Therapeutic Drug Monitoring of Pregabalin in a Critically ill Patient with Acute Kidney Injury undergoing Continuous, Prolonged Intermittent, and Intermittent Kidney Replacement Therapy
Francesca Di Mario*,1,4, Eleonora Galosi2, Paolo Greco1,4, Caterina Maccari1,4, Brenda Menegazzo1,3, Teresa Coccini4, Elisa Roda4, Enrico Fiaccadori1,3
1UO Nefrologia, Azienda Ospedaliero-Universitaria Parma, Dipartimento di Medicina e Chirurgia, Università?′ di Parma, Parma, Italy
2Dipartimento di Neuroscienze umane, Università degli Studi di Roma “La Sapienza”, Roma, Italy
3Scuola di Specializzazione in Nefrologia, Dipartimento di Medicina e Chirurgia, Università di Parma, Parma, Italy
4Laboratory of Clinical and Experimental Toxicology, Pavia Poison Centre - National Toxicology Information Centre, Toxicology Unit, Istituti Clinici Scientifici Maugeri IRCCS, 27100 Pavia, Italy
*Corresponding author: Francesca Di Mario. Nephrology Operational Unit, Parma University Hospital, Parma, Italy, Via Gramsci 14, 43100 Parma, Italy Received: 20 July 2023; Accepted: 01 August 2023; Published: 26 October 2023
Article Information
Citation: Francesca Di Mario, Eleonora Galosi, Paolo Greco, Caterina Maccari, Brenda Menegazzo, Teresa Coccini, Elisa Roda, Enrico Fiaccadori. Therapeutic Drug Monitoring of Pregabalin in a Critically ill Patient with Acute Kidney Injury undergoing Continuous, Prolonged Intermittent, and Intermittent Kidney Replacement Therapy. Journal of Pharmacy and Pharmacology Research. 7 (2023): 220-226.
View / Download Pdf Share at FacebookAbstract
Pregabalin is an anti-epileptic drug which also represents one of the most frequently prescribed medications for neuropathic pain management worldwide. Moreover, in recent years its use has widely increased also in critically ill patients in the setting of multimodal analgesia. Commonly available as capsules and oral solution, it is characterized by a predominant kidney elimination. Consequently, in patients with kidney failure posology adjustments are needed. According to the pharmacokinetic parameters (low molecular weight and volume of distribution, negligible protein binding), pregabalin is expected to undergo a significant extracorporeal clearance, which should be taken into account when one of the different Kidney Replacement Therapy (KRT) modalities is required for Acute Kidney Injury (AKI). The case of a critically ill patient with AKI undergoing Therapeutic Drug Monitoring of Pregabalin in course of Continuous, Prolonged Intermittent KRT (CKRT and PIKRT, respectively), and conventional intermittent hemodialysis (IHD) is presented here for the first time.
Keywords
<p>Acute Kidney Injury (AKI), Therapeutic Drug Monitoring (TDM), CKRT and PIKRT, Intermittent Hemodialysis (IHD), Intensive Care Units (ICUs)</p>
Article Details
1. Background
Pregabalin is an anti-epileptic drug representing one of the most frequently prescribed medications for neuropathic pain management worldwide, whereas its use as an anti-convulsant is currently limited [1]. Despite being an analogue of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA), like gabapentin, pregabalin shows no binding activity to GABA receptors. Instead, it binds with high affinity to the alpha-2-delta subunit of voltage-gated calcium channels, located at neuronal presynaptic endings at different levels in the nervous system, decreasing the depolarization-induced influx of calcium into neurons and ultimately reducing the synaptic release of excitatory neurotransmitters [2]. The reduction of abnormal neuronal excitability within the brain may account for its anticonvulsant and anxiolytic effects, while a decrease in synaptic release of several neuromediators at the spinal cord level, such as glutamate, Calcitonin gene-related peptide (CGRP), and substance P, is likely to be responsible for its analgesic effects [2]. Pregabalin is available in US and Europe as capsules (25 mg, 50 mg, 75 mg, 100 mg, 150 mg, 200 mg, 225 mg, 300 mg) and as an oral solution (20 mg/ml), with recommended doses between 150 mg and 600 mg a day, split into 2 or 3 separate doses. Extended-release tablets for single daily administration are also available (82.5 mg, 165 mg, 330 mg) [2]. It has rapid absorption following oral administration, with peak plasma concentrations occurring between 0.7 and 1.3 hours, and shows 90% bioavailability, independently of dose and administration frequency. Half-life is approximately 6 hours, with steady state achievement within 1 to 2 days [3, 4].
