Enhanced Adhesion Molecules are Early Inflammatory Driver in Symptomatic Patients with Preserved Ejection Fraction Prior to Diastolic Dysfunction
Carlos Plappert1,2,3*, Henrike Arfsten4, Jan-Niklas Dahmen1,2, Anna Feuerstein1,2, Veronika Zach1,2, Heinz-Peter Schultheiss5, Frank Edelmann1,2,6, Felicitas Escher1,2,5
1Department of Internal Medicine and Cardiology, German Heart Center at Charité, Campus Virchow Klinikum, Berlin, Germany
2DZHK (German Centre for Cardiovascular Research), partner site Berlin, Berlin, Germany
3Division of Cardiology, Department of Medicine, Northwestern University Feinberg School of Medicine, Chicago, Illinois, USA
4Division of Cardiology, Department of Internal Medicine II, Medical University of Vienna, Vienna, Austria
5Institute for Cardiac Diagnostics and Therapy (IKDT), Berlin, Germany
6Berlin Institute of Health (BIH), Berlin, Germany
*Corresponding author: Carlos Plappert, MD, Department of Internal Medicine and Cardiology, German Heart Center at Charité, Campus Virchow Klinikum, Augustenburger Platz 1, 13353 Berlin, Germany.
Received: October 25, 2024; Revision received: November 19, 2024; Accepted: November 20, 2024; Published: December 24, 2024
Article Information
Citation: Carlos Plappert, Henrike Arfsten, Jan- Niklas Dahmen, Anna Feuerstein, Veronika Zach, Heinz-Peter Schultheiss, Frank Edelmann, Felicitas Escher. Enhanced Adhesion Molecules are Early Inflammatory Driver in Symptomatic Patients with Preserved Ejection Fraction Prior to Diastolic Dysfunction. Cardiology and Cardiovascular Medicine. 8 (2024): 521-530.
View / Download Pdf Share at FacebookAbstract
Background: Patients with diastolic dysfunction exhibit signs of chronic myocardial inflammation. However, which inflammatory markers are crucial in the early inflammatory process in symptomatic patients with preserved ejection fraction leading to the development of diastolic dysfunction remain elusive.
Methods: We retrospectively analyzed n=72 (49 male/23 female) consecutive patients with heart failure symptoms according to the New York Heart Association (NYHA) classification stages II-IV. Only patients with preserved left ventricular ejection fraction (LVEF) > 50% and without echocardiographic findings of diastolic dysfunction including E/e’ < 14 or left atrial volume index (LAVI) < 34 ml/m2 were enrolled. All patients underwent endomyocardial biopsies (EMBs).
Results: The mean LVEF was 58±2%. According to EMBs, immunohistological signs of inflammatory processes were shown in n=29 (40%) patients. In univariable regression analysis, age (OR: 1.410, 95% CI: 1.040-1.751, p <0.001) and adhesion molecules ICAM-1 (OR: 1.395, 95% CI: 1.029-1.780, p <0.05) were significantly associated with E/e’ in patients with microvascular inflammatory processes, respectively. The association of age and ICAM-1 with E/e’ remained virtually unchanged after adjustment for both variables. In contrast, in patients without inflammatory processes, we observed in univariable regression analysis, age (OR: 1.619, 95% CI: 1.430-1.951, p <0.001), female sex (OR: 1.449, 95% CI: 1.164-1.733, p <0.05) and low-grade CD11+cells (OR: 1.548, 95% CI: 1.273-1.823, p <0.05) were significantly associated with E/e’. After multivariable regression analysis, age (OR: 1.597, 95% CI: 1.337- 1.856, p <0.001) remained significant.
Conclusion: ICAM-1 is a key marker in the early inflammatory processes of symptomatic patients with preserved ejection fraction that are prior to diastolic dysfunction.
Keywords
<p>Inflammatory Markers; ICAM-1; Diastolic Dysfunction; Heart Failure with Preserved Ejection Fraction; Endomyocardial Biopsies</p>
Article Details
Introduction
The aging population and steadily rising prevalence of risk factors associated with cardiovascular diseases develop more and more to a global health burden [1].
Cardiovascular risk factors and comorbidities, such as obesity, metabolic syndrome, lipid disorders and hypertension trigger a low-grade proinflammatory state leading to an abnormal vascular endothelial function [2-4]. Cellular adhesion molecules (CAMs) such as vascular cell adhesion molecule-1 (VCAM-1), endothelial (E)-selectin, intracellular adhesion molecule-1 (ICAM-1) or perforin are biomarkers of endothelial activation and of particular importance in the initiation of an inflammatory response [2,5]. In this context, CAMs are expressed on the surface of endothelial cells and upregulated [5-8]. Higher circulating levels of CAMs initiate a cascade leading to a monocyte release that turn into macrophages and produce transforming growth factor-ß (TGF-ß), followed by an increased collagen and thereby scar tissue production. Detrimental effects on myocardial tissue include disturbances in diastolic myocardial relaxation due to left ventricular (LV) stiffness and subsequently diastolic dysfunction [9-14].
It has been demonstrated that endothelial cell activation and coronary microvascular dysfunction represent central mechanisms in the pathogenesis of heart failure with preserved ejection fraction (HFpEF) [2,7,8]. Several human studies and animal models addressed the major negative impact of upregulated CAMs on myocardial structural and functional alterations [15-18].
