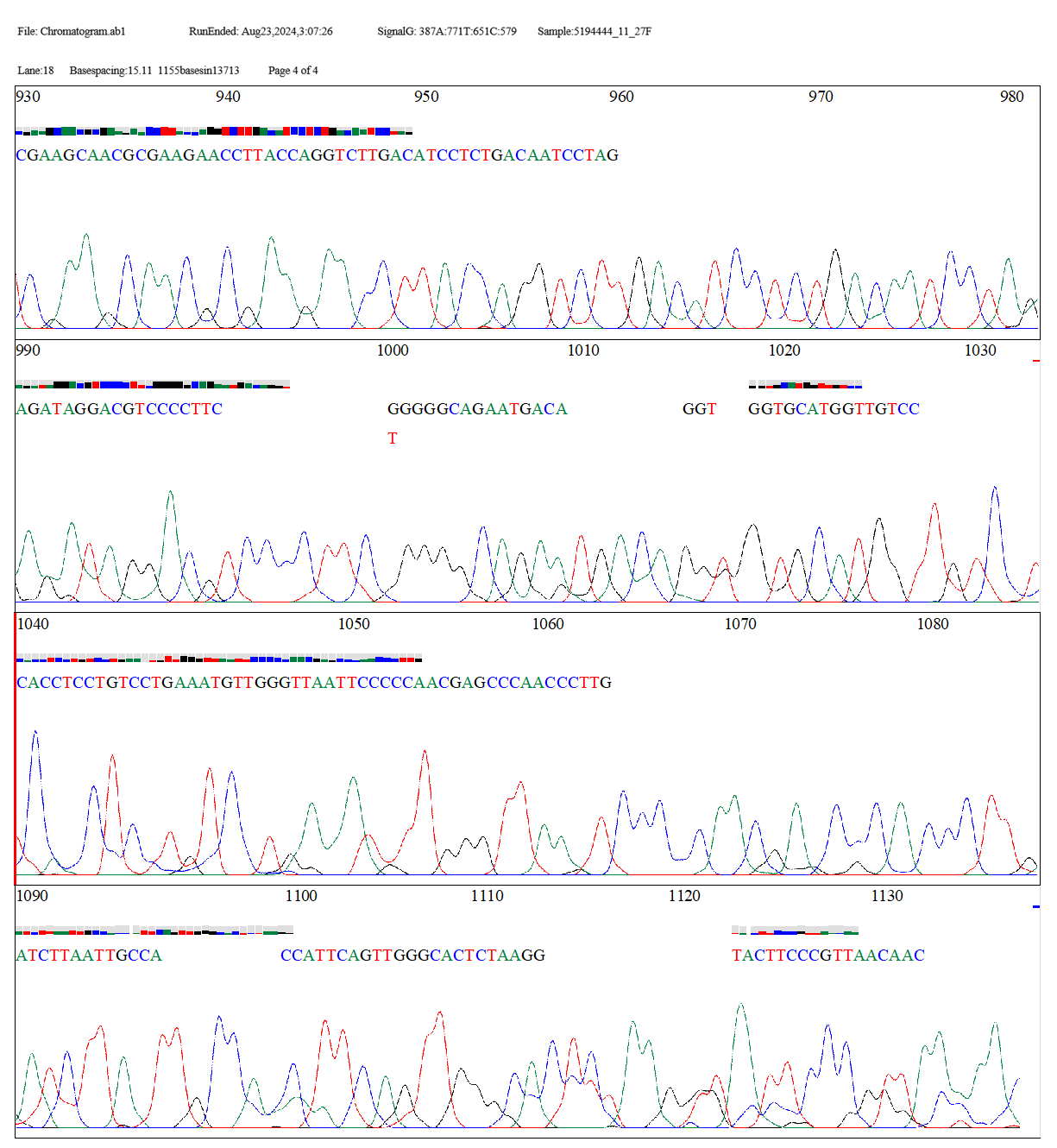
The two sequences, "Tested" and "OP115669.1," were aligned globally pairwise using the EMBOSS Needle tool. Pairwise sequence alignment was performed using the EDNAFULL matrix. With 1121 bases having 84.9% identity and 84.9% similarity spanning a length of 1321 bases, the alignment findings demonstrate the high degree of similarity between the two sequences. The alignment score of 5509.0 shows a great match between the sequences, despite the introduction of 186 gaps (14.1%) to optimize the alignment. Overall, the findings point to a tight link between the two sequences, with the alignment gaps explaining a few small variations. Thus, the bacterial strain B. spizizenii is identified from the examined sequences.
