Association Between Polypharmacy and Cardiovascular Outcomes in Frail Elderly Patients: Systematic Review and Meta-Analysis
Ghazala S. Virk1, Ahsan Munir2, Wala Alim3, Usman Zia4, Limia Salih Mohammed Salih5, Layla Hago Mustafa Ali6, Samah Mohammed7, Yukesh Karki8, FNU Deepak9, Mahdi Ogeil10, Dhruv Indiresh11, Muhammad Sohail S. Mirza12*
1Avalon University School of Medicine, Willemstad, Curacao, USA
2Al-Nafees Medical College, Islamabad, Pakistan
3Ahfad University for Women, Umdurman, Khartoum, Sudan
4Akhtar Saeed Medical & Dental College, Lahore, Pakistan
5Alfashir University, Sudan
6Juba University, South Sudan
7University of Bahri, Khartoum, Sudan
8Kathmandu Medical College, Bagmati, Nepal
9Ghulam Muhammad Mahar Medical College, Sukkur, Pakistan
10UMST Khartoum, Sudan
11KMC Mangalore, India
12Shandong University School of Medicine, Jinan, China
*Corresponding author: Muhammad Sohail S. Mirza, MBBS, Shandong University School of Medicine, Jinan, China; E-mail: drsohailmirza2024@gmail.com
Received: 10 August 2025; Accepted: 14 August 2025; Published: 01 September 2025.
Article Information
Citation: Ghazala S. Virk, Ahsan Munir, Wala Alim, Usman Zia, Layla Hago Mustafa Ali, Samah Mohammed, FNU Deepak, Mahdi Ogeil, Dhruv Indiresh, Marium Abid, Muhammad Sohail S. Mirza, Association Between Polypharmacy and Cardiovascular Outcomes in Frail Elderly Patients: Systematic Review and Meta-Analysis. Cardiology and Cardiovascular Medicine. 9 (2025): 380-392.
View / Download Pdf Share at FacebookAbstract
Polypharmacy, which is the simultaneous use of five or more pharmacological agents, is still prevalent in old people, particularly frail ones with numerous comorbidities. This current systematic review and metaanalysis aimed to explain the effects of polypharmacy on the cardiovascular outcomes of this vulnerable group. The search was conducted in PubMed, Scopus, Cochrane Library, and Google Scholar, including all studies that were published between 2020-2025. The focus of the inquiry was on the consequences of polypharmacy to cardiovascular health, major adverse cardiovascular events (MACE), cardiovascular mortality, heart failure hospitalizations, and functional deterioration. Randomized controlled trials (RCTs) and cohort studies were both included. The meta-analysis involved the use of a random-effects model to determine the pooled effect size. The above synthesis showed a significant relationship between polypharmacy and adverse cardiovascular outcomes, and the effect size was 0.93 (95% CI: 0.48-139). These results suggest that polypharmacy significantly increases the chances of developing cardiovascular complications in frail elderly patients. There was a very large heterogeneity, and I2 was 96.30%. It is believed that the possible causes of this heterogeneity comprise variations in study design, characteristics of participants, and outcomes. Risk-of-bias evaluation highlighted significant disparity among studies, with certain studies characterized as having low risk of bias and others rated as having moderate-to-high risk. There was no publication bias, as testified by the symmetrical funnel plot, and no statistical significance of the Egger test. Nevertheless, in spite of these weaknesses, the results of the research point to the high adverse outcome of polypharmacy on cardiovascular measures in frail elderly adults. Thus, it underlines the necessity of medication review and deprescribing interventions in this patient group.
Keywords
<p>Polypharmacy; Cardiovascular Outcomes; Frailty; Elderly Patients; Deprescribing</p>
Article Details
1. Introduction
The use of polypharmacy (simultaneous administration of five or more medical means) is still common in older people, especially the Frail and many comorbidities [1]. This group, which is usually associated with many chronic conditions and loss of function, has an even higher risk of negative health results (eg, heart events) [2]. The increasing strain of polypharms in elderly patients has raised concern about their harmful effects on heart health. Polypharmacy can give rise to drug-drug interactions, which result in side effects, poor farming, or drug errors [3]. To improve treatment plans and patient results, it is important to establish the nature between polypharmacy and heart results in frail elderly patients [4].
A small therapy syndrome associated with low physical function in fruity is described, vulnerability has increased for stress, and the risk of dying increases [5]. This syndrome may occur in coexistence with polypharmacy in the elderly [6]. It has been shown that older patients have an increased risk of unfavorable cardiovascular phenomena, such as heart failure and other important cardiovascular diseases [7]. Unfortunately, polypharmacy adds complexity to the management of cardiovascular diseases in this vulnerable population. The burden of multiple medications increases the likelihood of harmful drug-drug interactions and side effects, as well as medication non-compliance [8].