However, the pathophysiological understanding of the exact inflammatory processes linked to endothelial activation in patients at greater risk for HFpEF remains poor. Furthermore, relatively little is known about potential markers that play a key role in HFpEF development over time [2,19,20].
The aim of this analysis was to improve the immunohistological understanding based on endomyocardial biopsies (EMBs) regarding inflammatory processes in symptomatic patients with preserved ejection fraction prior to the development of diastolic dysfunction.
Materials and Methods
Study population
Seventy-two consecutive patients with heart failure symptoms, but without prior LV dysfunction, undergoing EMBs at Charité-University hospital, Berlin, Germany within the period of 5 years, were retrospectively analyzed. Patients were classified according to the New York Heart Association (NYHA) stages II-IV. Patients with coronary artery disease and other possible causes of the clinical complaints (e.g. valvular heart disease, storage disorders) or active myocarditis had been excluded. All patients underwent an echocardiographic examination within four days regards to EMB sampling. Only patients with preserved left ventricular ejection fraction (LVEF) > 50%, but without clear evidence of diastolic dysfunction that was defined as elevated values of E/e’ > 14 or left atrial volume index (LAVI) > 34 ml/m2 were enrolled. Medical history, comorbidities and clinical status were recorded. Routine parameters from venous blood sampling were collected. Written informed consent was obtained from all study participants. The study protocol complies with the Declaration of Helsinki and was approved by the local ethics committee at the Charité-Universitätsmedizin Berlin.
Investigation methods
Echocardiography
Echocardiographic examination was performed. Cardiac chambers were quantified using standard four- and two- chamber views and LVEF was assessed based on the biplane Simson’s method. Tissue doppler was performed from the apical four-chamber view. Early diastolic mitral annulus peak velocity was measured and the ratio of the transmitral diastolic peak velocity to the mitral annular diastolic peak velocity (E/e’) was calculated. LAVI was calculated in the apical four-chamber view. Diastolic dysfunction was defined as elevated values of E/e’ > 14 or LAVI > 34 ml/m2, respectively.
Histological and Immunohistological Staining for Assessment of Inflammation
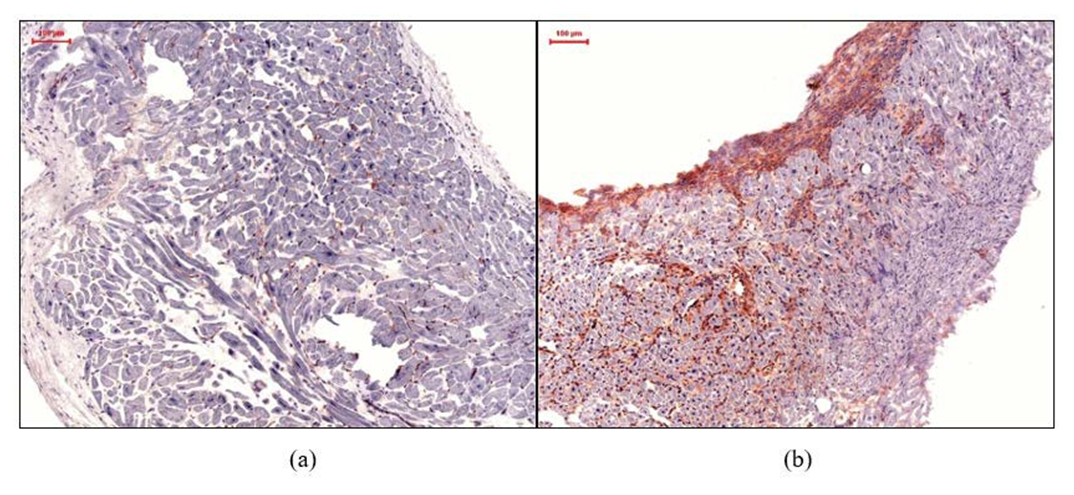
The EMB specimens were analyzed in the Institute for Cardiac Diagnostic and Therapy (IKDT) Berlin, Germany, including histology, immunohistochemistry and molecular virology. Histology was used to confirm appearance of active myocarditis as established by the Dallas Criteria. Immunohistochemistry was used for the characterization of inflammatory infiltrates. Antibodies used: CD3+ lymphocytes (Dako, Glostrup, Denmark), CD11a+/LFA-1+ lymphocytes (Immuno Tools, Friesoythe, Germany), CD45R0+ (Dako, Glostrup, Denmark), perforin+- cytotoxic infiltrates (BD Bioscience, San Jose, California, USA), CD11b+/Mac- 1+ macrophages (ImmunoTools, Friesoythe, Germany), ICAM-1/CD54 (ImmunoTools, Friesoythe, Germany), and VCAM-1/CD106 (ImmunoTools, Friesoythe, Germany). As secondary antibody we used enhancing EnVisionTM peroxidase-conjugated anti-mouse antibody (Dako, Glostrup, Denmark). Immunohistological staining was visualized using 3-amino-9-ethylcarbazole (Merck, Darmstadt, Germany) as chromogenic substrate. Finally, slides were counterstained in hematoxylin and mounted with Kaiser’s gelatinR (Merck, Darmstadt, Germany). The staining and peroxidase reactions in all samples were carried out identically and in parallel for all samples. Immunoreactivity was quantified by digital image analysis as described previously [21,22].
