Wood Vinegar From Eucalyptus As An Additive in Broiler Quail Feed
Géssica Vitalino Diógenes1, Elisanie Neiva Magalhães Teixeira1, Alexandre Santos Pimenta2*, Janete Gouveia Souza1, José Aparecido Moreira1, Andreza Lourenço Marinho1, Aline Veras1, Isidro Argentina Chemane1
1Agricultural Sciences Unit, Graduate Program in Animal Production (PPGPA), Rio Grande do Norte Federal University, Brazil
2Agricultural Sciences Unit Graduate Program in Forest Sciences (PPGCFL), Forest, Bioenergy and Environmental
Research Group, Rio Grande do Norte Federal University, Brazil
*Corresponding Author: Alexandre Santos Pimenta, Agricultural Sciences Unit Graduate Program in Forest Sciences (PPGCFL), Forest, Bioenergy and Environmental Research Group, Rio Grande do Norte Federal University, Brazil
Received: 29 June 2019; Accepted: 16 July 2019; Published: 26 July 2019
Article Information
Citation: Géssica Vitalino Diógenes, Elisanie Neiva Magalhães Teixeira, Alexandre Santos Pimenta, Janete Gouveia Souza, José Aparecido Moreira, Andreza Lourenço Marinho, Aline Veras, Isidro Argentina Chemane. Wood Vinegar From Eucalyptus As An Additive in Broiler Quail Feed. International Journal of Plant, Animal and Environmental Sciences 9 (2019): 164-181.
View / Download Pdf Share at FacebookAbstract
Wood vinegar (WV), a byproduct from wood pyrolysis, is a natural, safe, nontoxic, cheap and versatile product suitable for use as additive on animal diets, as growth promoters and preventive antibiotic. This study aimed to evaluate the effects of WV as additive to quail feed. Increasing levels of eucalyptus WV were added to quail feed and performance parameters and economic viability were assessed. Chemical composition of WV was determined by gas chromatography/mass spectrometry and main compounds were identified. An experiment was conducted with 400 quails (Coturnix coturnix) with age of 1 to 42 days, according to a 5 x 2 factorial design: 5 levels of WV addition and 2 types of bedding (fresh and recycled), with 10 treatments and 4 replicates. Weight gain, feed consumption and feed conversion ratio were determined. Furfural and phenolic compounds were the main components identified in the WV. The statistical analysis showed that for WV addition of 2.5% and quails housed with recycled bedding, weight gain increased in 10.6%, feed conversion ratio decreased in 19.4% and production efficiency factor increased in 23.8%. Regardless of using fresh or recycled bedding, WV addition of 2.5% is recommended during the breeding cycle of quails.
Keywords
<p>Broiler quail; Eucalyptus wood vinegar; Fresh and recycled bedding; GG100 Eucalyptus clone</p>
Article Details
1. Introduction
The use of growth promoters in poultry is considered a landmark in animal husbandry, but this widespread practice is not free from criticism, especially considering the indiscriminate use of antibiotics for this purpose. According to Ragland et al. [1], growth promoters can be classified into four groups: those that increase both growth and feed consumption, those that increase growth without altering feed consumption, those that do not alter growth but decrease feed consumption, and those that increase growth and decrease feed consumption. Performance enhancing additives such as antibiotics have been used in an attempt, by means of competitive exclusion, to keep a satisfactory balance in the gastrointestinal microbiota, reducing mortality and increasing productive efficiency of poultry [2-4].
After antibiotics as growth promoters for poultry were banned by the European Union, there has been increasing interest in research and development of natural or synthetic agents able to successfully substitute these antibiotics [5]. Among natural products, wood vinegar (from now on referred as WV) has good potential to substitute antibiotics as growth promoters. WV or pyroligneous acid is the aqueous fraction recovered from carbonization of wood and other lignocellulosic raw materials by trapping the pyrolysis gases through a condensing apparatus. This product is composed mainly of water and a dissolved organic fraction containing organic acids, furans, pyrans, ketones and phenols [2, 6-8]. A promising research line on WV appliance is its use as a supplement in ruminant and monogastric animal diets. WV is able to improve digestibility and nutrient absorption in poultry and cattle [9, 10] For poultry, better performance of laying hens fed with diets containing variable levels of WV has been reported as well [11, 12]. WV was also successfully used to substitute apramycin in weanling pig feed, as described by Choi et al. [13]. Results reported by Yamuchi et al. [14] showed stimulating effects of a commercial mixture of bamboo charcoal powder and WV as a dietary supplement on chicken performance associated with positive action on intestinal structure. As pointed out by Araújo et al. [7], this sort of strategy line involving WV in animal diets is important because the addition of antibiotics in animal feed is a practice that is facing increasing restrictions.