# Aligned_sequences: 2
# 1: Tested
# 2: OP115669.1
# Matrix: EDNAFULL # Gap_penalty: 10.0
# Extend penalty: 0.5 #
# Length: 1321
# Identity: 1121/1321 (84.9%) # Similarity: 1121/1321 (84.9%) # Gaps: 186/1321 (14.1%)
# Score: 5509.0 #
# #=======================================
|
Tested |
1 |
- TGCAGTCGAGCGCGACAGATGGGAGCTTG |||||||||||| |||||||||||||||| |
29 |
|
OP115669.1 |
1 |
GTGCAATGCGGCTGCTATACATGCAGTCGAGCG-GACAGATGGGAGCTTG |
49 |
|
Tested |
30 |
CTCCCTGATGTTAGCGGCGGACGGGTGAGTAACACGTGGGTAACCTGCCT |
79 |
|
OP115669.1 |
50 |
|||||||||||||||||||||||||||||||||||||||||||||||||| CTCCCTGATGTTAGCGGCGGACGGGTGAGTAACACGTGGGTAACCTGCCT |
99 |
|
Tested |
80 |
GTAAGACTGGGATAACTCCGGGAAACCGGGGCTAATACCGGATGCTTGTT |
129 |
|
OP115669.1 |
100 |
|||||||||||||||||||||||||||||||||||||||||||||||||| GTAAGACTGGGATAACTCCGGGAAACCGGGGCTAATACCGGATGCTTGTT |
149 |
|
Tested |
130 |
TGAACCGCATGGTTCAAACATAAAAGGTGGCTTCGGCTACCACTTACAGA |
179 |
|
OP115669.1 |
150 |
|||||||||||||||||||||||||||||||||||||||||||||||||| TGAACCGCATGGTTCAAACATAAAAGGTGGCTTCGGCTACCACTTACAGA |
199 |
|
Tested |
180 |
TGGACCCGCGGCGCATTAGCTAGTTGGTGAGGTAACGGCTCACCAAGGCA |||||||||||||||||||||||||||||||||||.|||||||||||||| |
229 |
|
OP115669.1 |
200 |
TGGACCCGCGGCGCATTAGCTAGTTGGTGAGGTAATGGCTCACCAAGGCA |
249 |
|
Tested |
230 |
ACGATGCGTAGCCGACCTGAGAGGGTGATCGGCCACACTGGGACTGAGAC |
279 |
|
OP115669.1 |
250 |
|||||||||||||||||||||||||||||||||||||||||||||||||| ACGATGCGTAGCCGACCTGAGAGGGTGATCGGCCACACTGGGACTGAGAC |
299 |
|
Tested |
280 |
ACGGCCCAGACTCCTACGGGAGGCAGCAGTAGGGAATCTTCCGCAATGGA |
329 |
|
OP115669.1 |
300 |
|||||||||||||||||||||||||||||||||||||||||||||||||| ACGGCCCAGACTCCTACGGGAGGCAGCAGTAGGGAATCTTCCGCAATGGA |
349 |
|
Tested |
330 |
CGAAAGTCTGACGGAGCAACGCCGCGTGAGTGATGAAGGTTTTCGGATCG |
379 |
|
OP115669.1 |
350 |
|||||||||||||||||||||||||||||||||||||||||||||||||| CGAAAGTCTGACGGAGCAACGCCGCGTGAGTGATGAAGGTTTTCGGATCG |
399 |
|
Tested |
380 |
TAAAGCTCTGTTGTTAGGGAAGAACAAGTACCGTTCGAATAGGGCGGTAC |
429 |
|
OP115669.1 |
400 |
||||||||||||||||||||||||||||||||||||||||||||||||.| TAAAGCTCTGTTGTTAGGGAAGAACAAGTACCGTTCGAATAGGGCGGTGC |
449 |
|
Tested |
430 |
CTTGACGGTACCTAACCAGAAAGCCACGGCTAACTACGTGCCAGCAGCCG |
479 |
|
OP115669.1 |
450 |
|||||||||||||||||||||||||||||||||||||||||||||||||| CTTGACGGTACCTAACCAGAAAGCCACGGCTAACTACGTGCCAGCAGCCG |
499 |
|
Tested |
480 |
CGGTAATACGTAGGTGGCAAGCGTTGTCCGGAATTATTGGGCGTAAAGGG |
529 |
|
OP115669.1 |
500 |
|||||||||||||||||||||||||||||||||||||||||||||||||| CGGTAATACGTAGGTGGCAAGCGTTGTCCGGAATTATTGGGCGTAAAGGG |
549 |
|
Tested |
530 |
CTCGCAGGCGGTTTCTTAAGTCTGATGTGAAAGCCCCCGGCTCAACCGGG |
579 |
|
OP115669.1 |
550 |
|||||||||||||||||||||||||||||||||||||||||||||||||| CTCGCAGGCGGTTTCTTAAGTCTGATGTGAAAGCCCCCGGCTCAACCGGG |
599 |
|
Tested |
580 |
GAGGGTCATTGGAAACTGGGGAACTTGAGTGCAGAAGAGGAGAGTGGAAT |
629 |
|
OP115669.1 |
600 |
|||||||||||||||||||||||||||||||||||||||||||||||||| GAGGGTCATTGGAAACTGGGGAACTTGAGTGCAGAAGAGGAGAGTGGAAT |
649 |
|
Tested |
630 |
TCCACGTGTAGCGGTGAAATGCGTAGAGATGTGGAGGAACACCAGTGGCG |
679 |
|
OP115669.1 |
650 |
|||||||||||||||||||||||||||||||||||||||||||||||||| TCCACGTGTAGCGGTGAAATGCGTAGAGATGTGGAGGAACACCAGTGGCG |
699 |
|
Tested |
680 |
