The Relationship between High Absolute Lymphocyte Counts and Favorable Prognosis in Eribulin Therapy is seen in First-Line Chemotherapy for Metastatic Breast Cancer: Combined Analysis of Two Phase 2 Studies
Kosei Kimura*, 1, Tsutomu Takashima2, Hiroyo Oku1, Ayana Ikari1, Tomo Tominaga1, Saki Takai1, Junna Sakane1, Michiaki Tanaka1, Hidemi Kawajiri3, Shinichiro Kashiwagi2, Shinya Tokunaga4, Shigehiko Nishimura5, Satoru Noda6, Mitsuhiko Iwamoto1
1Department of Breast and Endocrine Surgery, Osaka Medical and Pharmaceutical University, Osaka, Japan
2Department of Breast Surgical Oncology, Osaka Metropolitan University, Graduate School of Medicine, Osaka, Japan
3Department of Breast Surgery, Ishikiri Seiki Hospital, Osaka, Japan
4Department of Medical Oncology, Osaka City General Hospital, Osaka, Japan
5Department of Breast Surgery, Sumitomo Hospital, Osaka, Japan
6Department of Surgery, Ohno Memorial Hospital, Osaka, Japan
*Corresponding author: Kosei Kimura, Department of Breast and Endocrine Surgery, Osaka Medical and Pharmaceutical University, Osaka, Japan.
Received: 22 August 2022; Accepted: 02 September 2022; Published: 14 September 2022
Article Information
Citation: Kosei Kimura, Tsutomu Takashima, Hiroyo Oku, Ayana Ikari, Tomo Tominaga, Saki Takai, Junna Sakane, Michiaki Tanaka, Hidemi Kawajiri, Shinichiro Kashiwagi, Shinya Tokunaga, Shigehiko Nishimura, Satoru Noda, Mitsuhiko Iwamoto. The Relationship between High Absolute Lymphocyte Counts and Favorable Prognosis in Eribulin Therapy is seen in First-Line Chemotherapy for Metastatic Breast Cancer: Combined Analysis of Two Phase 2 Studies. Journal of Pharmacy and Pharmacology Research 6 (2022): 139-146
View / Download Pdf Share at FacebookAbstract
Background: While absolute lymphocyte count (ALC) and neutrophil-to-lymphocyte ratio (NLR) are associated with prolonged progression-free survival (PFS) and overall survival (OS), the influence of previous chemotherapy on blood cell counts may necessitate an evaluation of baseline ALC and NLR in first-line chemotherapy patients.
Methods: Patients with human epidermal growth factor receptor 2 (HER2)-negative metastatic breast cancer (MBC) who received first-line eribulin chemotherapy in two phase 2 trials (BIRICHEN and OMC-BC 03) were retrospectively analyzed. HER2-negative MBC patients who received first-line chemotherapy other than eribulin (treatment of physician’s choice; TPC) at Osaka Medical and Pharmaceutical University Hospital between March 2013 and March 2017 were also analyzed for comparison.
Results: In the eribulin group, the median OS (mOS) was 30.9 and 17.8 months in the high-(H-)ALC (≥1500/μL; n = 33) and low-(L-)ALC (<1500/μL; n = 26) groups, respectively (hazard ratio [HR], 0.52; 95% confidence interval [CI]: 0.27–1.01), whereas it was 30.9 and 15.4 months in the L-NLR (<2.5; n = 23) and H-NLR (≥2.5; n = 36) groups, respectively (HR, 0.49; 95% CI: 0.25–0.95). In the TPC group, neither ALC nor NLR was associated with OS or PFS extension. After propensity-score matching, the mOS in the eribulin group was 32.0 and 19.6 months in the H-ALC and L-ALC groups, respectively (HR, 0.43; 95% CI: 0.18–0.99), while the mOS in the L-NLR and H-NLR groups showed no significant differences in the eribulin group (HR, 0.65; 95% CI: 0.27–1.58).
Conclusions: ALC is a prognostic marker for first-line eribulin chemotherapy, but not for other agents.