In Brazil, use WV in agriculture is relatively recent, only starting about two decades ago. On the other hand, in Japan the product has been widely used since the 1930s [15]. According to Miyasaka et al. [2], WV can be added to animal feed in proportions from 0.3 to 1.0%, where it improves nutrient assimilation due to the increment in gastrointestinal microflora. As reported by Butaye et al. [16], experiments indicate that the action of antimicrobial growth promoters is mediated by their antibacterial effect. This way, the good antibacterial activity is one of the required qualities for a growth promoter and this activity is well confirmed for WV. Several authors have described the effective antimicrobial action of WV against both bacteria and fungi [7, 17-20]. However, as highlighted by Ragland et al. [1], many factors contribute to the low cost of poultry meat to consumers, but none has contributed as much financial profit for producers and low cost for consumers as have antibiotic growth promoters. Thus, not only the technical efficiency of potential substitutes for antibiotics has to be evaluated, but also their final cost, economic feasibility and ease of administration. Thus, a growth promoter may be effective from a technical point of view, but if it is too expensive or its use requires significant changes in the farm routine, its application becomes impractical [1, 21].
Unfortunately, in most countries, market for WV is underdeveloped and only small farmers currently use the product as an agriculture input. In Brazil, likewise, although agribusiness is an important and flourishing economic sector, for both domestic consumption and export. Brazil is leading exporter of beef, pork and chicken meat. For example, the Brazilian poultry sector produces about 40% of the total chicken meat consumed in the world, 4,320 million of tons in 2017, exporting products to over 150 countries (Brazilian Association of Animal Protein – ABPA 2018) [22]. In the same way, the Brazilian market for broiler and laying quails is well developed, reaching 15.1 million birds in 2016 [23]. Brazilian poultry sector has potential to absorb large amounts of WV as growth promoter, with the advantage of being a natural, nontoxic, cheap and easy to use product. However, WV application to feed poultry requires further investigation to establish the correct levels of addition and parameterize the effects on meat production and the respective economic impact and viability to replace conventional growth promoters. Since there is no information on literature about the application of WV in quail diets, the present work aimed to assess the effects of adding increasing WV levels in quail feed on performance parameters and economic viability, considering animals housed with two types of bedding.
2. Material and Methods
2.1. Wood vinegar production and refiningWood for the experiment was obtained from a planted forest of Eucalyptus urograndis GG100 clone (hybrid of Eucalyptus urophylla x Eucalyptus grandis), usually referred in Brazil as Eucalyptus urograndis. 1.0 m length wood logs were placed inside a masonry kiln in batches of about 0.65 ton and were carbonized. The kiln was equipped with a device designed to trap and collect the condensable portion of pyrolysis gases, and during all carbonization runs, the condenser was water cooled and maintained at 25°C. Ten carbonization runs were carried out by applying a heating rate of 9.4°C/hour, until final temperature of 450°C. Raw pyrolysis liquids were collected in the temperature range from 180 to 450°C, being that temperatures measured directly in the center of the carbonization bed by using a thermocouple. After the carbonization were concluded, the condensed liquids were stored in a refrigerator at 2°C for further processing. Raw liquids from 10 pyrolysis runs were mixed to form a single composite sample. Then the composite sample was vacuum bi-distilled in a stainless steel equipment to obtain the refined WV, according methodology described by Pimenta et al. [8].
