A Breakthrough in Cardiology: Machine Learning's Power in Unmasking Long QT Syndrome
Syed Owais Akhtar1, Hemanth Kesani Venkata2, Khunsha Mujaddadi1, Anosha Aslam Kamal3, Taranpreet Singh4, Natasha Anum5, Saif Khalid6, Fazeela Ansari7, Salih Zada8*, Adees Wirtan Sarkees Bedros9
1MBBS Student, Jinnah Sindh Medical University, Karachi, Pakistan
2Narayana Medical College and Hospital, Nellore, Andhra Pradesh, India
3Department of Cardiology, Dow University of Health Sciences, Karachi, Pakistan
4MBBS, Mahatma Gandhi Medical College, Navi Mumbai, India
5Liaquat University of Medical and Health Sciences, Pakistan
6Department of Medicine, Royal College of Surgeons Dublin, Ireland
7MBBS, Dubai Medical College, Dubai
8ICS & IT University of Agriculture, Peshawar, Pakistan
9School of Medicine, The University of Jordan, Amman, Jordan
*Corresponding author: Salih Zada, ICS & IT University of Agriculture, Peshawar, Pakistan.
Received: 01 October 2024; Accepted: 10 October 2024; Published: 28 October 2024
Article Information
Citation: Syed Owais Akhtar, Hemanth Kesani Venkata, Khunsha Mujaddadi, Anosha Aslam Kamal, Taranpreet Singh, Natasha Anum, Saif Khalid, Fazeela Ansari, Salih Zada, Adees Wirtan Sarkees Bedros. A Breakthrough in Cardiology: Machine Learning's Power in Unmasking Long QT Syndrome. Cardiology and Cardiovascular Medicine 8 (2024): 455-465.
View / Download Pdf Share at FacebookKeywords
<p>Cardiology; Machine Learning; Long QT syndrome; ECG; Artificial intelligence</p>
Article Details
1. Introduction:
Long QT syndrome (LQTS) is a hereditary primary arrhythmia syndrome. The surface electrocardiogram (ECG) shows this cardiac ion channel repolarization abnormality as an extension of the corrected QT interval, which is caused by a delayed repolarization of the cardiomyocyte action potential [1]. Clinical manifestations include anoxic seizures, palpitations, and syncope due to ventricular arrhythmia, commonly known as torsade de pointes [2].
- • Long QT syndrome (LQTS) is a collection of inheritable conditions associated with cardiac repolarization dysfunction. Our knowledge of LQTS has grown significantly since it was first described in 1957. According to estimates, the prevalence of LQTS is 1:2,000, with a minor female predominance [3]. Long QT Syndrome (LQTS) presents several diagnostic challenges due to its often hidden or subtle nature. This complexity contributes to the condition frequently being undiagnosed or misdiagnosed.
- • The primary technique for diagnosing Long QT Syndrome (LQTS) is electrocardiography; however, because the condition presents differently in different patients, it may not give a clear picture for every patient. Physiological assessments can be improved by family history and genetic testing, which can help to clarify the diagnosis and direct further treatment [4]. Key factors contributing to the concealed nature of LQTS include:
- 1) A sizable fraction of people with LQTS may not show any symptoms at all or only occasionally display non-specific symptoms.
- 2) LQTS symptoms, such as syncope or vertigo, can happen infrequently or in response to triggers (such as physical activity or emotional stress). The sporadic character of the symptoms makes diagnosis more difficult.
- 3) Palpitations and dizziness, two common clinical signs of LQTS, are frequently linked to several other illnesses. A false diagnosis may result from this overlap in symptomatology.
- 4) There are several genetic subtypes included in LQTS. The diagnosis process may be made more difficult by the possibility that some people have genetic predispositions that do not show up as obvious clinical symptoms.
- 5) In addition, medications, electrolyte imbalances, heart rate, and other physiological factors can all affect the variability of the QT interval. This variability may obscure QT prolongation in standard ECG recordings.
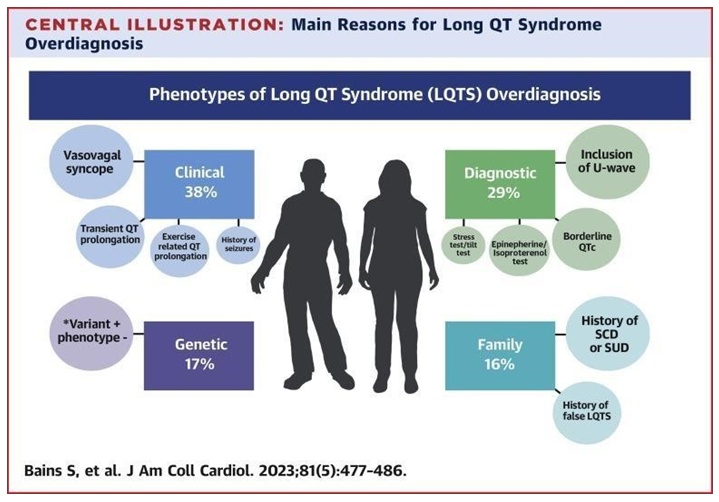
Figure 2: Knowing the 5 main determinants of discordance between a previously rendered diagnosis of LQTS and full diagnostic reversal or removal (vasovagal syncope, “pseudo”-positive genetic test result in LQTS-causative genes, family history of SCD, transient QT prolongation, and misinterpretation of the QTc interval) increases awareness and provides critical guidance to reduce this burden of overdiagnosed LQTS. The current diagnosis of Long QT Syndrome (LQTS) entails a thorough review that includes clinical presentation, family history, ECG interpretation, and, in some cases, genetic testing. Interestingly, roughly 50% of individuals with LQTS-related mutations may remain asymptomatic, and around 25 percent of patients may have a normal QTc interval on their ECG [5,6].