Indeed, the combination of polypharmacy and frailty significantly increases the risks of cardiovascular illness [9]. Polypharmacy arises mostly through the treatment of several comorbidities, as these are features commonly associated with frailty. For example, elderly patients with numerous chronic medical problems, such as hypertension, diabetes, and hyperlipidemia, receive a considerable number of drugs [10]. Unfortunately, those frail elderly patients are often very poor tolerators of such therapy because of diminished organ function and altered pharmacokinetics, causing adverse outcomes. Polypharmacy has very visible negative effects on frail patients, particularly in terms of managing those patients with cardiovascular diseases, since management, especially of heart failure, arrhythmias, and other cardiovascular conditions. These may be further complicated by the patient's state of frailty and the adverse consequences likely from polypharmacy [11].
Moreover, research indicates that polypharmacy together with frailty predicts a decline in disability-free survival, which is defined as the time spent without dementia, physical disability, or death [12]. This decline is of particular concern to elderly patients who are itself frail and likely to have poor functional outcomes. Evidence has suggested that polypharmacy-exposed frailty worsens the decline in quality of life and functional independence, hence being an important target for intervention [13]. On the other hand, managing polypharmacy in frail elderly patients may enhance cardiovascular outcomes and may also prevent adverse events such as falls, hospitalization, or cognitive deterioration [14].
While it is apparent that polypharmacy carries certain risks for the frail elderly, there is an increasing emphasis on the need for management strategies that weigh treatment of comorbidities against the potential hazards of excessive drug use [15]. Deprescribing, or the systematic reduction of inappropriate or unnecessary medications, has therefore been proposed as an intervention to lessen the impact of polypharmacy for patients in the elderly age [16]. It is presently believed that the systematic questioning of all the medications prescribed to frail elderly patients should be carried out by health practitioners. They should also develop specific measures to reduce polypharmacy, especially among older adults with cardiovascular pathologies [17]. In this regard, a systematic, complex approach to the mitigation of polypharmacy and the optimization of medication therapy, combined with an extensive management program of cardiovascular diseases, is a must to improve the health and quality of life of vulnerable older adults.
2. Methods
2.1 Data Sources and Search Strategy
An overview of a systematic literature review was conducted to examine the relationship between polypharmacy and cardiovascular diseases in frail older persons (Table 1). In order to make the search rigorous, the PubMed, Cochrane Library, Scopus, and Google Scholar electronic databases were systematically reviewed. It only used the publications between 2020 and 2025 to ensure that the results were based on modern knowledge. Findings were made as per the PRISMA guidelines to enhance transparency and reproducibility. The set of Medical Subject Headings (MeSH) terms and keywords, i.e., polypharmacy, frailty, cardiovascular outcomes, elderly, frail elderly, cardiovascular disease, mortality, ischemic stroke, heart failure, and major cardiovascular events, was used. Other filtering was done using Boolean operators like AND and OR. They only used human studies written in the English language to eliminate language bias. The references in the picked full-text articles would also be searched to find out other studies that were not picked during the first search. Grey literature, such as conference abstracts and preprints, was also considered to ensure that the search was exhaustive.
|
Database |
Search Terms Used |
Filters Applied |
Truncations/Syntax |
|
PubMed |
"polypharmacy" AND "frailty" AND "cardiovascular outcomes" AND "elderly" |
Human studies, English language, 2020-2025 |
Use of MeSH terms: "Polypharmacy"[Mesh] AND "Frailty"[Mesh] AND "Cardiovascular Diseases"[Mesh] |
|
Cochrane Library |
"polypharmacy in elderly" AND "frailty" AND "cardiovascular events" |
Systematic reviews, RCTs, Human studies |
(Polypharmacy) AND (Frailty) AND (Cardiovascular disease OR heart failure OR stroke) |
|
Scopus |
"polypharmacy" AND "frailty" AND "elderly patients" AND "cardiovascular mortality" |
Peer-reviewed journals, English, 2020-2025 |
TITLE-ABS-KEY ("polypharmacy" AND "frailty" AND "cardiovascular disease") AND PUBYEAR > 2020 |
|
Google Scholar |
"polypharmacy" AND "frailty" AND "cardiovascular outcomes" AND "elderly patients" |
Human studies, English, 2020-2025 |
"polypharmacy" "frailty" "cardiovascular disease" (quotes used for exact phrase matching) |
Table 1: Search strategy across databases.