Keywords
<p>Metastatic breast cancer, Overall survival, Eribulin, Treatment of physician’s choice, Absolute lymphocyte count</p>
Article Details
1. Introduction
Eribulin was shown to improve the overall survival (OS) of human epidermal growth factor receptor 2 (HER2)-negative metastatic breast cancer (MBC) patients without severe non-hematologic adverse events in the EMBRACE trial [1]. A recent ad hoc analysis of the trial reported that absolute lymphocyte count (ALC), an immune response marker, was a predictive marker of OS after treatment with eribulin [2]. Interestingly, ALC was not a prognostic marker in the treatment of physician’s choice (TPC) group [2]. The neutrophil-to-lymphocyte ratio (NLR), a marker of systemic immunity, is a significant prognostic factor for early-stage breast cancer [2, 3]. An ad hoc analysis of the EMBRACE trial showed an association between NLR and favorable progression-free survival (PFS) and OS in both eribulin and TPC groups [2]. However, the blood cell count must have been influenced by previous chemotherapy because the trial targeted late-line treatment. Thus, the influence of previous chemotherapy on blood cell counts may necessitate an evaluation of baseline ALC and NLR in patients receiving first-line chemotherapy.
We previously conducted two phase 2 trials that estimated the efficacy of eribulin as first-line chemotherapy for HER2-negative MBC in Japan, and both trials demonstrated remarkable efficacy [4, 5]. Here, we examined baseline ALC and NLR in first-line eribulin-treated patients who participated in these trials and those in first-line TPC patients treated at the same time to test the hypothesis that ALC is a prognostic marker for first-line eribulin therapy, but not for TPC.
2. Patients and Methods
2.1 Patients
We evaluated two groups (eribulin and TPC groups) in this study. The eribulin group included 59 patients with HER2-negative MBC, including 35 patients in the BIRICHEN trial (UMIN000006086) who received first-line chemotherapy with eribulin [4] and 24 patients who received first-line chemotherapy in the OMC-BC 03 trial targeting first- and second-line chemotherapy (UMIN000009568) [5]. For the TPC group, we retrospectively and constitutively recruited 48 patients with HER2-negative MBC who received first-line chemotherapy with agents other than eribulin at Osaka Medical and Pharmaceutical University Hospital at the same time as the OMC-BC 03 study (March 1, 2013, to March 1, 2017). Patients who received endocrine therapies before first-line chemotherapy were included, but those who received molecular-targeted therapy (e.g., CDK4/6 inhibitors or mTOR inhibitors) were excluded.
2.2 Treatment
Details of the dosing schedule of eribulin have been published in our previous study [4, 5]. In the TPC group, treatments included FEC (epirubicin 100 mg/m2, 5-fluorouracil [5-FU] 500 mg/m2, and cyclophosphamide 500 mg/m2 every 3 weeks), bevacizumab (Bmab) plus paclitaxel (PTX) (Bmab 10 mg/kg on days 1 and 8 and PTX 80 mg/m2 on days 1, 8, and 15 every 4 weeks), weekly PTX (75 mg/m2 every week), oral 5-FU (capecitabine 900–1,200 mg orally twice daily on days 1–21 every 4 weeks or S-1 40–60 mg orally twice daily on days 1–14 every 3 weeks), and vinorelbine (25 mg/m2 on days 1 and 8 every 3 weeks).
2.3 Assessment
Pretreatment blood cell count data were collected on or just before the first day of chemotherapy administration and compared with survival data. The cutoff value for ALC was set at 1500/mm3 and that for NLR was set at 2.5, based on the median value of each parameter and a previous study [6].
2.4 Statistical analysis
Means, medians, and interquartile ranges (IQRs) were calculated for continuous variables. Counts and percentages were reported for dichotomous and polychotomous variables. In both eribulin and TPC groups, the median values with 95% confidence intervals (CIs) for PFS and OS curves for ALC and NLR were estimated using the Kaplan–Meier method and analyzed using the log-rank test. Hazard ratios (HRs) for high to low ALC or low to high NLR were estimated using Cox proportional hazards models. The median follow-up duration was defined as the median follow-up duration for censored cases.
Comparative analyses were conducted using unadjusted and propensity-score matching (PSM) methods. One-to-one (1:1) PSM between the high- (H-) and low- (L-) ALC or H-NLR and L-NLR groups in each of the eribulin and TPC groups was conducted using the nearest-neighbor matching method to minimize baseline confounders [7-9]. The matched variables were age (≥65 or <65 years), performance status (PS) (0 or ≥1), subtype (estrogen receptor-positive or triple-negative breast cancer), and disease-free interval (DFI) (<2 years, ≥2 years, or de novo). After matching, standardized differences (SDs) were calculated, and values less than 0.1 were considered to indicate adequate variable balance after PSM [10]. All statistical analyses were performed using the JMP version 13 (SAS Institute Inc., Cary, NC, USA).