2.2. CG-MS analysis of WVInitially, 1.5 mL of concentrated ammonium hydroxide solution (Caledon, Canada) was added to 5 mL aliquots of WV samples in order to increase the pH to about 5. Then extractions were carried out by adding 3 mL of ethyl acetate (Merck, Brazil). After liquid-liquid extraction was carried out, 1 mL of the organic fraction was transferred to a GC vial and analyzed. The GC-MS analysis were achieved by means of a Shimadzu QP 2010 system. Chromatographic separation was performed in a CP-Wax column (Restek 52 DB with 30 m length, 0.25 mm diameter and 0.25 μm of film thickness). Injector temperature was kept the injector at 250°C. The sample (1 µL) was injected in a split ratio of 1:10, and the oven temperature program was 50°C during 2 minutes with a heating rate of 2°C min−1 applied from 50 to 240°C, holding the final temperature for 2 min. Carrier gas was helium used at a constant flow rate of 1 mL min-1. The main chemical components were detected and identified based on their typical mass spectra by comparison with the NIST library. All the chemical compounds listed herein had mass spectrum similarity above 85%. After WV refining, its properties were
determined, as follows: pH; titratable acidity by using a Methrom Titrando device (0.1 mol.L-1 NaOH titration solution); density (Koehler K86201 automatic density meter) and color.
2.3. Bird managementThe experiment was carried out in the poultry sector under experiment protocol number 016.007/2017, approved by the Animal Use Ethics Committee of the Rio Grande do Norte Federal University. Average temperature and relative humidity at the place of the experiment were 30°C and 80%, respectively. Four hundred non-sexed quails were used for the experiment, each one having an initial weight of about 9 + 3 g. Before the experiment, all the animals were vaccinated against Marek’s disease, following the standard protocol used in São Paulo State (Brazil). The quails were allotted in groups of ten and were housed in concrete floor boxes with dimensions of 1.00 m width x 1.50 m length. All the boxes were provided with heating and a continuous lighting program was adopted (24 hours natural light plus artificial light). The experiment was conducted according an entirely randomized design in a factorial scheme – 5 levels of WV feed addition (0.0, 1.0, 1.5, 2.0 and 2.5% related to total feed mass) and two types of bedding (fresh and recycled) composed of wood shavings, with four replicates. In brief: 5 WV levels x 2 types of bedding x 4 replicates with 10 animals for each replicate, totalizing 400 birds.
A recycled bed was used to provide a sanitary challenge. This material was obtained from a group of chickens reared shortly before the experiment without any previous treatment. This way, recycled bedding contained feces and feather remains. Regarding to feed formulation, for each experimental treatment, the WV was added to a mixture composed of a premix component, micronutrients and a portion of the total corn. After this, the resulting mixture was homogenized with the total feed formulation. Table 1 describes the different experimental diets that were formulated to satisfy the nutritional requirements of quails in each rearing phase, as recommended by Silva and Costa (2009).
¹Recommended by Costa and Silva [24]. ²Levels per kg of product: vitamin A 5,000,000 UI, vitamin B1 500 mg, vitamin B12 3,000 mcg, vitamin B2 1,500 mg, vitamin B6 500 mg, vitamin D3 1,100,000 UI, vitamin E 4,900 UI, vi tamin K3 1,000 mg, biotin 10 mg, coline 43 g, niacin 10 g, folic acid 100 mg, pantothenic acid 4,600 mg, cobalt 50 mg, copper 2,849
mg, iron 25 g, iodine 500 mg, manganese 23 g, selenium 100 mg, zinc 23 g, methionine 250 g, Bacillus subtilis 75 x10e9 UFC, Zinc bacitracin 12.5 g.
Table 1: Composition of feed without and with WV addition (1-21 and 22-42 days of age)¹.
At the end of the experimental period, at 42 days of age, 80 animals were slaughtered (two per box), corresponding to the average weight per box (270 + 20 g). Before slaughter, the quails were submitted to 24 hours of fasting. They were slaughtered by cervical dislocation, bled, deplumed, eviscerated and had their carcasses evaluated. The following
performance parameters were evaluated: carcass and cut yields, weight gain, and feed consumption and feed conversion. The occurrence of mortality was corrected in the data as recommended by Sakomura and Rostagno [25].
2.4. Statistical analysisStatistical analysis of experimental data was performed by using the GLM routine of the SAS software (Statistical Analysis System – https://www.sas.com/en_us/software/stat.html), by decomposing the sum of square treatments into orthogonal contrasts. The data group was analyzed in independent form and regression models were obtained by using the REG routine.