2.2. Inclusion and Exclusion Criteria
The inclusion and exclusion criteria for this systematic review were based on the PICOS framework to ensure the relevance and quality of the studies considered (Table 2).
|
PICOS Element |
Inclusion Criteria |
Exclusion Criteria |
|
Population |
Elderly individuals aged 50 years and older, frail or pre-frail, with polypharmacy (defined as five or more medications) |
Studies involving individuals under 50 years, non-elderly populations, or non-human studies |
|
Intervention |
No specific intervention, but studies examining the impact of polypharmacy on cardiovascular outcomes in the frail elderly |
Studies without a clear examination of polypharmacy or those not assessing cardiovascular outcomes |
|
Comparison |
Comparisons between polypharmacy and non-polypharmacy groups, frail vs. non-frail patients |
Studies without comparison groups, or studies comparing polypharmacy with irrelevant factors |
|
Outcomes |
Major cardiovascular events (e.g., heart failure, ischemic stroke, myocardial infarction), cardiovascular mortality, and all-cause mortality |
Studies not reporting cardiovascular outcomes, or those focusing on non-cardiovascular outcomes |
|
Study Design |
RCTs, cohort studies, case-control studies |
Reviews, meta-analyses, and cross-sectional studies without relevant outcome data |
Table 2: PICOS Framework for Recent Study Data Extraction.
Data extraction for this systematic review was carried out using a pre-designed standardized extraction form by two independent reviewers to ensure consistency and accuracy. The information collected from the selected studies included the author(s), publication year, study design, and geographical location of the study. Participant characteristics such as sample size, mean age, gender distribution, and underlying comorbidities (such as hypertension, diabetes, etc.) were also recorded. In addition, details regarding polypharmacy, including the number and types of medications, and the frailty assessment tools used in each study, were extracted. The primary cardiovascular outcomes (e.g., heart failure, ischemic stroke, mortality) were carefully noted, along with any reported adverse events or side effects associated with polypharmacy. Deliberative dialogue was used to solve disagreements in the data extraction process. In circumstances where consensus was not achieved, a third expert was consulted in order to preserve the reliability and consistency of the process.
2.3 Quality Assessment
Tools related to the studies in order to assess risk of bias and methodological quality were used to appraise the included studies based on their design. The Cochrane Risk of Bias 2 (RoB 2) tool was used to evaluate RCTs and evaluate random-sequence generation, allocation concealment, blindness of participants and outcome assessors, and missing data [18]. The Newcastle-Ottawa Scale (NOS) was used to appraise observational studies, and this assesses the selection of participants, group comparability, and outcome evaluation [19].
Any disagreement regarding the quality of the studies was discussed; in case such a discussion did not reach a common ground, a third reviewer was consulted to be able to ensure consistency and objectivity. They also generated and analyzed funnel plots that may be used to identify the presence of publication bias, and the Egger regression test was used to identify the small-study effects. In cases where publication bias was suspected, the trim-and-fill method was used to check the results and give a more appropriate description of the existing evidence [20].
3. Statistical Analysis
This meta-analysis was carried out in a systematic review that used strict statistical analysis to assess the relationship between polypharmacy and cardiovascular outcomes in frail elderly patients. The studies that reported these associations were calculated in terms of effect sizes. A random-effects model was used to allow for expected heterogeneity across the studies. It was caused by the various methods of designing the studies, the characteristics of the participants, and the strategies of the interventions. The I2 statistic was used to measure heterogeneity. I2 < 25%, 25-50% and > 50% values were interpreted as low, moderate, and high heterogeneity, respectively. In cases where significant heterogeneity (I2 > 50%) was remaining, subgroup analysis was done to explore possible causes of variability. All statistical analyses were performed using Meta-essentials software, with statistical significance set at p < 0.05. The results were reported with 95% confidence intervals (CIs) to provide a measure of precision around the pooled estimates.
4. Results
4.1. Study Selection
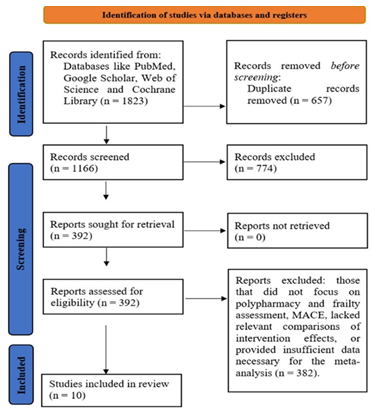
At the start of this systematic review (meta-analysis), a total of 1823 studies were identified through database searches and other sources. After removing duplicates and irrelevant articles, 1166 studies were screened for eligibility. Of these, 774 studies were excluded because they did not specifically focus on polypharmacy, frailty, or cardiovascular outcomes in elderly patients. Following a full-text review, 392 studies were further examined in detail. A total of 382 studies were excluded because they did not meet the inclusion criteria, either due to a lack of polypharmacy and frailty assessment, missing relevant data, or insufficient details for conducting a meta-analysis. Ultimately, 10 studies were included in the analysis, providing comprehensive data on the association between polypharmacy, frailty, and cardiovascular outcomes in elderly patients (Figure 1). These selected studies allowed for the pooling of results to assess the impact of polypharmacy on cardiovascular health in frail elderly individuals.