In conclusion, clinical symptoms and ECG results might lead to diagnostic mistakes, delaying effective management or therapy. Therefore,
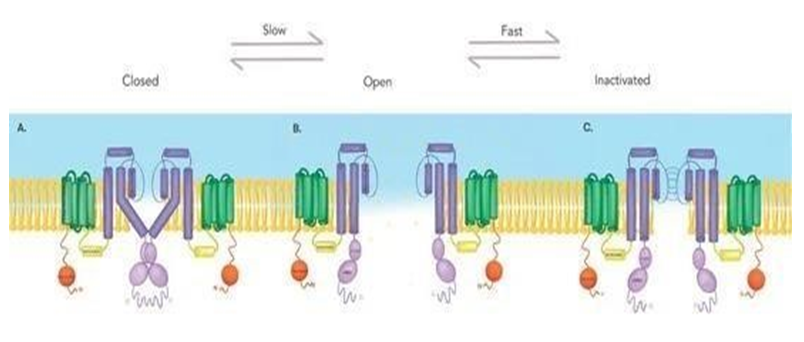
An important characteristic defining Kv11.1 is its rapid inactivation and recovery from inactivation, as opposed to the slow process of deactivation (closure) [7]. Figure adapted from Lahrouchi et al. [8]. (A) Kv11.1 in the closed state, the four S6 domains crisscross near the cytoplasmic domain to narrow the opening and avoid ion flow. Contains a voltage sensor domain (green) encompassed in the first four transmembrane helices (S1– S4), a pore (dark purple) that serves as the conducting ionic potassium pathway. Each alpha subunit of this tetrameric channel (only two subunits are shown) contains an amino terminus (orange) and a carboxyl terminus (light purple), C-linker and cNBD (cyclic nucleotide-binding homology domain). Figure adapted from: Lahrouchi et al. [8]. (B) Kv11.1 in the open state, the four S6 domains spread outwards to allow the passage of potassium ions. Figure adapted from: Lahrouchi et al. [8]. (C) Kv11.1 in the inactive stat
As a result, there is an increasing demand for more objective and efficient diagnostic tools to aid in the detection and management of Long QT Syndrome (LQTS).
The potential advancement in addressing the limitations of traditional methods is represented by the application of Artificial Intelligence (AI) in Long QT Syndrome (LQTS). The field has witnessed a surge in interest in utilizing artificial intelligence (AI) and machine learning (ML) in recent times. Compared to traditional computer algorithms, AI algorithms have demonstrated efficacy in differentiating between different ECG patterns and diagnoses, which may offer clinicians a preliminary interpretation that is more accurate [9], and can effectively process large volumes of ECG data, extract meaningful patterns and features, and make predictions based on the learned patterns. AI techniques, particularly deep learning convolutional neural networks, have facilitated rapid, human-like interpretation of ECGs.
The ability of these multilayer AI networks to identify patterns and signals that are frequently undetectable to human interpreters increases the usefulness of the ECG as a potent, non-invasive biomarker [9].
Through machine learning (ML), AI systems can continuously learn from fresh data and modify their algorithms to perform better over time, increasing the accuracy of diagnoses [11].
Artificial Intelligence (AI) in Long QT Syndrome (LQTS) diagnosis and treatment has the potential to revolutionize patient care by offering more precise, effective, and customized methods.
The ability of AI, particularly neural networks, to overcome certain shortcomings of conventional diagnostic techniques makes it clinically significant for the interpretation of ECGs. First, real-time assessments and the prompt identification of individuals at high risk for sudden cardiac death are made possible by AI algorithms' rapid analysis of ECG data. This skill is especially helpful in emergency scenarios where prompt decision-making is crucial. Second, AI-based systems can deliver consistent and standardized ECG interpretations, minimizing interobserver variability and enhancing diagnostic accuracy.
Aside from lowering the chance of misdiagnosis, the application of AI has other advantages, including the rapid and precise detection of issues such as arrhythmias and other hidden cardiac disorders. It has the potential to help physicians with interpretation, diagnosis, risk assessment, and illness treatment, ultimately contributing to the prevention of catastrophic arrhythmic events [13].
The Challenge of Diagnosing Long QT Syndrome (LQTS): A Race Against Time
Long QT Syndrome (LQTS) is a heart condition that affects the electrical activity of the heart, increasing the risk of dangerous arrhythmias and sudden cardiac arrest [11]. Early and accurate diagnosis is critical, yet identifying LQTS remains difficult due to limitations in current diagnostic methods. Conventional approaches, like electrocardiograms (ECGs) and genetic testing, face several obstacles, especially in borderline or asymptomatic cases. This underscores the pressing need for advancements in diagnostic tools.
Figure 6: The QT interval occupies a pivotal role in drug development as a surface biomarker of ventricular repolarization. The electrophysiologic substrate for QT prolongation coupled with reports of noncardiac drugs producing lethal arrhythmias captured worldwide attention from government regulators eventuating in a series of guidance documents that require virtually all new chemical compounds to undergo rigorous preclinical and clinical testing to profile their QT liability [14].
2. Limitations of Current Diagnostic Methods
Electrocardiograms (ECGs): The Challenges
The electrocardiogram (ECG) is the most used method for diagnosing LQTS. It records the heart's electrical activity, particularly the QT interval, which measures the time the heart's ventricles take to recharge between beats [13]. A prolonged QT interval is a key indicator of LQTS, making the ECG essential to the diagnostic process. However, the ECG has notable limitations.