2.5 Ethics Committees
The ethics committees of Osaka Medical and Pharmaceutical University and Osaka Metropolitan University approved this study. Informed consent was obtained in the form of an opt-out at all institutions or the websites of each institution. Patients who did not provide consent were excluded.
3. Results
3.1 Patients
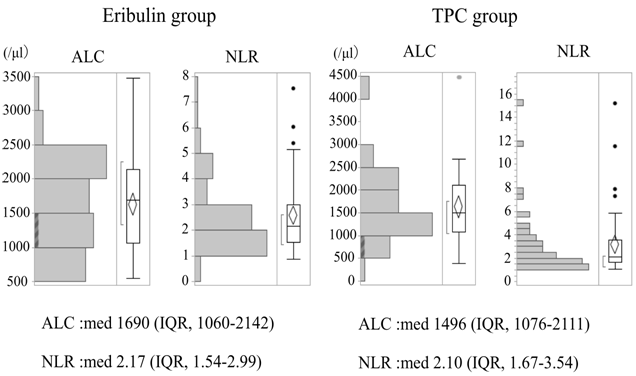
Baseline characteristics of patients in the eribulin and TPC groups are shown in Table 1. In the TPC group, 16 patients (33.3%) received anthracycline (FEC), 12 (25.0%) received Bmab + PTX, three (6.3%) received weekly PTX (6.3%), 11 (22.9%) received S-1, 5 (10.4%) received capecitabine, and only 1 patient (2.1%) received vinorelbine. After TPC failure, 60% of the patients received eribulin in either treatment line. The median (IQR) baseline ALCs in the eribulin and TPC groups were 1690/μL (1060–2142/µL) and 1496/μL (1076–2111/µL), respectively. The median (IQR) baseline NLRs in the eribulin and TPC groups were 2.2 (1.5–3.0) and 2.1 (1.7–3.5), respectively (Figure 1).
ALC absolute lymphocyte count; IQR interquartile range; med, median; NLR neutrophil-to-lymphocyte ratio; TPC treatment of physician’s choice.
Table 1: Demographics and baseline characteristics of the patients
|
Eribulin |
(n = 59) |
TPC |
(n = 48) |
||
|
Age (years) |
Median (IQR) |
65 (38-75) |
64 (38-82) |
||
|
Sex |
Female |
59 |
100% |
48 |
100% |
|
PS |
0 |
44 |
75% |
27 |
56% |
|
≥1 |
15 |
25% |
21 |
44% |
|
|
Subtype |
ER-positive |
44 |
75% |
38 |
79% |
|
TN |
15 |
25% |
10 |
21% |
|
|
DFI |
De novo |
14 |
24% |
27 |
56% |
|
<2 y |
21 |
36% |
2 |
4% |
|
|
≥2y |
24 |
41% |
19 |
40% |
|
|
Regimen |
Eribulin |
59 |
100% |
0 |
0% |
|
Anthracycline |
0 |
0% |
16 |
33% |
|
|
Bmab + PTX |
0 |
0% |
12 |
25% |
|
|
PTX |
0 |
0% |
3 |
6% |
|
|
S-1 |
0 |
0% |
11 |
23% |
|
|
Capecitabine |
0 |
0% |
5 |
10% |
|
|
Vinorelbine |
0 |
0% |
1 |
2% |
|
|
Eribulin on either treatment line |
No |
59 |
100% |
29 |
60% |
|
Yes |
0 |
0% |
19 |
40% |
|
TPC: treatment of physician's choice; PS: performance status,
DFI: disease-free interval; IQR: interquartile range; ER, estorogen receptor,
TN: triple-negative; Bmab: bevacizumab; PTX: paclitaxel
In both the eribulin and TPC groups, PS0 and DFI over 2 years were higher in H-ALC or L-NLR cases (Table 2). The median follow-up duration was 31.7 months (range, 17.0–65.6 months) in the eribulin group and 23.6 months (range, 2.3–90.7 months) in the TPC group.