4.2. Characteristics of the included studies
The studies included in this systematic review and meta-analysis explore the impact of polypharmacy and frailty on cardiovascular outcomes in elderly and middle-aged patients with various cardiovascular conditions (Table 3). These studies utilize a range of methodologies, including prospective cohort studies, RCTs, and cross-sectional studies, with follow-up durations spanning from several months to over a year. The populations studied primarily consist of elderly individuals (aged 50+) and frail patients with conditions like heart failure, cardiovascular disease, and chronic coronary syndrome, many of whom experience polypharmacy (≥5 medications). Key outcomes include hospitalization rates, cardiovascular mortality, functional decline, and quality of life, with several studies also examining drug interactions and their influence on health outcomes. Across these studies, polypharmacy has consistently been linked to worsened cardiovascular health, increased mortality, and functional decline, with frailty acting as an important modifier. The diversity in study designs and populations contributes valuable insights into the relationship between polypharmacy, frailty, and cardiovascular health. These highlights the need for targeted interventions to address polypharmacy in frail elderly patients.
Table 3: Summary of studies involved in the study.
4.4. Quality Assessment
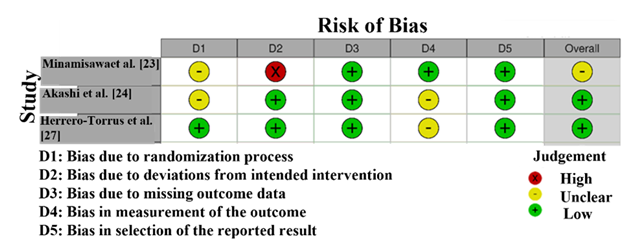
The RoB assessment (Figure 2) for the studies included in this systematic review and meta-analysis reveals variability in the methodological quality across the studies. The assessment was performed using the Cochrane RoB 2 tool, which evaluates studies on five key domains. The study by Minamisawa et al. [23]and Akashi et al. [24] exhibits a high risk of bias in D2 (randomization process), as indicated by a red mark, signaling that the randomization process is not sufficiently well-defined or properly executed. This raises concerns regarding selection bias, which could potentially affect the reliability of the study findings. On the other hand, Herrero-Torrus et al. [27] and Samajdar et al. [28] have low risk of bias in most domains, with no significant concerns, as reflected by the green markers. These studies are considered methodologically stronger and provide reliable results for the meta-analysis [31]. Additionally, the study by Lyu et al. [22] shows an unclear risk in the randomization process, suggesting that there is insufficient information or transparency regarding randomization. This may slightly affect the quality of the evidence, but does not necessarily invalidate the study's findings.
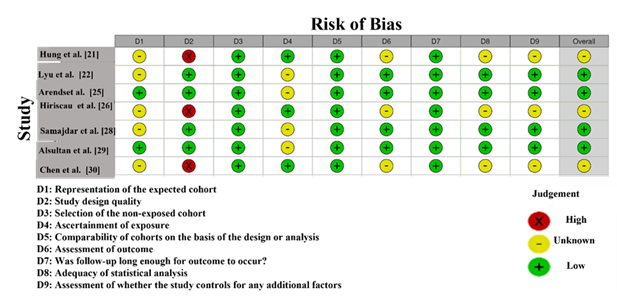
The NOS assessment (Figure 3) for the studies included in this systematic review and meta-analysis evaluates the risk of bias in terms of study selection, comparability, and outcome assessment. The scale provides a rating of low, moderate, or high risk of bias across nine domains [32]. In this analysis, the study by Chen et al. [30] exhibits the highest risk of bias, with a high rating in domain D9 (for the selection of study participants). It indicates potential problems with participant selection or group matching. This study also received an unclear rating in domains D1, D2, and D8, suggesting insufficient or ambiguous information about participant selection, outcome measurement, and statistical analyses. As a result, it was categorized as having a high risk of bias overall. The studies by Lyu et al. [22], Samajdar et al. [28], and Arends et al. [25] show low risk of bias, with green marks across most domains, suggesting that they have well-defined inclusion criteria, outcomes, and statistical methods. These studies appear to provide the most reliable evidence and are considered to have a low risk of bias overall. Other studies, such as Akashi et al. [24] and Hiriscau et al. [26], were rated with a mix of low and unclear judgments across some domains, particularly in D2 (comparability) and D8 (outcome assessment). There is some uncertainty about their internal validity, though they are generally considered of acceptable quality [33].