One major challenge with ECGs is the variability in measuring the QT interval. Several factors, especially heart rate, can affect the QT interval [15].
The AI-ECG interpretation provides a more specific and accurate interpretation of the ECG, while the computer-generated interpretation does not identify the first-degree AV block. While the AI-ECG algorithm can identify the AV conduction defect, it does not report a PR interval duration like traditional computer-generated algorithms.
As the heart rate increases, the QT interval shortens, and as the heart rate decreases, it lengthens. To address this, the QT interval is often corrected for heart rate (QTc), but these corrections are not always precise, potentially leading to inaccuracies.
Additionally, certain medications can artificially lengthen or shorten the QT interval, further complicating diagnosis. Drugs such as some antibiotics and antidepressants are known to prolong the QT interval [16], making it difficult to distinguish between drug-induced changes and LQTS.
Borderline cases add complexity. When the QT interval is within the upper range of normal or only slightly prolonged, it becomes challenging to determine whether LQTS is present or if it's just a normal variation, which can delay or mislead diagnosis.
Interpreting the QT interval is subjective, adding another layer of complexity to LQTS diagnosis. A prolonged QT interval refers to an extended duration of the QT interval on an electrocardiogram (ECG), which measures the time it takes for the heart's ventricles to recharge between beats. The QT interval begins at the start of the Q wave and ends at the end of the T wave, representing the time from the onset of ventricular depolarization (when the ventricles contract) to the completion of ventricular repolarization (when the ventricles reset for the next contraction).
There is no universal cutoff for what constitutes a "prolonged" QT interval, leading to differences in diagnosis among physicians [18]. Some may diagnose LQTS at the first sign of a prolonged QT, while others may wait for more pronounced symptoms, resulting in inconsistent diagnoses.
Moreover, factors such as age and sex can influence the QT interval. Women generally have slightly longer QT intervals than men, and QT intervals tend to lengthen with age [19]. Without standardized guidelines that account for these variations, misinterpretation becomes more likely, complicating the diagnostic process further.
3. Genetic Testing: Opportunities and Challenges
Genetic testing has become a valuable tool in diagnosing LQTS, as the condition is often caused by mutations in genes that regulate the heart's electrical activity [20]. While this method can confirm a diagnosis and guide treatment, it also has limitations.
One of the major barriers to genetic testing is its high cost. It is often expensive and not always covered by insurance, making it inaccessible to many patients [21]. As a result, some individuals with suspected LQTS may rely solely on ECGs, despite their limitations.
Additionally, genetic testing is time-consuming [23]. The process of collecting samples, running tests in a lab, and waiting for results can take weeks or months. For patients needing a quick diagnosis, this delay can be life-threatening.
While genetic testing can identify many mutations associated with LQTS, it does not cover all cases. Not all instances of LQTS are linked to identifiable genetic mutations. Up to 25% of individuals with LQTS may not have a detectable mutation, meaning a negative genetic test does not rule out the condition [24].
Moreover, even when a mutation is found, incomplete penetrance—where not all individuals with the mutation exhibit symptoms—can make it hard to interpret results. A positive genetic test does not guarantee the patient will experience symptoms, complicating decisions about treatment and potentially leading to overdiagnosis [25].
The Urgent Need for Improved Diagnostic Tools
Given the limitations of ECGs and genetic testing, there is a clear need for more reliable diagnostic tools for LQTS. Advances in machine learning to analyze ECG data more accurately, improvements in genetic testing, and comprehensive clinical guidelines could help mitigate these challenges. Ultimately, better diagnostic methods are crucial to ensuring that patients with LQTS are diagnosed and treated in time to prevent life-threatening complications.
Borderline QT Prolongation: Borderline QT prolongation refers to a QT interval that is longer than normal but does not yet reach the threshold for a definitive diagnosis of Long QT Syndrome (LQTS). This condition can warrant careful monitoring, as it may increase the risk of arrhythmias and other cardiovascular issues. Clinicians often consider factors such as patient history, family history, and additional diagnostic tests when evaluating borderline QT prolongation. The variability in ECG interpretation, lack of clear guidelines regarding the management of patients with borderline QT prolongation, relative lack of extensive research and not fully known genetic predisposition can lead to inconsistencies in diagnosing borderline QT prolongation.
Concealed LQTS: Patients carrying genetic variants associated with long QT syndrome (LQTS) but have normal QTc values [26]. Concealed Long QT Syndrome (LQTS) refers to cases where individuals have an abnormal QT interval that is not easily detected through standard ECG measurements or clinical assessments. Concealed Long QT Syndrome (LQTS) presents notable challenges in diagnosis and management. Many individuals are asymptomatic, complicating the identification of those at risk for life-threatening arrhythmias. Standard ECGs often show normal QT intervals, leading to missed diagnoses and insufficient monitoring. Variability in QT intervals under different conditions, such as exercise or medication, further complicates detection. Additionally, a lack of awareness regarding family histories of sudden cardiac death can result in missed opportunities for early intervention. Genetic testing is complex, with not all mutations being well understood, which hampers the identification of at-risk individuals. The absence of standardized management protocols leads to inconsistent treatment approaches, while the uncertainty surrounding concealed LQTS can cause anxiety for patients and families. These challenges highlight the need for improved diagnostic tools and comprehensive management strategies for those affected by concealed LQTS. Hence, many patients who have a normal QTc and lack a genetic confirmation of LQTS may actually be suffering from "concealed LQTS" [20].