Table 2: Demographics and baseline characteristics of patients categorized by ALC or NLR
TPC, treatment of physician's choice; ALC, absolute lymphocyte count; NLR, neutrophil-to-lymphocyte ratio; SD, standardized difference,
PS, performance status; DFI, disease-free interval; IQR, interquartile range; ER, estorogen receptor; TN, triple-negative
3.2 Eribulin group
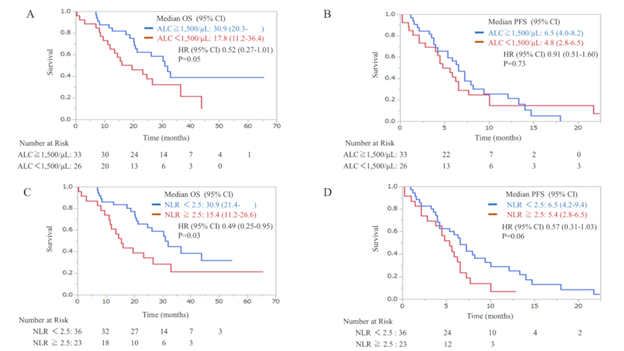
In comparisons based on the ALC, median OS (mOS) was 30.9 months in the H-ALC group (≥1500/μL; n = 33) and 17.8 months in the L-ALC group (<1500/μL; n = 26); the HR was 0.52 (95% CI: 0.27–1.01) but was not statistically significant. Median PFS (mPFS) was 6.5 months in the H-ALC group and 4.8 months in the L-ALC group; HR was 0.91 (95% CI: 0.51–1.60) and was not statistically significant (Figure 2a, b). In comparisons based on NLR, mOS was 30.9 months in the L-NLR group (<2.5; n = 36) and 15.4 months in the H-NLR group (≥2.5; n = 23); HR was 0.49 (95% CI: 0.25–0.95) and was statistically significant. In contrast, there was no statistical significance for PFS (mPFS was 6.5 months in the L-NLR group and 5.4 months in the H-NLR group, HR 0.57 [95% CI: 0.31–1.03]) (Figure 2c, d).
Figure 2: Kaplan–Meier plots of OS in relation to ALC (a), PFS in relation to ALC (b), OS in relation to NLR (c), and PFS in relation to NLR (d) in the eribulin group. ALC, absolute lymphocyte count; CI, confidence interval; HR, hazard ratio; NLR, neutrophil-to-lymphocyte ratio; OS, overall survival; PFS, progression-free survival
3.3 TPC group
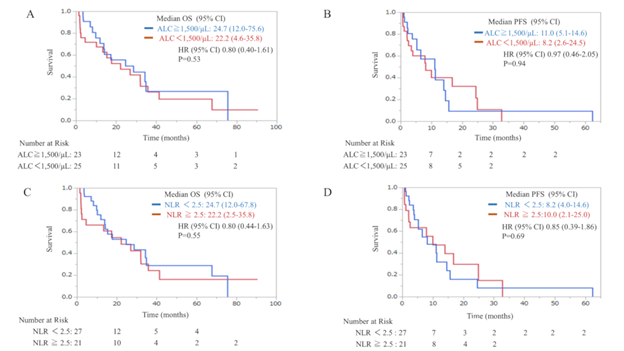
ALC-based comparisons showed no statistically significant differences in OS and PFS (mOS was 24.7 months in the H-ALC group [n = 23] and 22.2 months in the L-ALC group [n = 25], HR 0.80 [95% CI: 0.40–1.61], and mPFS was 11.0 months in the H-ALC group and 11.0 months in the L-ALC group, HR 0.97 [95% CI: 0.46–2.05]). (Figure 3a, b). Similarly, in comparisons based on NLR, mOS was 24.7 months in the L-NLR group (n = 27) versus 22.2 months in the H-NLR group (n = 21); HR was 0.80 (95% CI: 0.44–1.63), and mPFS was 8.2 months in the L-NLR group versus 10.0 months in the H-NLR group; HR was 0.85 (95% CI: 0.39–1.86) and was not statistically significant (Figure 3c, d).