3. Results and Discussion
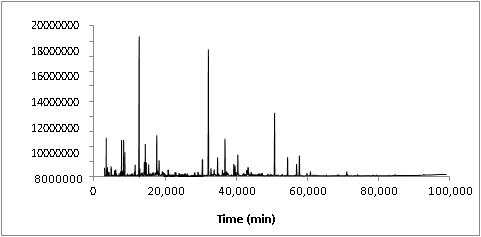
3.1. CG-MS analysis of WVRegarding to the sample extraction procedure, it was observed that the addition of ammonium hydroxide to WV sample is a decisive step because it was able to decrease the concentration of acetic acid in the organic phase. It also was able to increase the ionic strength of the solution, giving less solubility of organic compounds in the aqueous phase and then improving the efficiency of the liquid-liquid extraction step, which allowed the quantification of the chemical compounds even at low levels. Figure 1 shows the total ion chromatogram (TIC) of the WV from Eucalyptus urograndis.
In the chemical composition of WV, 70 main compounds could be identified, which corresponded to 96.26% of the total area percent in the TIC, as presented in Table 2. For better understanding, the chemical components identified in the WV were divided into the following groups: alcohols, aldehydes and ketones, furans and pyrans, phenolic compounds and organic acids. For instance, phenolic compounds corresponded to 42.36% of the extractable organic fraction while furfural alone corresponded to 15.67%. The properties of WV applied in the present work are shown in Table 3. It is important to highlight that the chemical composition depicted in Table 2 is related to the extractable organic phase of WV, which corresponded to 8.98% of the initial mass of WV (Table 3). A significant number of the compounds here identified in WV composition by GC/MS are the same cited in the literature as being components of liquid smoke used in the food industry [26-29].
Table 2: Main compounds identified in the WV from Eucalyptus urograndis GG100 clone.
Table 3: Properties of WV from Eucalyptus GG100 clone.
The main group identified in the WV was the phenolic compounds, the same major components reported by other researchers [27, 29]. This chemical group is also reported as majority in WV from different sources [2, 6, 9]. The high contents of furfural, guaiacol, phenol and cresols present in WVs seems to explain their biological and antibacterial/antifungal activities and, likewise, their effects as growth promoters in animal diets as pointed out by Myasaka et al. [2], Kook and Kim [9] and Kook et al. [10]. Besides that, the preservative properties of WV when applied as input in the food industry are closely connected to the presence of phenols in WV composition as reported by Montazeri et al. [27], Budaraga et al. [28] and Cadwallader [29]. Other component to be emphasized is furfural, which is found in virtually all types of spice as a flavor ingredient and can act as fungicide highlighted by Abdel-Kahr et al. [30] and a report from Environmental Protection Agency [31]. However, as observed by Yang et al. [20], antibacterial and antifungal activities of WV from different origins cannot be attributed simply to a unique chemical component, but instead to a synergistic combination of several ones, particularly the phenolic compounds.
Among the phenolic compounds present in WV chemical composition, the following ones stand out for their remarkable biological effects and properties. Guaiacol, for instance, is used medicinally as expectorant, antiseptic, and even as local anesthetic, according O’Neil [32]. Also, guaiacol and its derivatives have antioxidant properties and they are present in commercial liquid smokes used in food industries [27]. In its turn, 4-methyl-2-methoxy-phenol, also referred as creosol is a flavoring agent present in many foods and beverages and in liquid smoke. Phenol itself has therapeutic importance as a fungicide, antiseptic and disinfectant [32], with activity against a broad range of microorganisms including even some viruses. Phenol is also one of the components of commercial liquid smoke products as reported by Achmadi et al. [26] Montazeri et al. [27], Budaraga et al. [28] and Cadwallader [29]. The cresols, ortho, meta and para-cresol, are used as local
antiseptics, parasiticides, disinfectants and as intestinal antiseptics [32], and are also present as components in liquid smoke products [27-29]. Other compounds present in WV, such as 4-ethyl-2-methoxy-phenol, maltol, 2,6-dimethoxy-phenol (syringol) and its derivatives and also the xylenols, are used as flavoring agents but can present preservative properties [27- 29]. As brought out above, phenolic compounds along with furfural are the components of WV that more likely explain the action of WV as a natural antibiotic.
3.2. Bird ManagementAn interaction effect between the WV addition levels and the type of bedding was observed (at 5% significance by the F- test). According the interaction splitting, the quails housed with fresh bedding and fed with 1.0, 2.0 and 2.5% of WV showed higher feed consumption, whereas for the quails housed with recycled bedding the highest consumption was observed for the 0.0% treatment. Among all the experimental treatments evaluated, the highest feed consumption was observed at 1.0% WV addition, as shown in Table 4.