It mainly undergoes renal elimination (98% as unchanged drug), and posology adjustments are needed in patients with kidney failure (Table 1) [5]. Side effects are less severe respect to other anti-epileptic drugs, being the most frequently reported somnolence, dizziness, dry mouth, angioedema, blurred vision, and weight gain [1]. Pregabalin is currently approved in US and Europe for the treatment of neuropathic pain deriving from diabetic neuropathy, post-herpetic neuralgia, and spinal cord injury, besides being recognized as an adjunctive therapy of partial-onset seizures in adults. Therapeutic indications have been expanded to generalized and social anxiety disorders, whereas only in the US it is FDA-approved for the treatment of fibromyalgia syndrome [6]. Off-label pregabalin uses include bipolar disorder, insomnia, restless legs syndrome, and chronic pain conditions other than those above listed, such as cancer pain and post-surgical pain [6]. Patients with Chronic Kidney Disease (CKD) often take pregabalin with heterogeneous therapeutical indications, such as neuropathic pain, pruritus, and restless legs syndrome [7]. Not least, pregabalin is gaining an emerging role for pre-emptive preoperative multimodal analgesia, with different randomized clinical trials showing its efficacy in reducing post-operatory pain and opioid consumption after different types of surgical interventions [8, 9]. Mostly in this context, given the pharmacokinetic profile of pregabalin, the start of Kidney Replacement Therapy (KRT) for Acute Kidney Injury (AKI) usually adds further complexity related to the additional extracorporeal clearance. Despite the limited available data, the implementation of the use of Therapeutic Drug Monitoring (TDM) of pregabalin may represent a useful tool to accurately tailor the pharmacological prescription [10-12]. We report here a case of a critically ill patient undergoing different KRT modalities for severe AKI in whom pregabalin levels in the course of different modalities were monitored by TDM.
Table 1: Physicochemical and pharmacokinetic data of pregabalin with dose adjustments recommendations in patients with impaired kidney function
|
Pharmacokinetic parameters |
References |
|
|
Molecular weight, Da |
159 |
3 |
|
Protein binding, % |
Negligible |
3 |
|
Volume of distribution, L/Kg |
0.5 |
3 |
|
Oral bioavailability, % |
> 90 |
3 |
|
Time to peak concentration, h |
01-Feb |
3 |
|
Half-life, h |
05-Jul |
3 |
|
Therapeutic concentration range, microg/mL |
Not definitely established, reported 2 - 8 |
5, 7 |
|
Kidney function |
References |
|
|
Cr Cl ≥60 mL/min |
150-600 mg/day |
4 |
|
Cr Cl 30-59 mL/min |
75-300 mg/day |
4 |
|
Cr Cl 15-29 mL/min |
25-150 mg/day1 |
4 |
|
Cr Cl <15 mL/min |
25-75 mg/day |
4 |
|
PD |
25-75 mg/day |
4 |
|
IHD |
25-75 mg/day, after dialysis on dialysis days; a supplementary dose after IHD session may be considered (50-100% of the usual daily dose) if the usual dose is administered prior to the IHD session |
3,4 |
|
PIKRT |
50-75 mg/day, until a maximum of 300 mg/day on dialysis days |
Expert opinion* |
|
CKRT |
50-75 mg/day, until a maximum of 300 mg/day |
Expert opinion* |
Abbreviations: Cr Cl, Creatinine Clearance; PD, Peritoneal Dialysis; IHD, Intermittent Hemodialysis; PIKRT, Prolonged Intermittent Kidney Replacement Therapy; CKRT, Continuous Kidney Replacement Therapy.