Detection of viral genomes by nested PCR (nPCR) and reverse transcription-PCR (RT-PCR)
EMBs were subjected to molecular biological investigation of cardiotropic viral genomes according to the published techniques [23]. In brief, a PCR was performed on RNA extracted from EMBs for enterovirus, adenovirus, and on DNA for Epstein-Barr virus, Erythrovirus genomes and human Herpesvirus 6.
Statistical analysis
Baseline descriptive data included demographic, clinical, echocardiographic, immunohistological and laboratory characteristics. Continuous data were presented as mean and standard deviation (SD). Categorical data were presented as counts and percentages. All data were investigated for a normal distribution using the Shapiro-Wilk-test.
The analysis was performed in the entire patient cohort and separately according to immunohistological signs of inflammatory processes (patients with inflammatory processes versus those without). Differences between the groups were compared using Student t-test, Mann-Whitney U test, X2 test and Fisher exact test (when appropriate).
Pearson correlation test for normally distributed continuous variables and Spearman correlation test for non-normally distributed continuous variables were conducted to detect significant associations between patient’s characteristics and echocardiographic diastolic parameters encompassing E/e’ and LAVI. Furthermore, we tested significant associations between inflammatory markers and LVEF as a measure of cardiac systolic function using correlation analysis. Only variables that were significant in correlation analysis (p <.05) were subsequently included in univariable and multivariable regression analysis.
Univariable and multivariable linear and logistic regression were used to evaluate the independent associations between patient’s characteristics (independent variables) and echocardiographic indices of diastolic function
including E/e’ and LAVI (dependent variables). Due to different scale ranges, levels of data were standardized (per SD) for the regression models. Models were adjusted for age and sex and significant covariates obtained from correlation analysis were included. Univariable and multivariable effects were reported with calculation of the regression coefficient, odds ratio (OR) and 95% confidence interval (CI) for each observation.
Two-tailed p values <.05 were considering statistically significant. The statistical analysis was performed using R version 4.3.2 "Eye Holes" from October 2023 (R Foundation for Statistical Computing, Vienna, Austria) [24].
Results
Baseline characteristics
The demographic, clinical and echocardiographic data of the 72 patients, classified into two groups according to inflammatory or non-inflammatory processes, are demonstrated in Table 1. Mean age was 42.9 years and 68% were male. Mean LVEF was 58%, E/e’ was 8 and LAVI was 23ml/m2. The mean NT-proBNP was 494 ng/L and most of the patients presented in NYHA stage II (78%). Hypertension was the leading comorbidity in these patients (24%). Sixty- six patients (92%) received a left ventricular biopsy and six patients (8%) underwent a right ventricular biopsy. The most histomorphological changes were fibrosis (50%), followed by myocyte hypertrophy (35%) and atrophy (22%). The immunohistological and laboratory data of the 72 patients, classified into two groups according to inflammatory or non- inflammatory processes, are shown in Table 2.
Table 1: Clinical and echocardiographic parameters presented as mean±SD or counts and percentages (%).
|
Variables |
Inflammatory processes |
Non-inflammatory processes |
p value |
|
Number of patients, No. (%) |
29 (40) |
43(60) |
|
|
Men, No. (%) |
21 (72) |
28 (65) |
0.55 |
|
Women, No. (%) |
8 (28) |
15 (35) |
0.38 |
|
Age, mean±SD, (years) |
45.8±14.2 |
40.9±16.0 |
0.18 |
|
Laboratory parameters, mean±SD |
|||
|
NT-proBNP, ng/L |
708±1390 |
349±832 |
<0.05 |
|
Echocardiography, mean±SD |
|||
|
LVEF, % |
56±2.0 |
58.9±5.1 |
0.05 |
|
Average E/e’ |
8.3±2.2 |
7.1±2.0 |
<0.05 |
|
LAVI, ml/m2 |
23.5±5.7 |
22.2±5.7 |
0.37 |
|
NYHA-classification, No. (%) |
|||
|
NYHA-II |
23 (80) |
33 (77) |
0.18 |
|
NYHA-III |
4 (14) |
9 (21) |
0.17 |
|
NYHA-IV |
2 (6) |
1 (2) |
0.56 |
|
Medical history, No. (%) |
|||
|
Hypertension |
9 (31) |
8 (19) |
0.09 |
|
Obesity |
8 (28) |
7 (16) |
0.07 |
|
Diabetes |
1 (3) |
1 (2) |
0.65 |
|
Dyslipidemia |
6 (21) |
6 (14) |
0.24 |
|
No., number; NT-proBNP, N-terminal pro-brain natriuretic peptide; LVEF, left ventricular ejection fraction; LAVI, left atrial volume index; NYHA, New York Heart Association; p value, significance level. |
|||
Table 2: Immunohistological and laboratory parameters presented as mean±SD or counts and percentages (%).