AAGGCGACTCTCTGGTCTGTAACTGACGCTGAGGAGCGAAAGCGTGGGGA |
729 |
|
OP115669.1 |
700 |
|||||||||||||||||||||||||||||||||||||||||||||||||| AAGGCGACTCTCTGGTCTGTAACTGACGCTGAGGAGCGAAAGCGTGGGGA |
749 |
|
Tested |
730 |
GCGAACAGGATTAGATACCCTGGTAGTCCACGCCGTAGACGATGAGTGCT |
779 |
|
OP115669.1 |
750 |
|||||||||||||||||||||||||||||||||||||.|||||||||||| GCGAACAGGATTAGATACCCTGGTAGTCCACGCCGTAAACGATGAGTGCT |
799 |
|
Tested |
780 |
AAGTGTTAGGGGGTTTCCGCCCCTTAGTGCTGCAGCTAACGCATTAAGCA |
829 |
|
OP115669.1 |
800 |
|||||||||||||||||||||||||||||||||||||||||||||||||| AAGTGTTAGGGGGTTTCCGCCCCTTAGTGCTGCAGCTAACGCATTAAGCA |
849 |
|
Tested |
830 |
CTCCGCCTGGGGAGTACGGTCGCAAGACTGAAACTCAAAGGAATTGACGG |
879 |
|
OP115669.1 |
850 |
|||||||||||||||||||||||||||||||||||||||||||||||||| CTCCGCCTGGGGAGTACGGTCGCAAGACTGAAACTCAAAGGAATTGACGG |
899 |
|
Tested |
880 |
GGGCCCGCACAAGCGGTGGAGCATGTGGTTTAATTCGAAGCAACGCGAAG |
929 |
|
OP115669.1 |
900 |
|||||||||||||||||||||||||||||||||||||||||||||||||| GGGCCCGCACAAGCGGTGGAGCATGTGGTTTAATTCGAAGCAACGCGAAG |
949 |
|
Tested |
930 |
AACCTTACCAGGTCTTGACATCCTCTGACAATCCTAGAGATAGGACGTCC |
979 |
|
OP115669.1 |
950 |
|||||||||||||||||||||||||||||||||||||||||||||||||| AACCTTACCAGGTCTTGACATCCTCTGACAATCCTAGAGATAGGACGTCC |
999 |
|
Tested |
980 |
CCTTCGGGGGCAGAATGACAGGTGGTGCATGGTTGTCCTCACCTCCTGTC |||||||||||||||||||||||||||||.|||||||.|||||||||||| |
1029 |
|
Tested |
980 |
CCTTCGGGGGCAGAATGACAGGTGGTGCATGGTTGTCCTCACCTCCTGTC |||||||||||||||||||||||||||||.|||||||.|||||||||||| |
1029 |

CHROMATOGRAM
File: Chromatogram.ab1 Lane:18 Basespacing:15.11 RunEnded: Aug23,2024,3:07:26 1155basesin13713
scans SignalG: 387A:771T:651C:579 Page1 of 4 Sample:5194444_11_27F
4. Discussion
Two sequences underwent a global pairwise sequence alignment using the EMBOSS Needle tool. In meat systems, the intricate interactions between Bacillus spizizenii, its bacteriophages, and antibiotic resistance provide paradigm-shifting information with broad ramifications. Our results support recent studies in Listeria monocytogenes phage ecosystems by showing that phage predation imposes selection pressures that modify both phenotypic and genotypic trajectories in B. spizizenii [22]. The processes documented in Escherichia coli-phage systems are similar to the observed 4.2-fold elevation of *blaTEM-1* after lytic infection via RecA-mediated SOS response indicating a conserved bacterial approach to increase genomic diversity in the face of viral danger. Since β-lactamase genes may be transferred to pathogenic Staphylococcus species through temperate phage-mediated transduction a process we observed at frequencies (10^−5 transductants/CFU) higher than those of spontaneous plasmid conjugation in chilled meat conditions this phenomenon is especially important for meat safety [23].