*Expert opinion reported on www.uptodate.com © 2022 UpToDate, Inc. and/or its affiliates. All Rights Reserved.
2. Case presentation
An adult obese man (age range 65-70 years; usual body weight 110 Kg, BMI 35 kg/m2) was admitted to the Renal Intensive Care Unit (ICU) for oliguric AKI on stage 3b CKD (usual serum creatinine [Scr] concentration, 2 mg/dL, CKD-EPI eGFR 32.6 mL/min/1.73 m2) associated with septic shock due to newly diagnosed infective endocarditis. His drug treatment included pregabalin, 50 mg twice daily, for severe neuropathic pain secondary to diabetic neuropathy. On admission, he complained of severe dyspnea and fatigue. Physical examination revealed a pyretic 118-Kg oliguric patient with severe peripheral edema and diffuse basal pulmonary rales. Blood pressure was 105/70 mmHg while on norepinephrine 16 mcg/min, with pulse rate 100 beats/min. Breaths were 28/min, with peripheral oxygen saturation at 97% while on non-invasive mechanical ventilation (BPAP modality, PSV 12 cmH2O, PEEP 8 cmH2O, FiO2 50%). APACHE II score was 30.
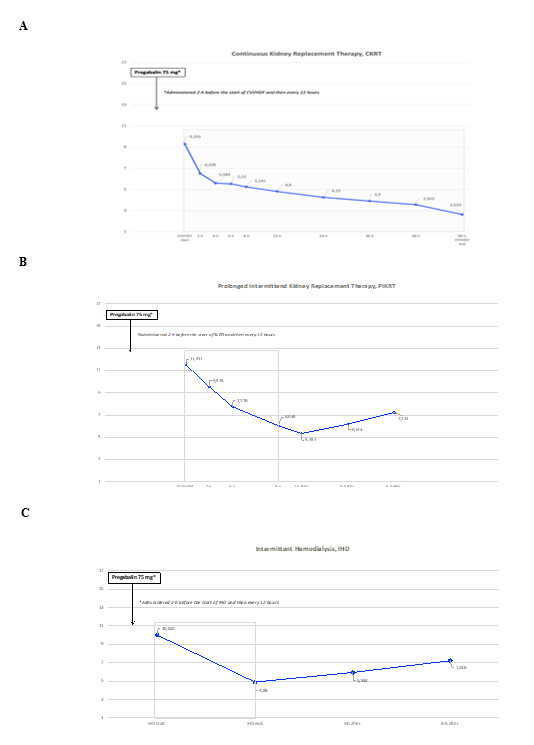
Laboratory findings showed a stage 3 AKI with mild metabolic acidosis. Given the persistent oliguria associated with severe fluid overload unresponsive to the high-dose loop diuretic therapy and hemodynamic instability, KRT was started as 72-hrs Continuous Venovenous Hemodiafiltration (CVVHDF) with regional citrate anticoagulation (RCA), using the Prismax system (Baxter Renal Care, USA) and a polyacrylonitrile AN69 hemofilter (ST 150, 1.5 m2, Baxter Renal Care, USA). A low concentration citrate solution (18 mmol/L; Regiocit, Baxter) was combined with a phosphate-containing solution, used as both dialysis and post-dilution replacement fluid (Biphozyl, Baxter) for a prescribed dialysis dose of 30 ml/Kg/h. After 58 hrs the KRT session was prematurely interrupted because of patient’s hemodynamic intolerance and, in the absence of emergent indications, the dialysis session was not restarted. Three days after, given the slow but progressive improvement of fluid overload, KRT modality was shifted to Sustained Low-Efficiency Dialysis (SLED) by using the same system and the same dialysis solutions, with a prescribed effluent volume of 100 ml/min. On the fourth ICU day, patient’s hemodynamic status definitely improved, and KRT was thereafter continued as every other day conventional Intermittent Hemodialysis (IHD). Given the reported neuropathic pain and aiming at reducing opioids consumption, chronic therapy with pregabalin was confirmed. Moreover, given the expected extracorporeal clearance, the prescribed pregabalin dose was increased at KRT start to 75 mg every 12 hours. Blood samples for pregabalin measurement were collected at the same circuit sampling points on the arterial line, stored at -80°C and subsequently measured. The evaluation of TDM of pregabalin in course of different KRT modalities was then performed by Liquid Chromatography coupled with tandem mass spectrometry (LC-MS/MS system) (Shimadzu Italia, Milano) (Supplemental Material 1) [11]. At the same dose administered (75 mg every 12 hours) a progressive reduction of pregabalin serum levels in course of prolonged KRT modalities (CVVHDF and SLED) was observed, while a rapid decrease was observed during IHD with a negligible post treatment rebound (Figure 1). Fifteen days after the ICU admission, the patient’s clinical condition progressively improved and he was discharged home with a thrice-weekly dialysis schedule. The pregabalin prescription was then restarted at the initial dose of 50 mg twice daily, prescribed after dialysis on dialysis day.