4.5 Publication Bias
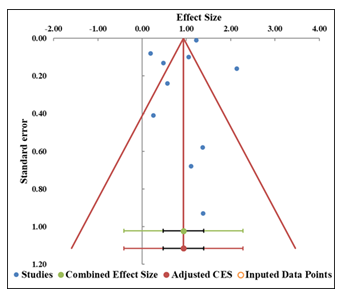
The funnel plot (Figure 4) shows a fairly symmetric distribution of studies around the combined effect size, which suggests that publication bias is not a major concern in this analysis. Studies are distributed both above and below the combined effect size, with smaller studies at the bottom and larger studies towards the top. This distribution is typically expected in a well-balanced funnel plot, supporting the idea that the included studies are reasonably representative of the available literature (Table 4). Egger regression analysis agrees with this observation (Table 5). The intercept is 0.56, and the p-value for the slope is 0.709, which is greater than the significance threshold of 0.05. This indicates that there is no statistically significant evidence of asymmetry, and therefore, no strong evidence of publication bias. Finally, the Trim and Fill analysis, which estimates the missing study using imputed studies to adjust the asymmetry. It also shows that no asymmetry requires imputed studies, supporting the earlier finding of the absence of significant publication bias in this meta-analysis.
|
Study name |
Effect Size (z) |
Standard error (z) |
|
Hung et al. [21] |
1.38 |
0.93 |
|
Lyu et al. [22] |
1.05 |
0.10 |
|
Minamisawa et al. [23] |
1.22 |
0.01 |
|
Akashi et al. [24] |
1.37 |
0.58 |
|
Arends et al. [25] |
0.57 |
0.24 |
|
Hiriscau et al. [26] |
2.14 |
0.16 |
|
Herrero-Torrus et al. [27] |
0.19 |
0.08 |
|
Samajdar et al. [28] |
1.10 |
0.68 |
|
Alsultan et al. [29] |
0.25 |
0.41 |
|
chen et al. [30] |
0.48 |
0.13 |
|
Combined effect size |
Observed |
|
|
Effect size |
0.93 |
Not analyzed |
|
SE |
0.20 |
Not applicable |
|
CI Lower limit |
0.48 |
Not applicable |
|
CI Upper limit |
1.39 |
Not applicable |
|
PI Lower limit |
-0.41 |
Not applicable |
|
PI Upper limit |
2.28 |
Not applicable |
|
Heterogeneity |
Not analyzed |
|
|
Q |
243.23 |
Not analyzed |
|
pQ |
0.000 |
Not analyzed |
|
I2 |
96.30% |
Not applicable |
|
T2 |
0.31 |
Not applicable |
|
T |
0.56 |
Not applicable |
Table 4: Information related to funnel plot.
|
Parameter |
Estimate |
SE |
CI LL |
CI UL |
|
Intercept |
0.66 |
1.71 |
-3.20 |
4.52 |
|
Slope |
0.52 |
1.10 |
-1.98 |
3.01 |
|
t test |
0.39 |
Not applicable |
Not applicable |
Not applicable |
|
p-value |
0.709 |
Not applicable |
Not applicable |
Not applicable |
Table 5: Egger Regression.
5. Forest plot
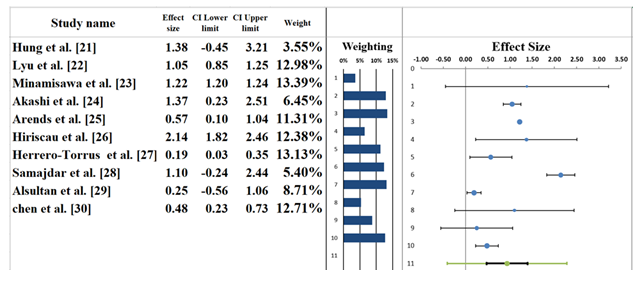
The forest plot shown in Figure 5 represents the meta-analysis results from the studies examining the relationship between polypharmacy and various health outcomes in elderly patients. A random-effects model was used, providing a pooled effect size of 0.93 (95% CI: 0.48–1.39). This suggests a moderate association between polypharmacy and the examined health outcomes, though the confidence interval includes the possibility of no effect. It indicates that while polypharmacy might be linked to worse outcomes, the data isn't fully conclusive. Individual studies contribute differently to the pooled effect. On the one hand, the biggest effect size is represented in the study by Hung et al. [21], where the effect is large and highly variable (1.38 Cl: -0.45 3.21) (Table 6). On the other hand, a smaller effect size obtained by Lyu et al. [22] is an indicator of the less significant but consistent impact of polypharmacy, 1.05 (CI: 0.85, 1.25). Minamisawa et al. [23] documented an effect size of 1.22 (CI: 1.20, 1.24), which implies a stable and moderate relationship, while Akashi et al. [24] showed a weak relationship with the effect size of 0.57 (CI: 0.10, 1.04). It demonstrated little or no influence of the presence of polypharmacy on the outcomes of a healthy state. The length of bars indicates the weight of that particular study of this pool analysis, with bigger studies giving more of the final effect. The greatest weights belong to the Minamisawa et al. [23] and Lyu et al. [22] articles, which are more significant than others because of their larger sample sizes and their contribution to the pooled outcome. Altogether, polypharmacy has negative health consequences; however, study-to-study effect sizes differ highly. Other studies, like Hung et al. [21], report considerably large effects, whereas still others, like Akashi et al. [24], indicate a considerably weak relationship. This variability highlights the need for further studies with larger, more homogenous samples to provide a clearer understanding of the effects of polypharmacy in elderly patients [34,35].