The Dire Consequences of Missed or Delayed Diagnoses in Long QT Syndrome (LQTS)
Increased Risk of Sudden Cardiac Death
Long QT Syndrome (LQTS) is a genetic disorder that can lead to abnormal heart rhythms (arrhythmias), increasing the risk of sudden cardiac death (SCD), especially in young individuals and athletes. If LQTS is undiagnosed or diagnosed late, the risk of a fatal arrhythmic event escalates significantly. Sudden cardiac death often occurs without warning and can be triggered by physical exertion, stress, or even sleep. In athletes, intense exercise is a well-documented risk factor for triggering ventricular arrhythmias in those with LQTS, which can lead to sudden cardiac arrest on the field [27]. The consequences of undiagnosed LQTS are particularly devastating in younger populations. Many children and adolescents with LQTS may experience no noticeable symptoms, leading to the mistaken belief that they are healthy. However, this lack of early diagnosis and intervention leaves these individuals vulnerable to sudden cardiac events. Studies indicate that LQTS accounts for a significant portion of sudden unexplained deaths in otherwise healthy young people, particularly athletes [27]. The impact is not only personal but also societal, as families and communities deal with the grief and loss of young lives cut short.
Missed Opportunities for Life-Saving Interventions
The timely diagnosis of LQTS can be life-saving. Early identification allows for the implementation of preventive measures that can significantly reduce the risk of fatal arrhythmias. For example, beta-blockers, a class of medications that help reduce the heart's workload and control its rhythm, are highly effective in preventing life-threatening events in individuals with LQTS [28]. Without diagnosis, patients miss the opportunity to start these critical medications, leaving them vulnerable to fatal cardiac events that could have been avoided.
Additionally, lifestyle modifications are a key component of managing LQTS. Those diagnosed early can avoid high-risk activities, such as competitive sports or swimming, which are known to trigger dangerous arrhythmias in individuals with the condition. Furthermore, patients can be educated on the importance of avoiding certain medications that prolong the QT interval, such as specific antibiotics or antidepressants, which can exacerbate the condition and lead to arrhythmias. In more severe cases, early diagnosis can lead to the placement of an implantable cardioverter-defibrillator (ICD), a device that monitors heart rhythms and delivers shocks to restore normal rhythm if a life-threatening arrhythmia occurs. ICDs are especially beneficial in high-risk patients, as they can intervene in real-time to prevent sudden death [29]. When diagnosis is delayed, patients miss the opportunity to receive this potentially life-saving intervention, increasing their risk of SCD.
Ultimately, the failure to diagnose LQTS early represents missed opportunities for these critical interventions. A proactive approach involving family history assessment, genetic testing, and electrocardiogram (ECG) screening in at-risk populations could significantly reduce the mortality associated with LQTS [29]. Early diagnosis provides a window of opportunity to prevent sudden cardiac death and enable individuals to lead relatively normal lives with appropriate management.
4. II Machine Learning: Deciphering the Heart’s Silent Code
Machine learning (ML) has emerged as a groundbreaking tool in healthcare, enabling clinicians and researchers to unlock insights from vast amounts of complex data. In the field of cardiology, ML’s ability to process and interpret intricate patterns has demonstrated its potential to revolutionize the diagnosis and treatment of conditions like Long QT Syndrome (LQTS) [30]. LQTS, a hereditary cardiac disorder, predisposes individuals to life-threatening arrhythmias and sudden cardiac death (SCD). The disorder often eludes traditional diagnostic methods, as symptoms may be mild or absent. This is where ML offers transformative potential, identifying subtle data patterns and enhancing diagnostic accuracy [31].
Machine Learning as a Powerful Tool for Complex Data Analysis
Machine learning excels at analyzing massive datasets and uncovering relationships that might be overlooked by traditional methods. For example, ML models can detect nonlinear patterns and complex dependencies within data, which are difficult for human interpretation and conventional statistical techniques to recognize [32]. These models learn and optimize from large volumes of training data, improving their predictive performance with increased data exposure. This capability makes ML particularly valuable in diagnosing conditions like LQTS, where clinical presentations may be subtle or confounding factors may be present [33].
LQTS diagnosis has traditionally relied on electrocardiograms (ECGs), family history, and genetic testing. However, the condition can be challenging to identify through these methods alone due to its variable presentation. The ECG, which measures the electrical activity of the heart, remains a cornerstone in LQTS diagnosis, but interpreting subtle variations and risk markers is not always straightforward. ML, by contrast, can analyze not only the QT interval (the time it takes for the heart’s ventricles to repolarize) but also various other markers to produce a more comprehensive risk assessment [34]. This allows ML to surpass traditional diagnostic approaches, offering precision and speed in identifying at risk individuals.
5. How ML Can Revolutionize LQTS Diagnosis
ECG Pattern Recognition
A fundamental aspect of diagnosing LQTS is recognizing patterns in the electrical activity of the heart as captured by ECGs. Cardiologists have long relied on manual interpretation of ECGs to detect QT prolongation, but this approach has limitations. Borderline cases or instances of “concealed” LQTS—where the QT interval may appear normal but arrhythmogenic risk is still present—are easily missed. ML algorithms trained on vast ECG datasets can identify nuanced patterns indicative of LQTS that may be imperceptible to human clinicians. For example, ML models are adept at detecting differences in LQTS subtypes, such as LQT1, LQT2, and LQT3, which are often difficult to differentiate using traditional QT interval measurements alone. By analyzing more complex features of the ECG—such as the shape, slope, and timing of the heart’s electrical signals—ML can distinguish between healthy individuals and those with different LQTS subtypes, offering a more refined and accurate diagnosis [35]. This capability can reduce the number of false negatives, ensuring that individuals who might otherwise go undiagnosed receive timely intervention.