CV – coefficient of variation (%); WV – wood vinegar; NS: statistically not significant by F-test; *Linear effect
Table 4: Performance of quails with increasing WV levels and type of bedding from 1 to 42 days of age.
There was no significant effect (at 5% significance by the F-test) for final weight, feed consumption, weight gain and feed conversion ratio when increasing levels of WV were applied to birds housed with fresh bedding. These negative results found here are in accordance with the results reported by Pessoa et al. [33]. They assessed the effect of a 1.0% WV level on the performance of Japanese quails reared in cages with no sanitation challenge and also found no effect on feed consumption. According to Paz et al. [21], when fresh bedding is used combined with feed additives, the lack of effect on
performance parameters can be explained by the experimental environment, good management conditions, nutritional quality of the feed provided, and mainly by the non-exposure of the birds to a sanitation challenge.
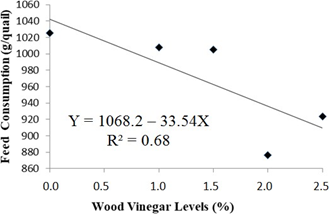
Feed consumption of quails housed with recycled bedding showed a decreasing linear effect until the level of 2%, according the regression analysis, as depicted in Figure 2. During a period of immunological stress of birds, it is not surprising for a drop in feed consumption to occur, which makes it advisable to manipulate the nutritional plans, depending on the stage of rearing [34]. Bioactive components of diets can interact with the immune response, since they have potential to lessen the susceptibility of birds to infectious diseases [35]. Therefore, in this experiment the chemical components present in the WV may have stimulated the absorption of nutrients, reducing their consumption.
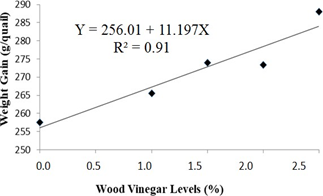
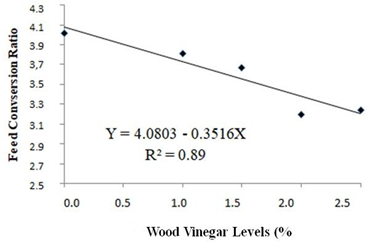
According to the regression analysis of experimental data, weight gain and feed conversion of the quails housed with recycled bedding presented increasing (at 5% significance by the F-test) and decreasing linear effect, as shown, respectively in Figures 3 and 4. This means that weight gain and feed conversion improved as the level of WV increased. In the maximum level of WV addition of 2.5%, for quails housed with recycled bedding, weight gain increased in 10.6% and feed conversion ratio decreased in 19.4%. Probably this positive effect is associated with the antiseptic effect of Eucalyptus WV, since it has been shown to have antibacterial and antifungal activities [7]. Those activities are probably associated with phenols in WV’s chemical composition, such as cresols and guaiacol. Despite having antiseptic chemicals in its composition, WV has a chemical composition quite similar to liquid smokes currently used in the food industry, as pointed out by Pimenta et al. [8], and no harmful effects are associated with liquid smoke ingestion [27, 29]. However, not only the antiseptic effect has to be taken into consideration regarding to benefits of WV, but also some synergism among its chemical compounds, since Teixeira et al. [36], using fennel oil, a well-known antibacterial and antifungal agent [37], observed worse performance of broilers housed with recycled bedding comparing to animals bred with fresh bedding.
Even when sanitation challenge is considered, the performance parameters of quails housed with recycled bedding were better compared to those housed with fresh bedding. Likewise, the positive effect of WV on animals submitted to adverse conditions was shown by Zhu [38], who demonstrated a clear detoxification action of WV regarding the presence of aflatoxin B1 in feed of broiler chickens. The author did not observe significant difference between the performance parameters for the negative control group of broilers (with negative presence of aflatoxin B1), but observed a positive effect of WV on the positive control group (with aflatoxin B1), for which the increasing levels of WV improved weight gain and feed conversion ratio.