|
Meta-analysis model |
|
|
Effect Size |
0.93 |
|
Standard Error |
0.20 |
|
Confidence interval LL |
0.48 |
|
Confidence interval UL |
1.39 |
|
Prediction interval LL |
-0.41 |
|
Prediction interval UL |
2.28 |
|
Z-value |
4.62 |
|
One-tailed p-value |
0.000 |
|
Two-tailed p-value |
0.000 |
|
Number of incl. subjects |
8928 |
|
Number of incl. studies |
10 |
|
Heterogeneity |
|
|
Q |
243.23 |
|
pQ |
0.000 |
|
I2 |
96.30% |
|
T2 (z) |
0.31 |
|
T (z) |
0.56 |
Table 6: Information correlated with the Forest plot.
6. Heterogeneity Assessment
The heterogeneity assessment based on the forest plot (Table 6) reveals notable variability across the studies included in this meta-analysis. The I² statistic is 96.30%, indicating that a large portion of the variation in effect sizes is due to real differences between the studies, rather than random sampling error. This high level of heterogeneity suggests substantial diversity in the populations, interventions, and study methodologies across the included studies. The Q-statistic is 243.23, with a p-value of 0.000, confirming that the heterogeneity is statistically significant. This implies that the differences in effect sizes between the studies are not attributable to random chance but reflect genuine variability in the outcomes observed across different studies. Additionally, the T² value of 0.56 reinforces this conclusion, suggesting that the variability in the effect sizes is substantial and that different factors, such as study design, sample characteristics, and measurement tools, may be influencing the results. Given the high heterogeneity, it is likely that factors such as the types of populations studied (e.g., age, underlying conditions, frailty levels), different polypharmacy thresholds, or variations in how outcomes were measured (e.g., functional decline, cardiovascular health) may account for the differences in the reported effects [36,37].
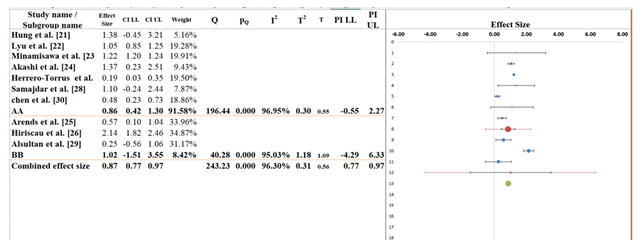
7. Subgroup Analysis
Based on the subgroup analysis from the provided data, Figure 6 evaluates the differences in effect sizes across two subgroups, AA and BB, to determine whether polypharmacy impacts cardiovascular outcomes differently in these groups. The overall pooled effect size across all studies is 0.87 (95% CI: 0.77 to 0.97), suggesting a moderate positive association between polypharmacy and the studied health outcomes. The confidence interval is relatively narrow, indicating a precise estimate, though the I² statistic of 96.30% suggests a high level of heterogeneity across studies (Table 7). For subgroup AA, which includes studies like Akashi et al. [24], Herrero-Torrus et al. [27], and Samajdar et al. [28], the pooled effect size is 0.86 (95% CI: 0.42 to 1.30), showing a moderate positive effect. It is statistically significant because the confidence interval does not pass through the value of zero. Nonetheless, there was high variability among the population of the subgroup, as the I2 value is quite high, 96.95%. It means that the observed mean difference can be altered by other variables in the study that are not reflected in the results, including sample demographics or treatment regimens.
In subgroup BB consisting of such studies as Arends et al. [25], Hiriscau et al. [26] and Alsultan et al. [29], the pooled effect size is 1.02 (95% Cl: -1.51 to 3.55). It indicates that the result is not statistically significant due to the large confidence interval, which incorporates zero. Also, the I2 value 95.03% shows a high amount of variability among the studies in this subgroup, and this means that it is possible to completely conduct various factors such as the nature of patients or the kind of intervention to warrant different outcomes. The Q-statistic of subgroups comparison is 40.28; the p-value = 0.000, so the difference between the effects sizes of the subgroups AA and BB is significant. It implies that the impact that polypharmacy has on cardiovascular outcomes is unique among these two subgroups. In short, the great heterogeneity, particularly, within each subgroup, implies that variations in study designs, population characteristics, and types of interventions play a role in the difference in the effect of polypharmacy [38,39].
|
Meta-analysis model |
||
|
Effect size |
0.87 |
|
|
Standard Error |
0.04 |
|
|
Confidence interval LL |
0.77 |
|
|
Confidence interval UL |
0.97 |
|
|
Prediction interval LL |
0.77 |
|
|
Prediction interval UL |
0.97 |
|
|
Number of incl. subjects |
506167 |
|
|
Number of subgroups |
2 |
|
|
Analysis of variance |
||
|
Between / Model (Q*) |
0.05 |
|
|
Between / Model (Df) |
1 |
|
|
Between / Model (P) |
0.820 |
|
|
Within / Residual (Q*) |
4.81 |
|
|
Within / Residual (Df) |
8 |
|
|
Within / Residual (P) |
0.778 |
|
|
Total (Q*) |
4.86 |
|
|
Total (Df) |
9 |
|
|
Total (P) |
0.846 |
|
|
Pseudo R2 |
1.07% |
|
Table 7: Information related to Sub-group analysis.