Figure 1 A-C: Time course of serum pregabalin concentration at start, during and after one session of Continuous Kidney Replacement Therapy (CVVHDF modality) [1-A], one 8-hour session of Prolonged Intermittent Kidney Replacement Therapy (SLED-f modality) [1-B] and one conventional Intermittent Hemodialysis (IHD) [1-C]. Abbreviations: CVVHDF, Continuous Venovenous Hemodiafiltration; SLED-f, Sustained Low Efficiency Dialysis filtration.
3. Discussion
According to our knowledge, this is the first study reporting TDM of pregabalin in course of continuous and prolonged intermittent KRT in a critically ill patient. These modalities of KRT, often used as complementary therapies, are considered the preferred dialysis modalities for critically ill patients with AKI, especially in those with hemodynamic instability [13]. As each treatment session is performed over a long-time span, these KRT modalities allow for slower fluid and solute removal, with better hemodynamic tolerance and lower risk of rapid osmolal shift [14]. In addition, due to their extended duration, prolonged KRTs usually provides an efficient daily solute clearance [15]. As detailed in Table 1, pregabalin has small molecular weight, negligible protein binding and low volume of distribution, thus it is characterized by a significant theorical extracorporeal clearance by KRTs. Data regarding the target therapeutic concentration are still limited. Studies on patients with epilepsy with normal kidney function receiving 150-600 mg daily of the drug, reported a serum level ranging from 2 - 8 microg/mL [10-12, 16].
Optimal therapeutic plasma concentrations for neuropathic pain control have not been established yet. However, studies have demonstrated a dose-response relationship for pregabalin in the treatment of painful conditions, like post-herpetic neuralgia. Given the linear absorption of pregabalin, with plasma concentrations increasing proportionately with increasing dose, pregabalin plasma concentration stability likely plays a crucial role in analgesic effect maintenance [17]. Given the increased adoption of pregabalin also in the ICU, not only as a continuation therapy in patients with chronic neuropathic pain, but also in the setting of multimodal analgesia [6-8, 18], a great degree of attention should be required to avoid the risk of inadequate therapeutic concentrations. Indeed, given the prevalent renal metabolism, a dose adjustment is usually recommended in patients with impaired kidney function (Table 1). However, according to the pharmacokinetic profile and in line with the different KRT modalities techniques, our data showed a slow but progressive reduction of pregabalin serum levels in course of the prolonged KRTs (CKRT and SLED, Figure 1 A-B), while a more rapid and significant reduction during conventional IHD was observed (Figure 1 C). Indeed, despite the double daily administration, a slow but progressive decline in serum levels was observed, as reported in a case of gabapentin intoxication treated with CKRT [19] (Figure 1A). Our CKRT session was prematurely interrupted, but given the trend of pregabalin concentration, we can speculate that, when a modality of continuous KRT is applied for incident AKI (e.g. CVVHDF), the usual recommended pregabalin dose may become insufficient to maintain the therapeutic concentration range. While in course of the SLED session serum levels remained within the suggested therapeutic range (Figure 1B), a more severe reduction may be supposed with longer duration (e.g. 12-16 hours) and increased dialysis fluid rate (until 300 ml/min). Finally, as already previously reported in chronic hemodialysis patients [4, 5, 20], TDM data obtained during and after the IHD session confirmed a rapid reduction of serum concentration during the treatment session, along with a subsequent normalization after dialysis, also thanks to the double daily administration (Figure 1C). In conclusion, even though more data are needed to confirm our findings, the TDM of pregabalin may represent a useful therapeutic option in critically ill patients, especially in those requiring continuous or prolonged intermittent KRT. This approach could help to achieve the therapeutic effect while minimizing the risk of side effects.