|
Variables |
Inflammatory processes |
Non-inflammatory processes |
p value |
|
Immunohistology, mean±SD |
|||
|
CD11+cells/mm2 |
35.3±51.0 |
9.7±6.3 |
<0.001 |
|
Macrophages+cells/mm2 |
59.5±63.1 |
20.7±14.1 |
<0.001 |
|
CD3+T-cells |
9.5±2.6 |
2.4±2.0 |
<0.001 |
|
Perforin+cells/mm2 |
2.9±1.3 |
1.9±0.8 |
<0.001 |
|
ICAM-1/AF |
1.1±2.4 |
0.4±1.1 |
0.08 |
|
VCAM-1/AF |
0.03±0.02 |
0.06±0.15 |
0.66 |
|
Histomorphometry, No. (%) |
|||
|
Hypertrophy |
10 (34) |
15 (35) |
0.90 |
|
Fibrosis |
12 (41) |
24 (56) |
0.13 |
|
Atrophy |
5 (17) |
11 (26) |
0.17 |
|
Myocyte diameter, µm |
19.8±2.3 |
19.9±2.5 |
0.80 |
|
Laboratory parameters, mean±SD |
|||
|
C-reactive protein, mg/l |
23.7±50.0 |
10.4±20.1 |
0.34 |
|
Leucocytes, /nl |
8.1±3.1 |
7.4±2.6 |
0.33 |
|
No., number; ICAM, intracellular adhesion molecule; VCAM, vascular cell adhesion molecule; AF, area fraction; p value, significance level. |
|||
Correlation analysis of immunohistological markers
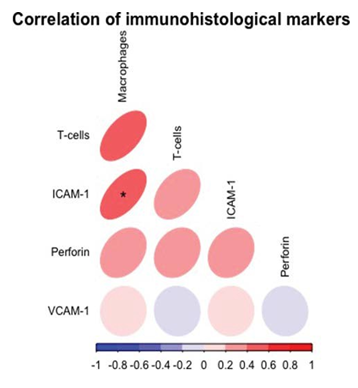
There was a moderate positive and significant correlation between ICAM-1 and macrophages (r=0.42, p <0.05) (Figure 1).
Correlation and regression analysis in patients with inflammatory processes
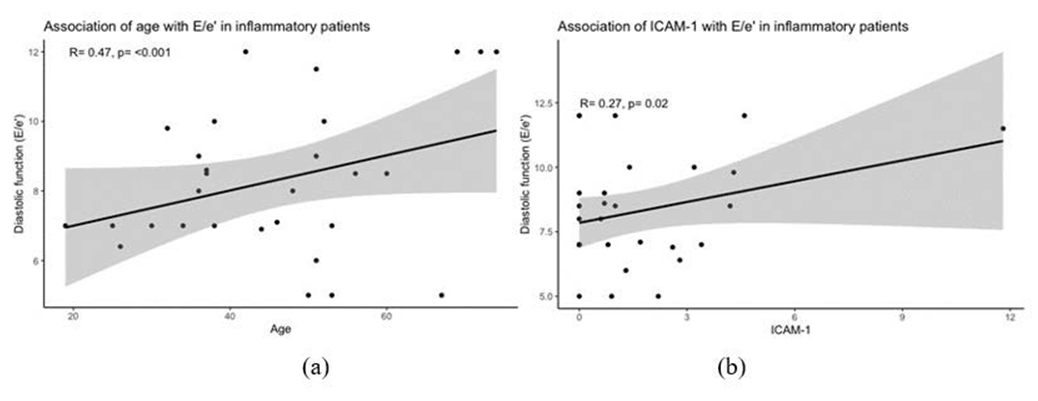
In the correlation analysis was a significant correlation between E/e’ and the following parameters: age (r=0.47, p <0.001), female sex (r=0.24, p <0.05), CD11+cells (r=0.37, p <0.001), CD3+T-cells (r=0.38, p <0.001), perforin+cells (r=0.34, p <0.05) and ICAM-1 (r=0.27, p <0.05) (Figures 2 and 3). In univariable linear and logistic regression analysis of the significant parameters obtained from correlation analysis, age (OR: 1.410, 95% CI: 1.040-1.751, p <0.05) and ICAM-1 (OR: 1.395, 95% CI: 1.029-1.780, p <0.05) remained significantly associated with E/e’ (Table 3).
There was a significant correlation between LAVI and the following parameters: age (r=0.15, p <0.05) and fibrosis (r=0.23, p <0.05). In univariable logistic regression analysis, LAVI was significantly associated with fibrosis (OR: 1.507, 95% CI: 1.163-1.851, p <0.05).
Correlation and regression analysis in patients without inflammatory processes
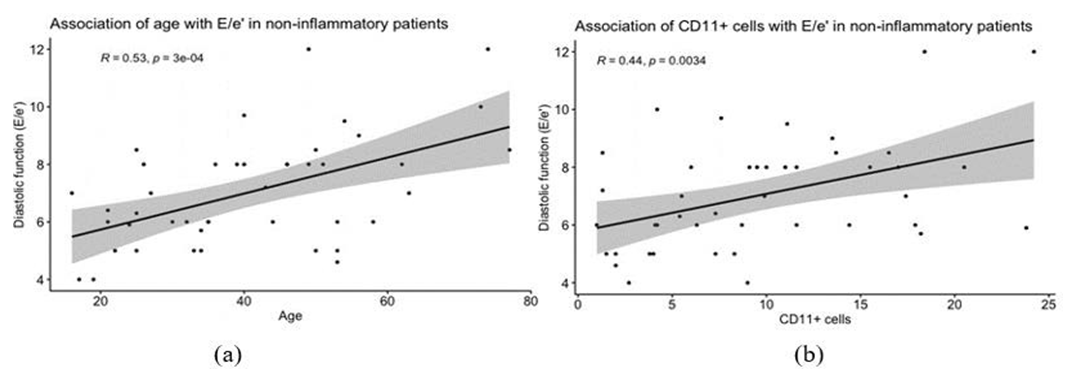
In the correlation analysis was a significant correlation between E/e’ and the following parameters: age (r=0.53, p <0.001), female sex (r=0.24, p <0.05) and CD11+cells (r=0.44, p <0.01) (Figure 4).