Table 5 shows the results of yields for hot carcass, chest, thigh plus drumstick and wing. There was no interaction between the WV levels and the type of bedding for yields of carcass and cuts. The carcass yield of quails housed with fresh bedding was higher than that observed for birds housed with recycled bedding. This result may have to been influenced by the low pH of WV, which possibly improved the assimilation of proteins, as demonstrated by the best yield. These results corroborate the experimental data published by Teixeira et al. [36], who observed that type of bedding did not influence the thigh + drumstick yield. As reported by Freitas et al. [39], if there is adequate ingestion of nutrients and the protein/energy and energy/amino acid ratios are not modified, rarely will changes occur in the carcass characteristics. Possibly this happened in the present study for the yields observed of birds housed with fresh bedding. As pointed out by Fascina et al. [40], the inclusion of growth promoters in feed, with no sanitation challenge, improves metabolizability of nutrients because the intestinal microflora becomes more homogeneous, directing nutrients to growth and muscular deposition.
CV: coefficient of variation (%); WV: wood vinegar; NS: statistically not significant by F-test; **Quadratic effect
Table 5: Carcass and cut yields of quails with increasing levels of wood vinegar and type of bedding from 1 to 42 days of age.
Considering carcass yield for quails housed with fresh bedding, a quadratic effect was observed (at 5% significance by the F-test), where the best level was 2.5% WV. When compared to birds housed with fresh bedding, carcass yields were lower, possibly a consequence of the weight of the internal organs and viscera, since the yield is calculated from the eviscerated carcasses. Regarding the cuts, similar results were found and no significant effect was observed. As a reference comparison, in an experiment with Japanese quails conducted by Genchev et al. [41], the authors observed carcass yield of 65%, thigh yield in the range 22 – 23.4% and thigh + drumstick yield in the range 20.5 – 20.9%, all of which are lower
values than those found in our experiment. Without the challenge related to the type of bedding, Otutumi et al. [42] did not find significant difference in carcass and cut yields by assessing the effect of probiotics in feeds with different crude protein levels on performance, carcass yield and nutritional requirement of crude protein for quails. Similarly, Fukayama et al. [43] did not find significant effect of oregano extract on chicken cut yields.
The weights of organs and viscera are shown in Table 6. A significant effect (at 5% significance by the F-test) of the interaction between WV levels and type of bedding was observed only for the gizzard weight, where the quails housed with recycled bedding and fed with basal feed (0.0% WV) had higher gizzard weight. Possibly higher mechanical stress to digest the feed may have occurred in such condition, resulting in the increased weight of the organ. Quails housed with recycled bedding had gizzard weight 3.84% higher than the birds housed with fresh bedding. Another noteworthy point is that no toxicity effect was observed among quails given feed with rising levels from 1.0 to 2.5%, since there was no statistical difference in liver weight among the experimental treatments.
CV: coefficient of variation (%), WV: wood vinegar, NS: statistically not significant by F test.
Table 6: Weight of organs and viscera of quails with increasing levels of wood vinegar and type of bedding from 1 to 42 days of age.
As demonstrated here, employment of WV as additive in quail feed is feasible from technical standpoint, but attention must be paid regarding use of only a refined product. Refinement of WV eliminates free of tar and polycyclic aromatic hydrocarbons (PAHs), which are potentially carcinogenic [8, 44]. Additionally, WV use can assure healthier condition when using of recycled bedding, a widespread practice in the poultry industry.
4. Conclusions
The wood vinegar from eucalyptus was able to improve the weight gain (10.6%), feed conversion (19.4%) and the productive efficiency factor (23.8%) of European quails raised in recycled litter. For quail reared with fresh bedding, WV improved carcass yield. Therefore, from a technical standpoint, it is recommended to include up to 2.5% of WV in European quail ration from 1 to 42 days of age regardless of the type of bedding used in breeding.
References
- Ragland WL, Janjecié Z, Franciosini MP, et al. Antibiotic growth promoters in poultry, and their potential alternatives. Zagreb Biotec D.O.O. Technical Report, Zagreb, Croatia (2015).
- Miyasaka S, Ohkawara T, Utsumi B. Ácido Pirolenhoso: uso e fabricação. Boletim Agro Ecológico 14 (1999).
- Castanon JIR. History of the use of antibiotic as growth promoters in European poultry. Feeds Poultry Science 86 (2007): 2466-2471.
- Cervantes HM. The future of antibiotic growth promoters in poultry production. Area: Nutrition and feed technologies. In: Annals of XX1V World’s Poultry Congress, Salvador, Brazil 5-9 (2012): 1-16.