In recent studies, ML has shown remarkable success in identifying patients with LQTS based on ECG data. One study by Khera et al. demonstrated that an ML algorithm could predict both QT prolongation and LQTS subtypes with high accuracy after being trained on thousands of ECGs. This type of ML-driven analysis could pave the way for more widespread use of AI tools in routine ECG screenings, improving diagnostic precision and reducing the risks of misdiagnosis.
Unmasking Hidden Markers
Beyond the traditional focus on QT intervals, ML algorithms can extract additional, often overlooked features from ECGs. These hidden markers, such as T-wave morphology (the shape and characteristics of the heart’s electrical recovery phase) and heart rate variability (HRV), play a critical role in understanding a patient’s arrhythmic risk [36]. T-wave morphology, for example, is an important diagnostic clue in LQTS, particularly in distinguishing between its subtypes. Subtle variations in the shape, duration, and amplitude of the T-wave can indicate specific arrhythmogenic risks associated with different LQTS genotypes [37]. While these variations may be too subtle for traditional diagnostic methods, ML algorithms can detect and analyze these features, producing a more comprehensive risk profile. In patients with borderline QT prolongation, these hidden markers can be key to early detection [38]. Similarly, heart rate variability (HRV) offers insight into the autonomic regulation of the heart. Reduced HRV is associated with higher susceptibility to arrhythmias and sudden cardiac events. ML algorithms can analyze HRV alongside ECG data to create a more nuanced assessment of a patient’s risk of arrhythmic events, improving the overall diagnostic process [39,40]. For example, patients with seemingly normal QT intervals but abnormal HRV could still be identified as high-risk by an ML model, prompting early intervention.
Integration with Genomic Data
In addition to ECG and HRV analysis, ML algorithms can also incorporate genetic data to further refine the diagnosis of LQTS. Certain gene mutations are known to cause LQTS, and ML can be used to analyze this genetic data alongside ECG patterns. By integrating clinical, electrophysiological, and genetic information, ML models can predict the likelihood of an individual developing LQTS with even greater accuracy [41]. This holistic approach could help personalize treatments, leading to more precise interventions based on the patient’s specific genetic and electrophysiological profile. The integration of genomics and ML represents the future of personalized medicine, where treatment plans and interventions are tailored to the specific needs of the individual based on their unique genetic and clinical markers. This allows for more targeted therapies and better long-term outcomes for patients with LQTS.
Predicting Drug-Induced LQTS with Machine Learning
Machine learning (ML) holds significant promise in predicting an individual’s susceptibility to drug-induced Long QT Syndrome (LQTS), a serious condition where certain medications can cause dangerous QT interval prolongation, leading to arrhythmias or sudden cardiac death. By leveraging large datasets encompassing genetic information, medication history, and ECG parameters, ML models can identify patients at a higher risk for developing QT prolongation in response to specific drugs [42]. This predictive capability enables personalized medicine, where clinicians can make more informed decisions about prescribing medications and avoiding drugs that could provoke QT prolongation in susceptible individuals. Moreover, this approach could revolutionize drug development by identifying potential risks earlier in the clinical trial phase, thereby enhancing patient safety [43]. A study by Khera et al. demonstrated that ML algorithms outperformed traditional risk assessment tools in predicting drug-induced LQTS by analyzing a combination of genetic and phenotypic data, achieving higher sensitivity and specificity. Such advancements hold the potential to prevent adverse drug reactions, which are a leading cause of morbidity and mortality in clinical practice. As ML continues to evolve, its application in this area will be instrumental in reducing the risks associated with QT-prolonging medications [44].
Superior Accuracy of ML Models in LQTS Diagnosis
ML models have demonstrated superior accuracy in diagnosing LQTS compared to conventional diagnostic methods. Studies have shown that ML algorithms trained on large ECG datasets can achieve higher sensitivity and specificity in identifying individuals at risk of LQTS. For example, models developed achieved higher diagnostic precision than traditional ECG analysis, allowing for earlier interventions and potentially life-saving treatments [45]. These models' enhanced accuracy translates to better patient outcomes, emphasizing the growing role of AI in improving healthcare delivery.
6. Overcoming Challenges, Realizing the Potential: A Collaborative Effort
Overcoming difficulties and achieving the potential of machine learning (ML) in detecting Long QT Syndrome (LQTS) is a multifaceted process [46]. Despite its promise, the use of machine learning (ML) in healthcare, particularly for disorders like LQTS, is still in its early phases [47]. Continuous algorithmic research and modification are critical for resolving present limits while improving accuracy and clinical value [48]. One of the major challenges in this area is the requirement for access to big, diversified datasets that contain a wide range of LQTS subtypes, demographics, and clinical presentations [49]. To construct strong and generalizable ML models, these datasets must include patients of diverse ethnicities as well as those with comorbidities [50]. Without this diversity, the algorithms may perform well in certain populations but fail to generalize across broader groups, which is a significant disadvantage in terms of global healthcare relevance [51]. In addition to data diversity, model interpretability is another significant challenge. ML models, especially those based on deep learning, often function as "black boxes," meaning that their decision-making processes are not transparent to clinicians [52]. This lack of transparency can make it difficult for healthcare professionals to trust these models, which may hinder their adoption in clinical practice [53]. Clinicians need to be able to understand how an ML model arrives at its predictions to validate its findings and make informed decisions regarding patient care [54]. Developing interpretable ML models is essential to ensure that these technologies can be seamlessly integrated into clinical workflows without sacrificing accuracy or trust. Interpretability not only boosts clinician confidence but also provides insights into new areas of cardiac care by offering a clearer understanding of the underlying processes driving the diagnosis [55].