In univariable linear and logistic regression analysis of the significant parameters obtained from correlation analysis, age (OR: 1.619, 95% CI: 1.430-1.951, p <0.001), female sex (OR: 1.449, 95% CI: 1.164-1.733, p <0.05) and CD11+cells (OR:
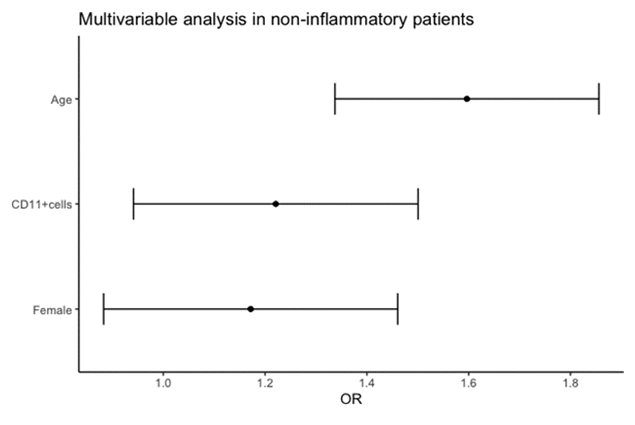
1.548, 95% CI: 1.273-1.823, p <0.05) remained significantly associated with E/e’ (Table 4). After multivariable-adjusted logistic regression analysis, age (OR: 1.597, 95% CI: 1.337- 1.856, p <0.001) remained significantly associated with E/e’ (Table 5, Figure 5). There was a significant correlation between LAVI and age (r=0.23, p <0.05), that remained significant in univariable analysis (OR: 1.332, 95% CI: 1.040-1.626, p <0.05).
Table 3: Univariable regression analysis in patients with inflammatory processes; LAVI, left atrial volume index; p value, significance level.
|
Variables |
Coefficient |
Adjusted OR (95% CI) |
p value |
|
E/e’ |
|||
|
Age |
3.335 |
1.410 (1.040, 1.751) |
<0.001 |
|
Sex (female) |
1.561 |
0.985 (0.607, 1.362) |
0.94 |
|
CD11+cells |
9.425 |
1.190 (0.724, 1.475) |
0.63 |
|
T-cells |
2.004 |
1.223 (0.852, 1.591) |
0.30 |
|
Perforin+cells |
1.091 |
1.115 (0.740, 1.490) |
0.57 |
|
ICAM-1 |
3.331 |
1.395 (1.029, 1.780) |
<0.05 |
|
LAVI |
|||
|
Age |
1.045 |
1.110 (1.735, 1.485) |
0.59 |
|
Fibrosis |
4.102 |
1.507 (1.163, 1.851) |
<0.05 |
Table 4: Univariable regression analysis in patients without inflammatory processes; LAVI, left atrial volume index; p value, significance
level.
|
Variables |
Coefficient |
Adjusted OR (95% CI) |
p value |
|
E/e’ |
|||
|
Age |
5.324 |
1.619 (1.430, 1.951) |
<0.001 |
|
Sex (female) |
3.493 |
1.449 (1.164, 1.733) |
<0.05 |
|
CD11+cells |
4.496 |
1.548 (1.273, 1.823) |
<0.05 |
|
LAVI |
|||
|
Age |
2.874 |
1.332 (1.040, 1.626) |
<0.05 |
Table 5: Multivariable regression analysis in patients without inflammatory processes; variables were standardized (per SD) before inclusion;
p value, significance level.
|
Variables |
Coefficient |
Adjusted OR (95% CI) |
p value |
|
E/e’ |
|||
|
Age |
4.679 |
1.597 (1.337, 1.856) |
<0.001 |
|
Sex (female) |
1.584 |
1.172 (0.883, 1.461) |
0.29 |
|
CD11+cells |
1.997 |
1.221 (0.941, 1.501) |
0.17 |
Discussion
The present analysis showed that (i) ICAM-1 plays an important role in inflammatory response in symptomatic patients with preserved ejection fraction prior to diastolic dysfunction. Moreover we showed that (ii) immunohistological markers interact significantly among each other (macrophages and ICAM-1) and correlated significantly with highly normal (reference < 14) E/e’ values.
Studies in human myocardium or patients prior to HFpEF development are limited [25]. The endothelium involves the endothelial cells of the coronary microvasculature and of the intramyocardial capillaries. Cardiovascular comorbidities such as obesity, metabolic syndrome or hypertension related to HFpEF leading to systemic and cardiac microvascular inflammation and subsequently to endothelial activation [5,11,26]. Pro-inflammatory properties dominate this condition affecting primarily the coronary microvascular endothelium [27-29].
Systemic and cardiac inflammation influence endothelial cell activity significantly in a way that CAMs are upregulated and overexpressed during these processes. Consistent with our findings, the role of CAMs like E-selectin, ICAM-1, perforin or VCAM-1 in the development of HF and especially HFpEF has been previously described [11,22, 30-34].