4. Learning Points
- Pregabalin is one of the most frequently prescribed medications for neuropathic pain management worldwide, but recently its use has widely increased also in critically ill patients in the setting of multimodal analgesia.
- Given the pharmacokinetic profile (low molecular weight and volume of distribution, negligible protein binding), pregabalin is expected to undergo a significant extracorporeal clearance, which should be taken into account when one of the different KRT modalities is required for AKI.
- The Therapeutic Drug Monitoring of pregabalin may represent a useful therapeutic option in high-risk patients, especially in those requiring continuous or prolonged intermittent KRT, to tailor the pharmacological prescription, in order to achieve the therapeutic effect while minimizing the risk of side effects.
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
Financial Disclosure Statement
Funding:
none.
References
- Verma V, Singh N, Singh Jaggi A. Pregabalin in neuropathic pain: evidences and possible mechanisms. Curr Neuropharmacol 12 (2014): 44-56.
- Taylor CP, Angelotti T, Fauman E. Pharmacology and mechanism of action of pregabalin: the calcium channel alpha2-delta (alpha2-delta) subunit as a target for antiepileptic drug discovery. Epilepsy Res 73 (2007): 137-50.
- Bockbrader HN, Radulovic LL, Posvar EL, et al. Clinical pharmacokinetics of pregabalin in healthy volunteers. J Clin Pharmacol 50 (2010): 941-50.
- Bouchard J, Yates C, Calello DP, et al; EXTRIP Workgroup. Extracorporeal Treatment for Gabapentin and Pregabalin Poisoning: Systematic Review and Recommendations from the EXTRIP Workgroup. Am J Kidney Dis 79 (2022): 88-104.
- Randinitis EJ, Posvar EL, Alvey CW, Sedman AJ, Cook JA, Bockbrader HN. Pharmacokinetics of pregabalin in subjects with various degrees of renal function. J Clin Pharmacol 43 (2003): 277-83.
- Goodman CW, Brett AS. A Clinical Overview of Off-label Use of Gabapentinoid Drugs. JAMA Intern Med 179 (2019): 695-701.
- Ishida JH, McCulloch CE, Steinman MA, Grimes BA, Johansen KL. Gabapentin and Pregabalin Use and Association with Adverse Outcomes among Hemodialysis Patients. J Am Soc Nephrol 29 (2018): 1970-1978.
- Zhang Y, Wang Y, Zhang X. Effect of pre-emptive pregabalin on pain management in patients undergoing laparoscopic cholecystectomy: A systematic review and meta-analysis. Int J Surg 44 (2017): 122-127.
- Rai AS, Khan JS, Dhaliwal J, et al. Preoperative pregabalin or gabapentin for acute and chronic postoperative pain among patients undergoing breast cancer surgery: A systematic review and meta-analysis of randomized controlled trials. J Plast Reconstr Aesthet Surg 70 (2017): 1317-1328.
- Patsalos PN, Berry DJ, Bourgeois BF, et al. Antiepileptic drugs--best practice guidelines for therapeutic drug monitoring: a position paper by the subcommission on therapeutic drug monitoring, ILAE Commission on Therapeutic Strategies. Epilepsia 49 (2008): 1239-76.
- Krasowski MD. Therapeutic Drug Monitoring of the Newer Anti-Epilepsy Medications. Pharmaceuticals (Basel) 11 (2010): 1909-1935.
- Hiemke C, Bergemann N, Clement HW, et al. Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology: Update 2017. Pharmacopsychiatry 51 (2018): 9-62.
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group (2012) KDIGO clinical practice guideline for acute kidney injury. Kidney Int 2 (2012): 1-138.
- Griffin BR, Liu KD, Teixeira JP. Critical Care Nephrology: Core Curriculum 2020. Am J Kidney Dis Mar 75 (2020): 435-452.