Figure 3: Kaplan–Meier plots of OS in relation to ALC (a), PFS in relation to ALC (b), OS in relation to NLR (c), and PFS in relation to NLR (d) in the TPC group. ALC, absolute lymphocyte count; CI: confidence interval; HR, hazard ratio; NLR, neutrophil-to-lymphocyte ratio; OS, overall survival; PFS, progression-free survival; TPC, treatment of physician’s choice
3.4 Propensity-score matching
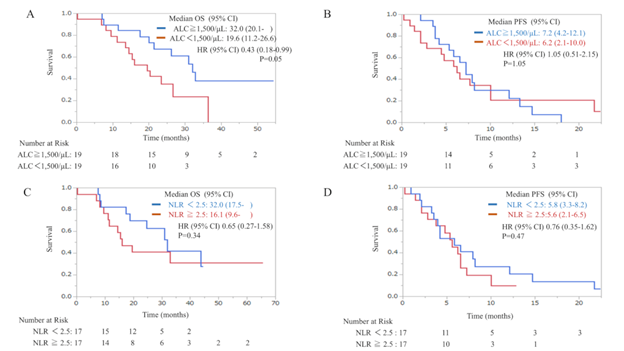
In the eribulin group, PSM was performed between the H-ALC and L-ALC groups. After matching, the H-ALC (n = 19) and L-ALC (n = 19) groups showed statistically significant differences in age, subtype, and DFI (Table 3). OS in the H-ALC and L-ALC groups showed statistical significance (HR, 0.43; 95% CI: 0.18–0.99): mOS was 32.0 months in the H-ALC group and 19.6 months in the L-ALC group. PFS in the H-ALC and L-ALC groups showed no statistical significance (HR, 1.05; 95% CI: 0.51–2.15 months): mPFS was 7.2 months in the H-ALC group and 6.2 months in the L-ALC group (Figure 4a, b). Similarly, after matching for NLR, the L-NLR (n = 17) and H-NLR (n = 17) groups showed statistically significant differences only for subtype and DFI (Table 3). OS in the L-NLR and H-NLR groups showed no statistical significance (HR, 0.65; 95% CI: 0.27–1.58): mOS was 32.0 months in the L-NLR group versus 16.1 months in the H-NLR group. PFS in the L-NLR and H-NLR groups showed no statistical significance (HR, 0.76; 95% CI: 0.35–1.62): mPFS was 5.8 months in the L-NLR group versus 5.6 months in the H-NLR group (Figure 4c, d).
Table 3: Demographics and baseline characteristics of patients categorized by ALC or NLR after PSM
TPC, treatment of physician's choice; ALC, absolute lymphocyte count; NLR, neutrophil-to-lymphocyte ratio; PSM, propensity-score matching;
SD, standardized difference; PS, performance status; DFI, disease-free interval; IQR, interquartile range; ER, estorogen receptor; TN, triple-negative
Figure 4: Kaplan–Meier plots of OS in relation to ALC (a), PFS in relation to ALC (b), OS in relation to NLR (c), and PFS in relation to NLR (d) in the eribulin group after propensity-score matching. ALC, absolute lymphocyte count; CI, confidence interval; HR, hazard ratio; NLR, neutrophil-to-lymphocyte ratio; OS, overall survival;
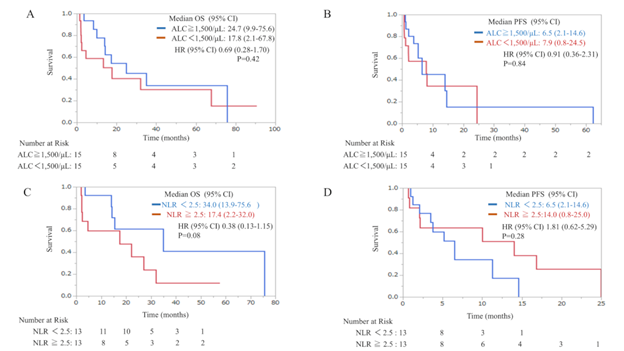
In the TPC group, after matching for ALC, the H-ALC (n = 15) and L-ALC (n = 15) groups showed statistically significant differences only for age and PS (Table 3). OS in the H-ALC and L-ALC groups showed no statistical significance (HR, 0.69; 95% CI: 0.28–1.70): mOS was 24.7 months in the H-ALC group versus 17.8 months in the L-ALC group. PFS in the H-ALC and L-ALC groups showed no statistical significance (HR, 0.91; 95% CI: 0.36–2.31): mPFS was 6.5 months in the H-ALC group versus 7.9 months in the L-ALC group (Figure 5a, b). Similarly, after matching for NLR, the L-NLR (n = 13) and H-NLR (n = 13) groups showed statistically significant differences only for age and DFI (Table 3). OS in the L-NLR and H-NLR groups showed no statistical significance (HR, 0.38; 95% CI: 0.13–1.15): mOS was 34.0 months in the L-NLR group versus 17.4 months in the H-NLR group. PFS in the L-NLR and H-NLR groups showed no statistical significance (HR, 1.81; 95% CI: 0.62–5.29): mPFS was 6.5 months in the L-NLR group versus 14.0 months in the H-NLR group (Figure 5c, d).