Recent studies showed increased ICAM-1 levels as significant driver in the development of HFpEF. Franssen et al. found, that ICAM-1 was upregulated in myocardial samples of HFpEF patients, but not in those with HFrEF [8]. Another very interesting finding is that from Salvador et al., where HFpEF murine models with a deficient ICAM-1 expression had less pro-inflammatory monocyte infiltration leading to less fibrotic changes [35]. In our study, patients with higher ICAM-1 levels had significantly increased NT-proBNP and E/e’ values. The higher prevalence of ICAM-1 revealed the endothelial activation and microvascular inflammation in these patients and the comorbidity-induced pro-inflammatory status [36]. Patel et al. showed that CAMs were associated with worse diastolic dysfunction but the effect was weakened by adjustment for covariates [37]. This finding strengthened the assumption that the relation of CAMs with diastolic dysfunction is explainable by the comorbidity burden [37]. Interestingly, we found that patients with higher ICAM-1 levels had significantly more comorbidities than those with lower ICAM-1 levels.
The expression of adhesion molecules favors myocardial infiltration of inflammatory cells. There is an increased promotion and activation of pro-fibrotic macrophages in HFpEF patients [36]. Furthermore, Hulsmans and colleagues highlighted the role of cardiac macrophages in the development of diastolic dysfunction [16]. Our findings regarding the macrophages were consistent with previous studies due to the increased macrophage level in the EMBs of our patients. There was a strong association of macrophages with ICAM-1 and higher E/e’ values. Our findings strengthened the assumption that these immunohistological markers can often be found in the early phase of inflammatory processes in HF development.
Macrophage activation can occur either in the M1 phenotype with pro-inflammatory properties or in the M2 phenotype with anti-inflammatory properties. Glezeva et al. demonstrated a M2 macrophage activation in the HFpEF pathogenesis [36]. We focused in our study on the macrophage number and did not consider the macrophage phenotype. Additionally, the endothelial activation triggers the endothelial-to-mesenchymal transition (EndMT), whereby endothelial cells are converted to mesenchymal- cells resulting in fibroblasts to develop cardiac fibrosis [38]. Moreo and colleagues proved in 252 patients that severe myocardial fibrosis correlated significantly with the degree of diastolic dysfunction [39]. Hypertrophy and fibrosis leading to cardiac function impairment are significantly more common in HFpEF patients compared to those without [25]. In our analysis, more than half of our patients had already fibrotic changes and not that much less hypertrophy. We found a significant correlation of LAVI with fibrosis in our study. Experimental models of diabetes induced an increased endothelial expression of endothelin-1 and therefore EndMT and fibrosis [40].
In our correlation and linear regression analysis, age was significantly associated with higher E/e’ values (OR: 1.410). HFpEF is often considered a disease of the elderly and its prevalence increases significantly with age, as reported in a sub-analysis of the EPICA study (8%-10% in women and 4%-6% in men > 80 years) [41,42].
There are considerable sex-differences in the area of HFpEF, particularly concerning sex-specific inflammatory mechanisms contributing to disease development and progression [41,43]. Results from the Framingham Heart Study showed the odds of HFpEF were 2.8-fold higher in women compared to men [44]. We found a trend in form of a significant correlation between female sex and higher E/e’ values, however results remained statistically non-significant in further analysis. This may be explainable due to the absence of diastolic dysfunction and clear evidence of HFpEF in our patients. Although no universally accepted consensus exists on sex-specific inflammatory mechanisms in HFpEF [41]. General hypotheses include greater endothelial dysfunction and systemic microvascular inflammation that occur earlier in women [41,45]. Beyond traditional risk factors of HFpEF that have been extensively discussed, female-specific risk factors involving sex hormones, pregnancy-related disorders and reproductive aspects may provoke a more severe inflammatory response [41]. Evidence suggests that sex hormones are of particular importance in the regulation of inflammatory response. While higher levels of estrogen downregulate inflammatory mechanisms such as nitric-oxide signaling and production of reactive oxygen species, the decline at menopause is linked to systemic inflammation and contribute to the pathogenesis of HFpEF [46]. Additionally, patients with pregnancy-related disorders like preeclampsia are at greater risk to develop HFpEF [47].
CAMs have been strongly correlated with HFpEF prevalence [8], but the chronological order from endothelial activation to subclinical alterations in cardiac function and the definitive manifestation of HFpEF remains to be clarified [37]. Interestingly, in the Coronary Artery Risk Development in Young Adults (CARDIA) trial was shown that ICAM-1and E-selectin levels in young adulthood preceded subclinical HFpEF in midlife, in the form of impaired systolic function measured by LV-global longitudinal strain (GLS) [37]. Both, diastolic and systolic dysfunction are driver in HFpEF development and have a decisive prognostic impact, as reported in larger clinical trials [48,49]. In our analysis, no significant associations between inflammatory markers and LVEF as a marker of cardiac systolic function were seen. Thus, LV-GLS add some valuable prognostic information beyond conventional LVEF to quantify LV contractile performance and to identify subclinical HFpEF [48-50].
Limitation of the study
There are limitations of a retrospective analysis which have to be considered when interpreting the obtained results and possible effects of selection bias cannot be denied. The number of patients is low that limits the power of our analysis. We investigated patients across different cardiovascular disease etiologies. This limits the accuracy of our results to describe a common inflammatory pathway in the development of HFpEF.