Ethical considerations further complicate the deployment of ML in healthcare. Ensuring the privacy and security of patient data is paramount, given the sensitive nature of the information used to train and validate these models [56]. Robust data de-identification techniques and secure data storage systems are necessary to protect patient confidentiality [57].
Furthermore, algorithmic bias is a pressing concern. ML algorithms are only as good as the data they are trained on, and if that data reflects existing healthcare disparities, the algorithms may perpetuate or even exacerbate these inequities [58]. For example, if an algorithm is trained predominantly on data from one demographic group, its performance may be less accurate when applied to underrepresented populations, leading to unequal healthcare outcomes. Addressing these biases by ensuring diverse, balanced datasets is crucial for developing equitable ML-based diagnostic tools [59]. The responsible implementation of ML in clinical settings also demands careful planning.
Integrating ML into clinical workflows should not disrupt healthcare professionals' day-to-day operations, and adequate training must be provided to ensure clinicians can interpret ML-generated predictions accurately [60]. Additionally, patients should be informed and give consent to the use of these technologies in their diagnosis and treatment. Ensuring that patients understand the role of ML in their care fosters transparency and trust, which are critical for the widespread adoption of these tools [61]. Overcoming these challenges will require interdisciplinary collaboration between clinicians, data scientists, ethicists, and policymakers [62]. This cooperative effort is essential to drive innovation while ensuring responsible and ethical development of ML tools. Clinicians can provide practical insights into the diagnostic process, while data scientists work on refining algorithms, and ethicists help navigate the moral implications of AI in healthcare. Policymakers, on the other hand, play a key role in developing regulations and standards for the use of ML in medical settings [63-65].
7. Conclusion: A Future Where LQTS is No Longer Silent
Machine learning (ML) has enormous potential for revolutionizing the diagnosis of Long QT Syndrome (LQTS). By employing complex algorithms and combining data from a variety of sources, machine learning can significantly enhance the precision and efficiency of diagnosing LQTS, resulting in better patient outcomes. However, realizing this promise needs coordinated collaboration among a wide range of professions, including doctors, data scientists, ethicists, and patient advocates. Machine learning has the potential to significantly alter the way LQTS is diagnosed. Its ability to handle large datasets enables it to identify subtle patterns and risk factors that traditional diagnostic approaches may overlook, considerably enhancing diagnostic accuracy and speed. Furthermore, ML's capacity to incorporate data from multiple sources, such as genetic information, clinical history, and lifestyle factors, allows for a more personalized approach to treatment, tailored to each patient's specific circumstances. As these models are constantly updated with fresh data, their accuracy improves over time, providing healthcare providers with the most up-to-date and relevant information about patient care. The use of machine learning into LQTS diagnostics marks a significant step forward in cardiovascular health, offering more effective disease management and overall patient care. To successfully implement ML techniques in clinical settings, it is critical to prioritize collaboration, ethical development, and continual professional education. These activities will contribute to the development of a healthcare system in which LQTS, a previously ignored disorder, may be managed more effectively through prompt and tailored treatment options. Realizing the full potential of ML in the diagnosis and treatment of LQTS will necessitate collaborative work. Clinicians, data scientists, ethicists, and healthcare policymakers must collaborate to create and ethically apply machine learning tools. This collaborative approach will address crucial issues such as algorithmic bias, data privacy, and the realities of incorporating these technologies into everyday medical practice, ultimately producing answers that improve patient care. Looking ahead, there is hope for a world in which LQTS is no longer a hidden threat but a condition that can be adequately managed. Advances in machine learning, combined with continuous interdisciplinary collaboration, provide optimism for a healthcare future in which LQTS is diagnosed early and treated individually, considerably improving the quality of life for persons affected by the condition. Working together, we can make this vision a reality, changing cardiovascular health and allowing patients to manage their disease confidently and effectively.
References
- Wallace E, Howard L, Liu M, et al. Long QT Syndrome: genetics and future perspective. Pediatric Cardiology 40 (2019): 1419-1430.
- Cohagan B, Brandis D. Torsade de Pointes. StatPearls - NCBI Bookshelf (2023).
- Krahn AD, Laksman Z, Sy RW, et al. Congenital Long QT syndrome. JACC. Clinical Electrophysiology 8 (2022): 687- 706.
- Wu M, Wang W, Zhang W, et al. The diagnostic value of electrocardiogram-based machine learning in long QT syndrome: a systematic review and meta-analysis. Frontiers in Cardiovascular Medicine 10 (2023).
- Baracaldo-Santamaría D, Llinás-Caballero K, Corso-Ramirez JM, et al. Genetic and Molecular aspects of Drug-Induced QT Interval Prolongation. International Journal of Molecular Sciences 22 (2021): 8090.
- Galic E. Congenital Long QT Syndrome: a Systematic Review. Acta Clinica Croatica (2021).
- Baracaldo-Santamaría D, Llinás-Caballero K, Corso-Ramirez JM, et al. Genetic and Molecular aspects of Drug-Induced QT Interval Prolongation. International Journal of Molecular Sciences 22 (2021b): 8090.