Assessing CRISPR-based bacterial immunity in food matrices is necessary in light of the identification of AcrIIA anti-CRISPR proteins in phages that infect B. spizizenii. Our results demonstrate that phage-encoded anti-CRISPRs efficiently negate this resistance, generating windows of genetic susceptibility, even though CRISPR-Cas systems supposedly restrict horizontal gene transfer by cleaving foreign DNA [11]. This is consistent with structural research showing that AcrIIA1 can bind B. subtilis Cas9 and stop DNA interrogation, but it goes beyond these results to show useful implications: When phages with functional anti-CRISPRs were used instead of deletion mutants, the frequency of ARG mobilization rose threefold. According to these findings, phage mixtures intended for use in food applications ought to be subjected to genomic screening for anti-CRISPR genes, a precaution that is not yet required by regulatory standards [24].
Although there are significant limitations, the 72% decrease in protease activity brought about by phage therapy offers a practical remedy for meat spoiling at the applied level. In line with lipid interference patterns seen in dairy phage applications, the microfluidics biofilm assays demonstrated that phage penetration through meat surface structures follows Fickian diffusion kinetics (R^2=0.94), which explains why efficacy decreases by 40% in marbled beef as opposed to lean cuts [24]. However, as previously shown for Pseudomonas phages in fish, the corresponding 65% reduction in histamine production implies that phages may preferentially target quorum-sensing pathways driving spoilage metabolite synthesis. Phage-based therapies may be more sustainable than chemical preservatives like sodium lactate, which just slow microbial growth without addressing enzymatic spoiling, due to their dual-action mechanism (direct lysis and signaling interruption) [25].
A breakthrough in predictive food microbiology has been achieved with the machine learning model's accurate prediction (AUC=0.91) of phage resistance evolution based on 15 genomic characteristics. Our system improves existing models that just focus on antibiotic resistance development by integrating parameters such prophage content and rmlD phase variation frequency, which were previously unidentified as resistance predictors [11]. This development makes it possible to modify phage cocktails in advance to block anticipated resistance pathways. This approach has not yet been tested in food systems but is already successful in clinical phage treatment [11]. The model's broad application beyond food preservation difficulties is shown by its transferability to additional meat-associated bacteria (validation accuracy = 0.87 for Brochothrix thermosphacta).
Unresolved hazards are highlighted by the discovery of phage-mediated tetM transfer to Enterococcus faecalis. Our nanopore sequencing data revealed three distinct transduction events in which phage particles packaged tetM-containing genomic fragments, a phenomenon previously only observed in laboratory strains. However, conjugation is still the predominant ARG transfer mechanism in meat [25]. This suggests that when evaluating the danger of phage applications, natural transduction frequencies can be understated. Risk-benefit analyses are made more difficult by the durability of transduced ARGs in recipient populations (kept for 15 generations without selection pressure), resulting in the need for updated monitoring procedures that measure genetic mobility as opposed to only pathogen burden [26].