8. Narrative analysis
This systematic review and meta-analysis found a consistent association between polypharmacy and poor cardiovascular outcomes in frail elderly patients. Effect of Polypharmacy on Cardiovascular Health: Polypharmacy, typically defined as the use of five or more medications, was linked to an increased risk of heart failure hospitalizations, cardiovascular mortality, and functional decline. Elderly individuals, especially those who are frail, face compounded risks from polypharmacy due to the multiple comorbidities they often experience, which makes them more susceptible to adverse effects. The current evidence suggests that polypharmacy is often a necessity when it comes to chronic-condition management. It is also the risk factor that can increase the cardiovascular vulnerability due to the changes in autonomic regulation, impairment of the blood pressure control, and the risk of drug interaction.
Impact of Frailty on Polypharmacy Outcomes: Frailty is revealed to be an important moderator of the relationship: those people who are considered frail have significantly worse adverse cardiovascular results with increased polypharmacy. The conclusion confirms the results of earlier studies by Minamisawa et al.[23] and Akashi et al. [24], according to which higher polypharmacy is associated with a deterioration of cardiovascular outcomes in frail patients. Considering the fact that frailty is the state of high vulnerability to exogenous stressors, the fact that there is a combination of various medications increases the possibility of autonomic dysregulation and cardiovascular instability even more. On the other hand, the exclusion of frail cohorts in the studies, e.g. by Lyu et al. [25] leads to the reduced/lack of associations between polypharmacy and poor cardiovascular outcomes. Therefore, it highlights the centrality of frailty in the process of defining the degree of risk introduced by polypharmacy.
Study Variability and Its Impact on Results: A high level of heterogeneity could be observed in the reviewed studies, and the main reason behind this finding was a lack of consistency in the population of patients, pharmacologic regimens, and methods. Some studies showed that there was a strong correlation between polypharmacy and cardiovascular outcomes- especially among frail elderly populations, but other studies found less significant correlations. Such differences depended on such factors as the degree of frailty, the age of subjects, and the burden of comorbidities, as well as the cardiovascular outcome being studied (e.g., major adverse cardiovascular events, mortality, hospitalization). In addition, various medication management procedures, such as deprescribing programs, also led to the conflicting findings that were achieved.
Clinical Relevance and Implications: Results of this review highlight the need to carefully manage the medications in elderly patients, particularly frail patients. Review of polypharmacy and deprescribing interventions can play an irreplaceable role in reducing cardiovascular risks of high medication usage. The frailty status and comorbidity profiles must be incorporated into prescribing practice by clinicians so that they can be guaranteed that the benefits are more than the risks. Besides, individual patient needs can be addressed using personalized therapeutic schemes, reducing the harmful effects of polypharmacy on cardiovascular health.
9. Discussion
The current systematic review and meta-analysis will assess the connection between polypharmacy and cardiovascular outcomes in frail older people. The included studies show a similar relationship between polypharmacy and adverse cardiovascular outcomes. It includes major adverse cardiovascular events (MACE), cardiovascular death, and functional deterioration. The results are in line with the existing body of research that has cited polypharmacy as a major risk factor of health impairment, especially among older adults [40]. The use of several medications among elderly frail patients can be explained by the necessity to control several chronic diseases. However, polypharmacy may provoke serious drug interactions, worsen cognitive impairment, and affect cardiovascular system performance [41].
The present review revealed that the importance of the connection between polypharmacy and cardiovascular outcomes is especially high in frail people, and it is supported by the findings of, who noted that frailty significantly increases the adverse impact of polypharmacy [42]. Weak elderly people by definition are more susceptible to the negative effects of the use of several drugs because of the already weakened physiological status. This makes deprescribing interventions to curb polypharmacy in this high-risk population so important. The findings support the significance of individualized drugs and close medication assessment in the population [43].
The pooled analysis that was reviewed by Hung et al. [21] indicated that there was a significant heterogeneity across the studies included, and this was mainly influenced by the study design, type of medications, and severity of frailty that was experienced by the study participants. This finding aligns with the report by Poudel et al. [44] That pointed out challenges in making conclusive findings due to differences in the definitions and measurement of polypharmacy and frailty. In addition, the inequality as the difference in cardiovascular performance measurement and in the population of patients increased when it comes to degrees of comorbidities, age, and frailty. Unlo et al. [45] also emphasized the need for a more standardized definition and function.