- Lahrouchi N, Tadros R, Crotti L, et al. Transethnic Genome-Wide Association study provides insights in the genetic architecture and heritability of long QT syndrome. Circulation 142 (2020): 324-338.
- Siontis KC, Noseworthy PA, Attia ZI, et al. Artificial intelligence-enhanced electrocardiography in cardiovascular disease management. Nature Reviews Cardiology 18 (2021): 465-478.
- Dehkordi NR, Dehkordi NR, Toudeshki KK, et al. Artificial intelligence in diagnosis of Long QT Syndrome: A review of current state, challenges, and future perspectives. Mayo Clinic Proceedings Digital Health 2 (2024): 21-31.
- Krishnan G, Singh S, Pathania M, et al. Artificial intelligence in clinical medicine: catalyzing a sustainable global healthcare paradigm. Frontiers in Artificial Intelligence 6 (2023).
- Maxwell YL. Machine learning ‘Sniffs’ out long QT otherwise unseen on ECG. TCTMD.com (2021).
- Muzammil MA, Javid S, Afridi AK, et al. Artificial intelligence-enhanced electrocardiography for accurate diagnosis and management of cardiovascular diseases. Journal of Electrocardiology 83 (2024): 30-40.
- Lester RM, Paglialunga S, Johnson IA. QT assessment in early drug Development: the long and the short of it. International Journal of Molecular Sciences 20 (2019): 1324.
- Andršová I, Hnatkova K, Šišáková M, et al. Heart rate influence on the QT variability risk factors. Diagnostics 10 (2020): 1096.
- Farzam K, Tivakaran VS. QT prolonging drugs. StatPearls - NCBI Bookshelf (2023).
- Rafie N, Kashou AH, Noseworthy PA. ECG interpretation: clinical relevance, challenges, and advances. Hearts 2 (2021): 505-513.
- Kahlon SS, Sikandar R, Tejovath S, et al. Diagnosing Torsades de pointes based on correlation to QT Interval: A Systematic review. Cureus (2022).
- Rossi M, Marzi F, Natale M, et al. Drug-Associated QTC prolongation in geriatric Hospitalized patients: A Cross-Sectional Study in Internal Medicine. Drugs - Real World Outcomes 8 (2021): 325-335.
- Zhu W, Bian X, Lv J. From genes to clinical management: A comprehensive review of long QT syndrome pathogenesis and treatment. Heart Rhythm O2 5 (2024): 573-586.
- Dusic EJ, Theoryn T, Wang C, et al. Barriers, interventions, and recommendations: Improving the genetic testing landscape. Frontiers in Digital Health 4 (2022).
- Aziz S, Ahmed S, Alouini M. ECG-based machine-learning algorithms for heartbeat classification. Scientific Reports 11 (2021).
- Doldi F, Plagwitz L, Hoffmann LP, et al. Detection of Patients with Congenital and Often Concealed Long-QT Syndrome by Novel Deep Learning Models. Journal of Personalized Medicine 12 (2022): 1135.
- Wilde AaM, Amin AS, Postema PG. Diagnosis, management and therapeutic strategies for congenital long QT syndrome. Heart 108 (2021): 332-338.
- Locati ET, Bagliani G, Cecchi F, et al. Arrhythmias due to Inherited and Acquired Abnormalities of Ventricular Repolarization. Cardiac Electrophysiology Clinics 11 (2019): 345-362.
- Yu Y, Deschenes I, Zhao M. Precision medicine for long QT syndrome: patient-specific iPSCs take the lead. Expert Reviews in Molecular Medicine 25 (2023).
- Prakash K, Swarnakari KM, Bai M, et al. Sudden cardiac arrest in athletes: a primary level of prevention. Cureus (2022).
- Yow AG, Rajasurya V, Ahmed I, et al. Sudden cardiac death. StatPearls - NCBI Bookshelf (2024).
- Hauwanga WN, Yau RCC, Goh KS, et al. Management of Long QT Syndrome: A Systematic Review. Cureus (2024).
- Sahu P, Acharya S, Totade M. Evolution of pacemakers and implantable cardioverter defibrillators (ICDs) in cardiology. Cureus (2023).
- Nosetti L, Zaffanello M, Lombardi C, et al. Early screening for long QT syndrome and cardiac anomalies in infants: a comprehensive study. Clinics and Practice 14 (2024): 1038-1053.
- Long QT syndrome - Symptoms and causes. (n.d.). Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/long-qt-syndrome/symptomscauses/sychttps://www.mayoclinic.org/diseases-conditions/long-qt-syndrome/symptomscauses/syc-2035251820352518
- https://www.researchgate.net/publication/377438052_Machine_Learning_in_the_Big_Data_Age_Advancem ents_Challenges_and_Future_Prospects
- Armoundas AA, Narayan SM, Arnett DK, et al. Use of artificial intelligence in improving outcomes in heart disease: a scientific statement from the American Heart Association. Circulation 149 (2024).
- Al-Akchar M, Siddique MS. Long QT syndrome. StatPearls NCBI Bookshelf (2022).
- Wu M, Wang W, Zhang W, et al. The diagnostic value of electrocardiogram-based machine learning in long QT syndrome: a systematic review and meta-analysis. Frontiers in Cardiovascular Medicine 10 (2023d).
- Aufiero S, Bleijendaal H, Robyns T, et al. A deep learning approach identifies new ECG features in congenital long QT syndrome. BMC Medicine 20 (2022b).
- Wu M, Wang W, Zhang W, et al. The diagnostic value of electrocardiogram-based machine learning in long QT syndrome: a systematic review and meta-analysis. Frontiers in Cardiovascular Medicine 10 (2023b).