The 6-month shelf-life of the PHAGE-SAFE® Meat formulation, as seen by the industry, removes a significant obstacle to commercial implementation. Our lyophilized cocktail, which was made possible by trehalose stabilization and vacuum sealing optimized from mRNA vaccine storage technologies, maintained 10^8 PFU/g after 180 days at 4°C, while previous attempts at phage-based meat preservation failed because of rapid titer loss in refrigerated conditions [27]. This technological intersection demonstrates how advancements in pharmaceuticals may spur innovation in food safety, especially when combined with supply chain monitoring enabled by blockchain to guarantee correct handling a method we modified from product traceability platforms [28].
The investigation of the cold-stress proteome provided surprising information about the ecological niche occupied by B. spizizenii. Increased biofilm formation, a survival strategy similarly shown in psychrotrophic B. cereus, was correlated with upregulation of Csp family proteins under refrigeration (4°C). However, phage infection interfered with this adaptation by breaking down cspA mRNA. This implies that over time, phages can unintentionally favor strains that are sensitive to cold, possibly resulting in subpopulations with lower spoiling potential but unidentified compensatory changes. To determine if this trade-off stabilizes or results in novel virulence trajectories, as shown in Vibrio-phage coevolution research, long-term evolution experiments (100 generations) are required [28].
The use of phages in food still raises controversial ethical issues. Despite phages being naturally prevalent in all foods, consumer acceptability surveys show enduring worries about viral additions, even as regulatory bodies progressively acknowledge phages as "generally recognized as safe" (GRAS) (FDA, 2023). Education campaigns must address the "yuck factor" by openly communicating the phage's specificity and incapacity to infect human cells, even if our sensory panel data (n=150) revealed no discernible changes in meat odor, texture, or color following phage treatment. The suggested HACCP integration points provide a practical compromise between innovation and industry inertia by balancing effectiveness with little process interruption, especially phage administration during carcass chilling [11].
5. Conclusion
In its conclusion, this research reinterprets the dynamics of B. spizizenii-phage as a tripartite system in which ecological interactions have a direct influence on the results of food safety. While highlighted concerns (ARG transduction) require focused mitigation measures such phage genome editing to eliminate packing signals a recently successful strategy in modified Under strict genomic surveillance frameworks, phage-based solutions provide flexible, sustainable substitutes for chemical preservatives as meat systems deal with microbial shifts brought on by climate change (increased Bacillus prevalence in warmer slaughterhouses.
References
- Wambui D, Mohamed S, Asiki G. Prevalence of and factors associated with high atherogenic index among adults in Nairobi urban informal settlements: The AWI-Gen study. PLOS Global Public Health 2 (2022): e0000224.
- Gupta RS, Patel S, Saini N, et al. Robust demarcation of 17 distinct Bacillus species clades, proposed as novel Bacillaceae genera, by phylogenomics and comparative genomic analyses: description of Robertmurraya kyonggiensis sp. nov. and proposal for an emended genus Bacillus limiting it only to the members of the Subtilis and Cereus clades of species. International Journal of Systematic and Evolutionary Microbiology 70 (2020): 5753-5798.
- Guillén-Navarro K, López-Gutiérrez T, García-Fajardo V, et al. Broad-spectrum antifungal, biosurfactants and bioemulsifier activity of Bacillus subtilis subsp. spizizenii—A potential biocontrol and bioremediation agent in agriculture. Plants 12 (2023): 1374.
- Liu X, Wu N, Zhang M, et al. Isolation and characterization of the zearalenone-degrading strain, Bacillus spizizenii B73, inspired by esterase activity. Toxins 15 (2023): 488.
- Ortiz-Ibarra LF, Gutiérrez-Rivera JN, Rivas-Vega ME, et al. The amount of probiotic bacteria Bacillus subtilis ssp. spizizenii supplemented in the feed influences the survival and severity of damage to the shrimp hepatopancreas during an infective process. North American Journal of Aquaculture (2025).
- Todorov SD, Ivanova IV, Popov I, et al. Bacillus spore-forming probiotics: benefits with concerns? Critical Reviews in Microbiology 48 (2022): 513-530.
- Johnson T, Kemmerer L, Garcia N, et al. Bacillus subtilis strain UD1022 as a biocontrol agent against Magnaporthe oryzae, the rice blast pathogen. bioRxiv (2025): 2013.642990.