- Lemos MJ, Calixto LFL, Torres-Cordido KAA, et al. Uso de aditivo alimentar equilibrador da flora intestinal em aves de corte e de postura. Arquivo do Instituto Biológico 83 (2016): 1-7.
- Encarnação F. Redução do impacto ambiental na produção de carvão vegetal e obtenção do ácido pirolenhoso como alternativa para proteção de plantas. Agroecologia e Desenvolvimento Rural Sustentável 2 (2001): 8-12.
- Araújo ES, Pimenta AS, Feijó FMC, et al. Antibacterial and antifungal activities of pyroligneous acid from wood of Eucalyptus urograndis and Mimosa tenuiflora. Journal of Applied Microbiology 124 (2017): 85-96.
- Pimenta AS, Fasciotti M, Monteiro TVC, Lima KMG. Chemical composition of pyroligneous acid obtained from Eucalyptus GG100 clone. Molecules 23 (2018): 426-432.
- Kook K, Kim KH. Effect of supplemental bamboo vinegar on production and meat quality of meat type ducks. Korean Journal of Animal Science and Technology 29 (2002):293-300.
- Kook K, Kim JE, Jung KH, et al. The effects of supplemental levels of bamboo vinegar on growth performance, serum profile and meat quality in fattening Hanwoo cow. Korean Journal of Animal Science and Technology 45 (2003): 57-60.
- Sakaida T, Enya K, Tanaka T. Effect of wood vinegar compound on egg production and egg quality of white leghorn hens. Japan Poultry Science 24 (1987): 44-49.
- Li HL, Ryu KS. Effect of feeding various vinegar on performance and egg quality of laying hens. Korean Journal of Animal Science Technology 43 (2001): 655-662.
- Choi JY, Shinde PL, Kwon IK, et al. Effect of wood vinegar on the performance, nutrient digestibility and intestinal microflora in weanling pigs. Asian-Australian Journal Animal Science 22 (2009): 267-274.
- Yamauchi K, Ruttanavut J, Takenoyama S. Effects of dietary bamboo charcoal powder including vinegar liquid on chicken performance and histological alterations of intestine. Journal of Animal and Feed Sciences 19 (2010): 257-268.
- Campos AD. Técnicas para produção de extrato pirolenhoso para uso agrícola. EMBRAPA (Empresa Brasileira de Agropecuária) Technical Report Pelotas-RS, Brazil (2007).
- Butaye P, Devriese LA, Haesebrouck F. Antimicrobial growth promoters used in animal feed: effect of less well known antibiotics on Gram-positive bacteria. Clinical Microbiology Reviews 16 (2003):175-188.
- Silva RM, Melo RM, Mazorche RM, Barros ROM, Queiroz VT, Póvoa HCC. Estudo da atividade antimicrobiana do licor pirolenhoso sobre bactérias dos gêneros E. coli e Klebsiella. Revista Científica da FAMINAS 3 (2007): 46.
- Rakmai J. Chemical determination, antimicrobial and antioxidant activities of Thai wood vinegars. Msc. Thesis, Prince of Songkhala University, Songkhala, Thailand (2009).
- Chan EWC, Chin HF, Kor XK, et al. Potent antibacterial activity of wood vinegar from Matang Mangroves, Malaysia. ISME GLOMIS Electron Journal 10 (2012): 10-12.
- Yang JF, Yang CH, Liang MT, et al.. Chemical composition, antioxidant and antibacterial activity of wood vinegar from Litchi chinensis. Molecules 21 (2016):
- Paz AS, Abreu RD, Costa MCMM. Aditivos promotores de crescimento na alimentação de frangos de corte. Revista Brasileira de Saúde e Produção Animal 11 (2010): 395-402.
- Brazilian Association of Animal Protein – ABPA. Annual Report, São Paulo, Brazil (2017).
- Avinews Avicultura.info. Plantel brasileiro de aves cresceu 1.9% apesar dos custos de produção. Avinews, Brazil. Available at https://avicultura.info/pt-br/plantel-brasileiro-de-aves-2016 (Access on February 20, 2019). (2017).
- Silva JHV, Costa FGP. Tabela para codornas japonesas e europeias. 2nd. Edition, Funep, Jaboticabal, Brazil (2009):
- Sakomura NK, Rostagno HS. Métodos de pesquisa em nutrição de monogástricos. Funep, São Paulo, Brazil (2016): 262 .