Conclusion
In summary, our data provide further evidence for the importance of ICAM-1 in the early stage of inflammatory response and endothelial dysfunction in symptomatic patients with preserved ejection fraction prior to diastolic dysfunction development.
Because the current success of treatments in terms of symptomatic improvement and prognosis for HFpEF patients has been limited, a personalized therapy option with suppression of microvascular inflammation and endothelial protective strategies may have potential benefit in preventing development of diastolic dysfunction. This hypothesis needs to be proven in large randomized trials.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding
This research was supported by a ProFIT grant cofounded by European Regional Development Fund, grant numbers 10169096.
Acknowledgments
The authors thank Susanne Ochmann, Kitty Winter, Jenny Klostermann, Katrin Errami (IKDT Berlin) for their skillful technical assistance.
Informed Consent / Ethics Statement
Informed consent was obtained from all subjects involved in the study and approved by Ethics Committee of Charité - University Medicine Berlin (EA4/236/20).
Supplementary Material:
None
Data Availability Statement
All relevant data is contained within the article:
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
References
- Bayes-Genis A, Bisbal F, Núñez J, et al., Transitioning from preclinical to clinical heart failure with preserved ejection fraction: a mechanistic Journal of Clinical Medicine 9 (2020): 1110.
- Paulus WJ, Tschöpe A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. Journal of the American College of Cardiology 62 (2013): 263-271.
- Sanders-van Wijk S, Tromp J, Beussink-Nelson L, et al., Proteomic Evaluation of the Comorbidity-Inflammation Paradigm in Heart Failure With Preserved Ejection Fraction: Results From the PROMIS-HFpEF Circulation. 2020;142(21):2029-2044.
- Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nature Reviews Cardiology. 11 (2014): 507-515.
- Kass DA, Bronzwaer JG, Paulus WJ. What mechanisms underlie diastolic dysfunction in heart failure? Circulation research 94 (2004):1533-1542.
- Ergatoudes C, Schaufelberger M, Andersson B, et al., Non-cardiac comorbidities and mortality in patients with heart failure with reduced vs. preserved ejection fraction: a study using the Swedish Heart Failure Clinical Research in Cardiology108 (2019): 1025-1033.
- Shah SJ, Lam CS, Svedlund S, et al., Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS- HFpEF. European heart journal 39 (2018): 3439-3450.
- Franssen C, Chen S, Unger A, et , Myocardial microvascular inflammatory endothelial activation in heart failure with preserved ejection fraction. JACC: Heart Failure 4 (2016): 312-324.
- Borbély A, Van Der Velden J, Papp Z, et , Cardiomyocyte stiffness in diastolic heart failure. Circulation. 111 (2005): 774-781.
- Westermann D, Lindner D, Kasner M, et al., Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection Circulation: Heart Failure 4 (2011): 44-52.
- Simmonds SJ, Cuijpers I, Heymans S, et al., Cellular and molecular differences between HFpEF and HFrEF: a step ahead in an improved pathological Cells 9 (2020): 242.
- Dai Z, Aoki T, Fukumoto Y, et , Coronary perivascular fibrosis is associated with impairment of coronary blood flow in patients with non-ischemic heart failure. Journal of cardiology 60 (2012): 416-421.
- Westermann D, Kasner M, Steendijk P, et , Role of left ventricular stiffness in heart failure with normal ejection fraction. Circulation 117 (2008): 2051-2060.
- Paulus WJ. Unfolding discoveries in heart failure. New England Journal of Medicine 382 (2020): 679-682.
- Kloch M, Stolarz-Skrzypek K, Olszanecka A, et , Inflammatory markers and left ventricular diastolic dysfunction in a family- based population study. Kardiologia Polska (Polish Heart Journal) 77 (2019): 33- 39.
- Hulsmans M, Sager HB, Roh JD, et , Cardiac macrophages promote diastolic dysfunction. Journal of Experimental Medicine 215 (2018): 423-440.
- Patel RB, Colangelo LA, Bielinski SJ, et al., Circulating Vascular Cell Adhesion Molecule-1 and Incident Heart Failure: The Multi-Ethnic Study of Atherosclerosis (MESA). Journal of the American Heart Association 9 (2020): e019390.
- Osborn L, Hession C, Tizard R, et al., Direct expression cloning of vascular cell adhesion molecule 1, a cytokine- induced endothelial protein that binds to lymphocytes. Cell 59 (1989): 1203-1211.
- Redfield Understanding" diastolic" heart failure. New England Journal of Medicine 350 (2004): 1930- 1931.
- Van Heerebeek L, Paulus W. Understanding heart failure with preserved ejection fraction: where are we today? Netherlands Heart Journal 24 (2016): 227-236.
- Escher F, Kühl U, Lassner D, et al., Long- term outcome of patients with virus-negative chronic myocarditis or inflammatory cardiomyopathy after immunosuppressive Clinical Research in Cardiology 105 (2016): 1011-1020.
- Escher F, Kühl U, Lassner D, et al., Presence of perforin in endomyocardial biopsies of patients with inflammatory cardiomyopathy predicts poor European journal of heart failure 16 (2014): 1066-1072.
- Escher F, Aleshcheva G, Pietsch H, et al., Transcriptional Active Parvovirus B19 Infection Predicts Adverse Long-Term Outcome in Patients with Non-Ischemic Biomedicines 9 (2021):1898.