Figure 5: Kaplan–Meier plot of OS in relation to ALC (a), PFS in relation to ALC (b), OS in relation to NLR (c), PFS in relation to NLR (d) in the TPC group after propensity-score matching. ALC, absolute lymphocyte count; CI: confidence interval; HR, hazard ratio; NLR, neutrophil-to-lymphocyte ratio; TPC, treatment of physician’s choice.
4. Discussion
This study revealed the relationship between high ALC and favorable prognosis in first-line eribulin chemotherapy, but not with TPC. In the post-hoc analysis of the EMBRACE trial [2], patients assigned to the eribulin group with an ALC ≥ 1500/μL had better OS than those with ALC < 1500/μL, but no difference was observed in PFS. Additionally, the TPC group did not show this relationship [2]. However, since the EMBRACE study evaluated a late-line setting, the findings could have been influenced by a pretreatment myelosuppressive effect. Therefore, Miyoshi et al. mentioned that “baseline ALC and NLR should be further evaluated in patients receiving first-line eribulin treatment [2].” Our study is the first to analyze the predictive value of baseline ALC and NLR in patients with MBC treated with eribulin or TPC as first-line treatment. In the EMBRACE trial, the median (IQR) baseline ALCs for eribulin and TPC were 1308/μL (1000–1814/μL) and 1307/μL (991–1697/μL), respectively. The median (IQR) baseline NLRs for eribulin and TPC were 3.1 (2.1–4.4) and 3.1 (2.1–4.2), respectively [2]. In contrast, in this first-line study population, the median (IQR) baseline ALCs for eribulin and TPC were 1690/μL (1060–2142/μL) and 1496/μL (1076–2111/μL), respectively. Median (IQR) baseline NLRs for eribulin and TPC were 2.2 (1.5–3.0) and 2.1 (1.7–3.5), probably due to less impaired bone marrow function. Interestingly, similar results were observed despite different treatment cohorts or different treatment line settings.
The choice between ALC and NLR as a biomarker for eribulin is still under debate. Watanabe et al. retrospectively evaluated the significance of ALC and NLR in MBC patients who underwent eribulin treatment and reported that ALC was a more useful immune-related marker than NLR for eribulin [6]. However, a post-hoc analysis of the EMBRACE trial [2] reported that NLR was associated with favorable PFS and OS in both the eribulin and TPC groups and may be a general prognostic factor [2]. In this study, NLR was associated with improved OS in the unadjusted cohort, but not with improved OS with first-line eribulin therapy in the PSM cohort. Therefore, we believe that ALC is a more useful marker than NLR in patients treated with eribulin.
Eribulin, a tubulin inhibitor, has been reported to have some different mechanisms of action than other agents, including reversal of epithelial-mesenchymal transition [11, 12], reoxygenation through vascular remodeling [13], and improved tumor immunity [14]. We previously examined the clinical significance of transforming growth factor-β (TGF-β), a local marker of host immunity, and ALC, a systemic marker, and found that patients who benefited from eribulin therapy had higher baseline ALC, and TGF-β levels were significantly decreased before and after eribulin therapy [15]. We conclude that eribulin improves the tumor immune microenvironment by downregulating TGF-β expression, which can be evaluated based on ALC. Therefore, it was speculated that patients with higher ALC in the peripheral blood maintain a favorable immune microenvironment that would benefit from eribulin therapy. However, it should be noted that this study did not compare the efficacy of eribulin to that of other agents, and the results do not indicate that eribulin is more suitable than other agents for patients with high ALC.
This study had several limitations. First, it was retrospective, observational, and not a randomized trial, and it had some biases. Second, we performed PSM to adjust for these biases, but PSM further reduced the sample size because of the small sample size of the unadjusted cohort. Third, in both the eribulin and TPC groups, between-group imbalances in some variables remained after PSM. Therefore, the findings of our study should be interpreted with caution, and any generalizations from these findings should be made cautiously.