- Maynar Moliner J, Honore PM, Sánchez-Izquierdo Riera JA, Herrera Gutiérrez ME, Spapen HD. Handling Continuous Renal Replacement Therapy-Related Adverse Effects in Intensive Care Unit Patients: The Dialytrauma Concept. Blood Purif 34 (2012): 177–185.
- Berry D, Millington C. Analysis of pregabalin at therapeutic concentrations in human plasma/serum by reversed-phase HPLC. Ther Drug Monit 27 (2005): 451-6.
- Bockbrader HN, Wesche D, Miller R, Chapel S, Janiczek N, Burger P. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin Pharmacokinet 49 (2010): 661-9.
- Alles SRA, Cain SM, Snutch TP. Pregabalin as a Pain Therapeutic: Beyond Calcium Channels. Front Cell Neurosci 14 (2020): 83.
- Guddati AK, Zafar Z, Cheng JT, Mohan S. Treatment of gabapentin-induced myoclonus with continuous renal replacement therapy. Indian J Nephrol 22 (2012): 59-61.
- Yoo L, Matalon D, Hoffman RS, Goldfarb DS. Treatment of pregabalin toxicity by hemodialysis in a patient with kidney failure. Am J Kidney Dis 54 (2009): 1127-30.
Therapeutic Drug Monitoring (TDM) of Pregabalin
Blood collection
Blood samples were collected in Vacutainer® tubes (Becton Dickinson; Plymouth, United Kingdom) without any additive and gel separator. For serum preparation, venous blood was immediately centrifuged at 3000 g for 15 min at 4 °C. Serum was separated and stored at -80 °C until analysis.
Therapeutic Drug Monitoring (TDM): quantitative determination of Pregabalin
TDM analysis was performed by a Shimadzu 8040 Liquid Chromatograph coupled with tandem mass spectrometry (LC-MS/MS system) (Shimadzu Italia, Milano), with electrospray ionization source, operating in positive mode, using the ClinMass® TDM Platform and the ClinMass® Add-on Set for Antiepileptic Drugs in serum (Recipe Chemicals & Instruments GmbH; Munich, Germany). The analyte and the isotope-labelled substance in the internal standard (IS) were identified and confirmed by their relative retention time (RRT), ion transitions and mass spectrum. In Table S1 are reported main MS/MS parameters. Concerning sample preparation, prior to LC-MS/MS analysis, a short procedure was carried out in order to remove the sample matrix and to spike samples with the IS. Next, samples were injected into the LC-MS/MS system for the chromatographic separation. The Pregabalin concentrations were calculated with the internal standard (IS) method via the peak areas. The respective calibration curve was obtained from the 4-levels calibrators by plotting the ratio of peak area “analyte/IS” against “analyte”. The analyte concentration in serum samples and two-level controls (run in the same analytical session) were calculated from the calibration curve and expressed as micrograms/mL.
Table S1: summarized important test data and reference ranges
|
Analyte /IS |
Quantifier MRM |
Qualifier MRM |
RRT |
||
|
Precursor (m/z) |
Product (m/z) |
Precursor (m/z) |
Product (m/z) |
(min) |
|
|
Pregabalin |
160.1 |
142 |
160.1 |
97 |
0.9 |
|
d4- Pregabalin |
164 |
146 |
0.88 |
||
Table S1. Mass transition and RRT of Pregabalin and isotope-labelled substance in IS measured with ESI positive mode.
|
Analyte |
Linearity (µg/mL) |
LOD (µg/mL) |
LLOQ (µg/mL) |
Laboratory Alert Level§ (µg/mL) |
|
Pregabalin |
0.05-96.5 |
0.017 |
10 |
Table S1. Linearity, detection limit, lower quantitation limit, and reference ranges. § according to Consensus Guidelines for therapeutic drug monitoring in Neuropsychopharmacology: Update 2017 (Hiemke et al., 2018).


 Impact Factor: * 3.3
Impact Factor: * 3.3 Acceptance Rate: 74.39%
Acceptance Rate: 74.39%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 