- Aufiero S, Bleijendaal H, Robyns T, et al. A deep learning approach identifies new ECG features in congenital long QT syndrome. BMC Medicine 20 (2022).
- Wu M, Wang W, Zhang W, et al. The diagnostic value of electrocardiogram-based machine learning in long QT syndrome: a systematic review and meta-analysis. Frontiers in Cardiovascular Medicine 10 (2023c).
- Ansari Y, Mourad O, Qaraqe K, et al. Deep learning for ECG Arrhythmia detection and classification: an overview of progress for period 2017–2023.Frontiers in Physiology 14 (2023).
- Smaranda AM, Dragoiu TS, Caramoci A, et al. Artificial Intelligence in Sports Medicine: Reshaping Electrocardiogram Analysis for Athlete Safety—A Narrative Review. Sports 12 (2024): 144.
- Duca S, Tudorancea I, Haba MSC, et al. Enhancing comprehensive assessments in chronic heart failure caused by ischemic heart disease: the diagnostic utility of Holter ECG parameters. Medicina 60 (2024): 1315.
- Kabra R, Israni S, Vijay B, et al. Emerging role of artificial intelligence in cardiac electrophysiology. Cardiovascular Digital Health Journal 3 (2022): 263-275.
- Simon ST, Mandair D, Tiwari P, et al. Prediction of Drug- Induced Long QT Syndrome using machine learning applied to harmonized electronic health record data. Journal of Cardiovascular Pharmacology and Therapeutics 26 (2021): 335-340.
- Yadav S, Singh A, Singhal R, et al. Revolutionizing drug discovery: The impact of artificial intelligence on advancements in pharmacology and the pharmaceutical industry. Intelligent Pharmacy 2 (2024): 367-380.
- Sachdeva P, Kaur K, Fatima S, et al. Advancements in myocardial Infarction Management: Exploring Novel Approaches and Strategies. Cureus (2023).
- Dehkordi NR, Dehkordi NR, Toudeshki KK, et al. Artificial intelligence in diagnosis of Long QT Syndrome: A review of current state, challenges, and future perspectives. Mayo Clinic Proceedings Digital Health 2 (2024b): 21-31.
- Armoundas AA, Narayan SM, Arnett DK, et al. Use of artificial intelligence in improving outcomes in heart disease: a scientific statement from the American Heart Association. Circulation 149 (2024).
- Alowais SA, Alghamdi SS, Alsuhebany N, et al. Revolutionizing healthcare: the role of artificial intelligence in clinical practice. BMC Medical Education 23 (2023).
- Jiang J, Vy HMT, Charney A, et al. Multimodal fusion learning for long QT syndrome pathogenic genotypes in a racially diverse population. Npj Digital Medicine 7 (2024).
- Arora A, Alderman JE, Palmer J, et al. The value of standards for health datasets in artificial intelligence-based applications. Nature Medicine 29 (2023): 2929-2938.
- Ueda D, Kakinuma T, Fujita S, et al. Fairness of artificial intelligence in healthcare: review and recommendations. Japanese Journal of Radiology 42 (2023): 3-15.
- Hassija V, Chamola V, Mahapatra A, et al. Interpreting Black-Box Models: A review on Explainable Artificial intelligence. Cognitive Computation 16 (2023): 45-74.
- Ahmed MI, Spooner B, Isherwood J, et al. A Systematic review of the barriers to the implementation of artificial intelligence in healthcare. Cureus (2023).
- Nasarian E, Alizadehsani R, Acharya U, et al. Designing Interpretable ML System to Enhance Trust in Healthcare: A Systematic Review to Proposed Responsible Clinician-AI-Collaboration Framework. Information Fusion 108 (2024): 102412.
- Ali S, Akhlaq F, Imran AS, et al. The enlightening role of explainable artificial intelligence in medical & healthcare domains: A systematic literature review. Computers in Biology and Medicine 166 (2023): 107555.
- Jeyaraman M, Balaji S, Jeyaraman N, et al. Unraveling the ethical enigma: Artificial intelligence in healthcare. Cureus (2023).
- McGraw D, Mandl KD. Privacy protections to encourage use of health-relevant digital data in a learning health system. Npj Digital Medicine 4 (2021).
- Ferrara E. Fairness and Bias in Artificial intelligence: A brief survey of sources, impacts, and mitigation strategies. Sci 6 (2023): 3.
- Ueda D, Kakinuma T, Fujita S, et al. Fairness of artificial intelligence in healthcare: review and recommendations. Japanese Journal of Radiology 42 (2023b): 3-15.
- Esmaeilzadeh P. Challenges and strategies for wide-scale artificial intelligence (AI) deployment in healthcare practices: A perspective for healthcare organizations. Artificial Intelligence in Medicine 151 (2024): 102861.
- Tilala MH, Chenchala PK, Choppadandi A, et al. Ethical Considerations in the Use of Artificial intelligence and Machine Learning in Health Care: A Comprehensive review. Cureus (2024).
- Patel AU, Gu Q, Esper R, et al. The crucial role of interdisciplinary conferences in advancing explainable AI in healthcare. BioMedInformatics 4 (2024): 1363-1383.
- Mennella C, Maniscalco U, De Pietro G, et al. Ethical and regulatory challenges of AI technologies in healthcare: A narrative review. Heliyon 10 (2024): e26297.








 Impact Factor: * 5.6
Impact Factor: * 5.6 Acceptance Rate: 74.36%
Acceptance Rate: 74.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 