- R Core R: A Language and Environment for Statistical Computing. Vienna, Austria (2017).
- Mohammed SF, Hussain S, Mirzoyev SA, et , Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 131(2015): 550-559.
- Burlew BS, Weber Cardiac fibrosis as a cause of diastolic dysfunction. Herz 27 (2002): 92-98.
- Tschöpe C, Van Linthout New insights in (inter) cellular mechanisms by heart failure with preserved ejection fraction. Current heart failure reports 11 (2014): 436-444.
- Van Heerebeek L, Hamdani N, Handoko ML, et , Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation 117 (2008): 43-51.
- Brunner H, Cockcroft JR, Deanfield J, et al., Endothelial function and Part II: Association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Fac- tors of the European Society of Hypertension. Journal of hypertension 23 (2005): 233-246.
- Shah SJ, Borlaug BA, Kitzman DW, et al., Research priorities for heart failure with preserved ejection fraction: national heart, lung, and blood institute working group Circulation 141 (2020): 1001-1026.
- Juni RP, Kuster DW, Goebel M, et , Cardiac microvascular endothelial enhancement of cardiomyocyte function is impaired by inflammation and restored by empagliflozin. JACC: Basic to Translational Science 4 (2019): 575-591.
- Widlansky ME, Gokce N, Keaney JF, et al., The clinical implications of endothelial dysfunction. Journal of the American College of Cardiology 42 (2003): 1149-1160.
- Katz SD, Hryniewicz K, Hriljac I, et , Vascular endothelial dysfunction and mortality risk in patients with chronic heart failure. Circulation 111 (2005): 310-314.
- Haraldsen G, Kvale D, Lien B, et al., Cytokine-regulated expression of E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) in human microvascular endothelial The Journal of Immunology 156 (1996): 2558-2565.
- Salvador AM, Nevers T, Velázquez F, et al., Intercellular adhesion molecule 1 regulates left ventricular leukocyte infiltration, cardiac remodeling, and function in pressure overload-induced heart failure. Journal of the American Heart Association 5 (2016): e003126.
- Glezeva N, Voon V, Watson C, et , Exaggerated inflammation and monocytosis associate with diastolic dysfunction in heart failure with preserved ejection fraction: evidence of M2 macrophage activation in disease pathogenesis. Journal of cardiac failure 21 (2015): 167-177.
- Patel RB, Colangelo LA, Reiner AP, et , Cellular adhesion molecules in young adulthood and cardiac function in later life. Journal of the American College of Cardiology. 2020;75(17):2156-2165.
- Zeisberg EM, Tarnavski O, Zeisberg M, et , Endothelial- to-mesenchymal transition contributes to cardiac fibrosis. Nature medicine 13 (2007): 952-961.
- Moreo A, Ambrosio G, De Chiara B, et al., Influence of myocardial fibrosis on left ventricular diastolic function: noninvasive assessment by cardiac magnetic resonance and Circulation: Cardiovascular Imaging 2 (2009):437-443.
- Widyantoro B, Emoto N, Nakayama K, et , Endothelial cell-derived endothelin-1 promotes cardiac fibrosis in diabetic hearts through stimulation of endothelial-to- mesenchymal transition. Circulation 121 (2010): 2407- 2418.
- Kaur G, Lau Sex differences in heart failure with preserved ejection fraction: From traditional risk factors to sex-specific risk factors. Women’s Health. 2022;18:17455057221140209.
- Ceia F, Fonseca C, Mota T, et al., Prevalence of chronic heart failure in Southwestern Europe: the EPICA study. European journal of heart failure. 2002;4(4):531-539.
- Smereka Y, Ezekowitz HFpEF and sex: understanding the role of sex differences. Canadian Journal of Physiology and Pharmacology. 2024;102(8):465-475
- Ho JE, Gona P, Pencina MJ, et , Discriminating clinical features of heart failure with preserved vs. reduced ejection fraction in the community. European heart journal. 2012;33(14):1734-1741.
- Beale AL, Meyer P, Marwick TH, et al., Sex differences in cardiovascular pathophysiology: why women are overrepresented in heart failure with preserved ejection Circulation. 2018;138(2):198-205.
- Sabbatini AR, Kararigas G. Menopause-related estrogen decrease and the pathogenesis of HFpEF: JACC review topic of the week. Journal of the American College of 2020;75(9):1074-1082.
- Williams D, Stout MJ, Rosenbloom JI, et , Preeclampsia predicts risk of hospitalization for heart failure with preserved ejection fraction. Journal of the American College of Cardiology. 2021;78(23):2281-2290.
- Kraigher-Krainer E, Shah AM, Gupta DK, et , Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. Journal of the American College of Cardiology. 2014;63(5):447-456.
- Shah AM, Claggett B, Sweitzer NK, et al., Prognostic importance of impaired systolic function in heart failure with preserved ejection fraction and the impact of Circulation. 2015;132(5):402-414.
- Brann A, Miller J, Eshraghian E, et , Global longitudinal strain predicts clinical outcomes in patients with heart failure with preserved ejection fraction. European Journal of Heart Failure. 2023;25(10):1755-1765.


 Impact Factor: * 5.6
Impact Factor: * 5.6 Acceptance Rate: 74.36%
Acceptance Rate: 74.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 