- Achmadi SS, Mubarik NR, Nursyamsi R, et al. Characterization of redistilled liquid smoke of oil-palm shells and its application as fish preservatives. Journal of Applied Sciences 13 (2013): 401-408.
- Montazeri N, Oliveira ACM, Himelbloom BH, et al. Chemical characterization of commercial liquid smoke products. Food Science and Nutrition 1 (2013): 102-115.
- Budaraga IK, Arnim YM, Bulanin U. Analysis of liquid smoke chemical components with GC MS from different raw materials variation production and pyrolysis temperature level. International Journal of ChemTech Research 9 (2016): 694-708.
- Cadwallader KR. Wood smoke flavor. In: Nollet LM, Boylston T, Chen F, Coggins PC, Gloria MB. (eds), Handbook of meat, poultry & seafood quality, Blackwell Publishing Ltd., New Jersey, USA (2007): 201-209.
- Abdel-Kahr MM, Hamman MMA, El-Mougy NS, et al. Pesticide alternatives for controlling root and root knot of cucumber under plastic house conditions. International Journal of Innovations Research Science, Engineering and Technology 4 (2015): 25-31.
- EPA - U.S. Environmental Protection Agency - Office of Pesticide Programs. (2018) OPP pesticide ecotoxicity database. EPA, USA, URL: http://www.ipmcenters.org/Ecotox.
- O’Neil MJ. The Merck index: An encyclopedia of chemicals, drugs, and biologicals. Royal Society of Chemistry, Cambridge, UK (2013).
- Pessoa RCL, Diógenes GV, Veras AG, et al. Uso de extrato pirolenhoso como melhorador de desempenho de codornas japonesas. In Anais ZOOTEC 2017, XXVII Congresso Brasileiro de Zootecnia, Santos, Brazil (2017): 22-24.
- Cardoso ALSP, Tessari ENC. Interaction between immunity and nutrition poultry: literature review. Revista Científica de Medicina Veterinária 24 (2015).
- Kogut MH, Klasing K. An immunologist’s perspective on nutrition, immunity, and infection diseases: introduction and overview. Journal of Poultry Research 18 (2009):103-110.
- Teixeira ENM, Silva JHV, Costa FGP, Silva CT, Goulart CC, Melo TS. Óleo essencial de erva-doce na ração de frangos de corte alojados em cama nova e reciclada. Arquivo Brasileiro de Medicina Veterinária e Zootecnia 65 (2013).
- Diao WR, Hu QP, Zhang H, et al. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.). Food Control 35 (2014):109-116.
- Zhu YZ. Detoxification effects of wood vinegar on Aflatoxin B1 in broiler chickens. Journal of Animal and Veterinary Advances 12 (2013): 1256-1259.
- Freitas ER, Sakomura NK, Ezequiel JMB, Neme R, Mendonça MO. Energia metabolizável de alimentos na formulação de ração para frangos de corte. Pesquisa Agropecuária Brasileira 41 (2006):107-115.
- Fascina VB, Sartori JR, Gonzales E, Carvalho FB, Souza IMGP, Polycarpo GV, Stradiotti AC, Pelícia VC. Phytogenic additives and organic acids in broiler chicken diets. Revista Brasileira de Zootecnia 41 (2012): 2189- 2197.
- Genchev A, Mihaylova G, Ribarski S, Pavlov A, Kabakchiev M. Meat quality and composition in Japanese quails. Trakia Journal of Sciences 6 (2008): 72-82.
- Otutumi LK, Furlan AC, Martins EN, et al. Efeito do probiótico sobre o desempenho, rendimento de carcaça e exigências nutricionais de proteína bruta de codornas de corte. Revista Brasileira de Zootecnia 38 (2009): 299- 306.
- Fukayma EH, Beterchini AG, Geraldo A, Kata RK, Murgas LDS. Extrato de orégano como aditivo em rações para frangos de corte. Revista Brasileira de Zootecnia 34 (2005): 2316-2326.
- Higashino TA, Shibata A, Yatagai Basic study for establishing specifications for wood vinegar by distillation
- Study of compounds contained in distilled wood vinegar. Journal of the Japan Wood Research Society 51 (2005):180-188.


 Impact Factor: * 4.1
Impact Factor: * 4.1 Acceptance Rate: 75.32%
Acceptance Rate: 75.32%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 