In conclusion, ALC is an excellent prognostic marker of eribulin not only in a late-line setting but also in first-line chemotherapy, and high ALC may be a predictive factor of OS even in first-line chemotherapy with eribulin. This association was not observed with other agents and may be associated with the unique effects of eribulin, such as improving the tumor immune microenvironment.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Disclosures and Declarations
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Conflicts of interests
Author Tsutomu Takashima received honoraria from Eisai Co., Ltd., Chugai Pharmaceutical Co. Ltd, Pfizer Japan Inc., Daiichi-Sankyo Co., Ltd., and Kyowa Kirin Co., Ltd. The remaining authors have no relevant financial or non-financial conflicts of interests.
Author contributions
All authors contributed to the conception and design of this study. Material preparation, data collection, and analysis were performed by Kosei Kimura and Tsutomu Takashima. The first draft of the manuscript was edited by Kosei Kimura, and all authors commented on the previous versions of the manuscript. All authors have read and approved the final manuscript.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to Ethical Guidelines for Medical and Health Research Involving Human Subjects but are available from the corresponding author on reasonable request.
Ethics approval
This study was performed in accordance with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Osaka Medical and Pharmaceutical University (2020-188, March 24, 2021) and Osaka Metropolitan University (April 06, 2021).
Consent to participate
Informed consent was obtained from all institutions or websites of each institution in the form of an opt-out. Patients who did not provide consent were excluded.
References
- Cortes J, O’Shaughnessy J, Loesch D, et al. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet 377 (2011): 914–923.
- Miyoshi Y, Yoshimura Y, Saito K, et al. High absolute lymphocyte counts are associated with longer overall survival in patients with metastatic breast cancer treated with eribulin-but not with treatment of physician’s choice-in the EMBRACE study. Breast Cancer 27 (2020): 706–715.
- Miyagawa Y, Araki K, Bun A, et al. Significant association between low baseline neutrophil-to-lymphocyte ratio and improved progression-free survival of patients with locally advanced or metastatic breast cancer treated with eribulin but not with nab-paclitaxel. Clin Breast Cancer 18 (2018): 400–409.
- Takashima T, Tokunaga S, Tei S, et al. A phase II, multicenter, single-arm trial of eribulin as first-line chemotherapy for HER2-negative locally advanced or metastatic breast cancer. SpringerPlus 5 (2016) :164.
- Kimura K, Iwamoto M, Tanaka S, et al. A Phase II, multicenter, single-arm trial of eribulin as first- or second-line chemotherapy for HER2-negative advanced or metastatic breast cancer: evaluation of efficacy, safety, and patient-reported outcomes. Cancer Chemother Pharmacol 81 (2018): 923–933.
- Watanabe J, Saito M, Horimoto Y et al. A maintained absolute lymphocyte count predicts the overall survival benefit from eribulin therapy, including eribulin re-administration, in HER2-negative advanced breast cancer patients: a single-institutional experience. Breast Cancer Res Treat 181 (2020): 211–220.
- Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med 33 (2014): 1242–1258.
- Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 70 (1983): 41–55.
- Gaffiney M, Mardekian J. Propensity scores in the analysis of observational studies (2009): 2–7
- Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput 38 (2009): 1228–1234.
- Yoshida T, Ozawa Y, Kimura T, et al. Eribulin mesilate suppresses experimental metastasis of breast cancer cells by reversing phenotype from epithelial-mesenchymal transition (EMT) to mesenchymal-epithelial transition (MET) states. Br J Cancer 110 (2014): 1497–1505.
- Funahashi Y, Okamoto K, Adachi Y, et al. Eribulin mesylate reduces tumor microenvironment abnormality by vascular remodeling in preclinical human breast cancer models. Cancer Sci 105 (2014): 1334–1342.
- Ueda S, Saeki T, Takeuchi H et al. In vivo imaging of eribulin-induced reoxygenation in advanced breast cancer patients: a comparison to bevacizumab. Br J Cancer 114 (2016): 1212–1218.
- Goto W, Kashiwagi S, Asano Y, et al. Eribulin promotes antitumor immune responses in patients with locally advanced or metastatic breast cancer. Anticancer Res 38 (2018): 2929–2938.
- Kashiwagi S, Asano Y, Goto W, et al. Validation of systemic and local tumour immune response to eribulin chemotherapy in the treatment of breast cancer. Anticancer Res 40 (2020): 3345–3354.


 Impact Factor: * 3.3
Impact Factor: * 3.3 Acceptance Rate: 74.39%
Acceptance Rate: 74.39%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 