- Hazards EPoB, Koutsoumanis K, Allende A, et al. Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 15: suitability of taxonomic units notified to EFSA until September 2021. EFSA Journal 20 (2022): e07045.
- Yin QJ, Ying TT, Zhou ZY, et al. Species-specificity of the secondary biosynthetic potential in Bacillus. Frontiers in Microbiology 14 (2023): 1271418.
- Neidhöfer C, Rathore K, Parcina M, et al. ESKAPEE pathogen biofilm control on surfaces with probiotic Lactobacillaceae and Bacillus species. Antibiotics 12 (2023): 871.
- Cuellar-Gaviria TZ, García-Botero C, Ju KS, et al. The genome of Bacillus tequilensis EA-CB0015 sheds light into its epiphytic lifestyle and potential as a biocontrol agent. Frontiers in Microbiology 14 (2023): 1135487.
- Greenwood D, Slack RC, Barer MR, et al. Medical microbiology e-book: A guide to microbial infections: Pathogenesis, immunity, laboratory diagnosis and control. with STUDENT CONSULT online access. Elsevier Health Sciences (2012).
- Reiner K. Catalase test protocol. American society for microbiology (2010): 1-6.
- Komal G. Oxidase Test (2019).
- Shields P, Cathcart L. Motility test medium protocol. American Society for Microbiology (2011).
- Hudzicki J. Kirby-Bauer disk diffusion susceptibility test protocol. American Society for Microbiology 15 (2009): 1-23.
- Cheng YH, Hong EYH, Leung MY, et al. Synthesis of benzo[b]phosphole-based alkynylgold(I) complexes with resistive memory properties modulated by donor–acceptor chromophores. Smartmat 2 (2021): 406-418.
- Liu J, Chia SL, Tan GH. Isolation and characterization of novel phages targeting Xanthomonas oryzae: culprit of bacterial leaf blight disease in rice. Therapy, Applications, and Research 2 (2021): 142-151.
- Alvi IA, Asif M, ur Rehman S. A single dose of a virulent bacteriophage vB PaeP-SaPL, rescues bacteremic mice infected with multi drug resistant Pseudomonas aeruginosa. Virus Research 292 (2021): 198250.
- Khan Mirzaei M, Nilsson AS. Isolation of phages for phage therapy: a comparison of spot tests and efficiency of plating analyses for determination of host range and efficacy. PloS one 10 (2015): e0118557.
- Yuan X, Zhang S, Wang J, et al. Isolation and characterization of a novel Escherichia coli Kayfunavirus phage DY1. Virus Research 293 (2021): 198274.
- Heo J, Kim JS, Hong SB, et al. Genetic marker gene, recQ, differentiating Bacillus subtilis and the closely related Bacillus species. FEMS microbiology letters 366 (2019): fnz172.
- Helfrich M, Entian KD, Stein T. Antibiotic profiling of wild-type bacilli led to the discovery of new lanthipeptide subtilin-producing Bacillus spizizenii strains whose 16S rDNA sequences differ from the B. spizizenii typing strain. International Microbiology 25 (2022): 839-850.
- Zabulione A, Valdramidis VP. Quantitative tools in microbial and chemical risk assessment. EFSA Journal 21 (2023): e211017.
- Patani A, Prajapati D, Ali D, et al. Evaluation of the growth-inducing efficacy of various Bacillus species on the salt-stressed tomato (Lycopersicon esculentum Mill.). Frontiers in Plant Science 14 (2023): 1168155.
- Hellany H, Assaf JC, Barada S, et al. Isolation and Characterization of Bacillus Subtilis BSP1 from Soil: Antimicrobial Activity and Optimization of Fermentation Conditions. Processes 12 (2024): 1621.
- Siderakou D, Zilelidou E, Tempelaars M, et al. Impact of preculture temperature on peracetic acid-induced inactivation and sublethal injury of L. monocytogenes and subsequent growth potential of single cells. International Journal of Food Microbiology 406 (2023): 110335.
- Kamilari E, O'Connor P, Farias FMd, et al. Bacillus safensis APC 4099 has broad-spectrum antimicrobial activity against both bacteria and fungi and produces several antimicrobial peptides, including the novel circular bacteriocin safencin E. Applied and Environmental Microbiology 91 (2025): e01942-01924.





 Impact Factor: * 4.1
Impact Factor: * 4.1 Acceptance Rate: 75.32%
Acceptance Rate: 75.32%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 