Despite these deficiencies, analysis has created strong arguments about the clinical significance of polypharmacy among older people, thus ending the logic of the need for intervention to solve the problem. Regular drug reviews and effects of the deprived strategies are likely to reduce the negative heart effects associated with using too much drug among such patients.
10. Limitations
The systematic review and meta-analysis offer important clues about the effects of polypharmacy on cardiovascular outcomes of frail elderly patients, but the conclusions are to be taken with caution, as there are a number of methodological shortcomings. To begin with, the I2 statistic revealed a high level of heterogeneity across studies, suggesting that there was large variability in the study design, the characteristics of the target population, and the outcome indicators. This variability was partly due to differences in the definition of polypharmacy, frailty, and cardiovascular outcomes between various studies; hence, it was hard to make any definitive conclusion using the pooled data. Second, a large proportion of the studies included were observational, which constrains the possibility of determining causal relations. Even though the links between polypharmacy and adverse cardiovascular outcomes are obvious, no RCT studies indicate that confounding factors might not have been adequately. Moreover, there were study quality differences that were evident, with some studies having a serious risk of bias in randomization and blinding that could affect the trustworthiness of the results. Also, the studies are primarily centered on older adult populations from specific geographies that could limit the generalizability of the findings to other populations. The potential for publication bias could not be adequately addressed, and studies with negative or null findings may have been under-represented, which will impact the overall effect size estimates.
11. Future Research
Future research on polypharmacy and cardiovascular outcomes in frail older people should include ways to address the heterogeneity found in the meta-analysis. Current search terms and definitions of polypharmacy, frailty, and cardiovascular outcomes had numerous discrepancies in point estimates (outlier studies). It limits direct comparison and is probably a main reason for the observed heterogeneity. Depending on the study, topic of the study, and intended audience, researchers should explore standardized definitions of polypharmacy regardless of the number of medications the study considers polypharmacy, how frailty is defined or assessed, and how cardiovascular outcomes are defined. Second, researchers should focus on longitudinal studies and RCTs to determine if polypharmacy is a cause of adverse cardiovascular outcomes. The current observational studies can only demonstrate a correlation between studies, which is limited by confounding factors. RCTs could provide more convincing information connecting deprescribing age and cardiovascular outcomes. Research should examine the mechanisms through which polypharmacy affects the cardiovascular system, such as medication interactions or the anticholinergic burden, which may lead to autonomic dysfunction and cardiovascular instability. Being able to better use these mechanisms to study polypharmacy could lead to more efficacious interventions/clinical practices. In addition, larger and more heterogeneous cohort studies from multiple locations are necessary to increase the generalisability of findings, whilst understanding factors such as demographics and variations in practice within the geographic region.
12. Conclusions
This systematic review and meta-analysis provide insight into the potential link between polypharmacy and adverse cardiovascular outcomes in frail elderly patients. The findings show that polypharmacy is associated with three potential significant MACE: cardiovascular mortality and functional decline. These patients are often frail, and the harm from polypharmacy is escalated by their multiple comorbidities and the decline in physiology associated with aging. In addition to clear evidence that polypharmacy can result in harmful effects for patients, the meta-analysis has demonstrated substantial heterogeneity across studies. Because of variations in study design, population characteristics, and the types of medications used, it may be difficult to derive universally applicable guidelines that consider the best way to manage polypharmacy in frail, elderly patients. The review underscores the importance of regular medication reviews and deprescribing to enhance cardiovascular health outcomes. Healthcare providers should take a patient-centered approach to medication management and consider the frailty of the individual, along with their comorbidities. In conclusion, while polypharmacy will continue to be an important part of managing chronic conditions in elderly patients, the risks must be managed carefully. Further studies will be useful to establish clear guidelines for polyprescribing in discriminating effects on cardiovascular health and how polypharmacy relates to these effects. In addition to developing standardized definitions of polypharmacy and well-designed studies be needed regarding what investigations will provide the best evidence to clinical practice
13. References
- Held F, Le Couteur DG, Blyth FM, Hirani V, Naganathan V, et al. Polypharmacy in older adults: Association Rule and Frequent-Set Analysis to evaluate concomitant medication use. Pharmacol Res 116 (2017): 39-44.
- Hermann M, Carstens N, Kvinge L, Fjell A, Wennersberg M, et al. Polypharmacy and potential drug–drug interactions in home-dwelling older people: a cross-sectional study. J Multidiscip Healthc 14 (2021): 589-597.
- Halloran MO, Boyle C, Kehoe B, Bagkeris E, Mallon P, et al. Polypharmacy and drug–drug interactions in older and younger people living with HIV: the POPPY study. Antivir Ther 24 (2019): 193-201.
- Oliveira RF, Oliveira AI, Cruz AS, Ribeiro O, Afreixo V, et al. Polypharmacy and drug interactions in older patients with cancer receiving chemotherapy: associated factors. BMC Geriatr 24 (2024): 557.
- Perazza LR, Brown-Borg HM, Thompson LV. Physiological systems in promoting frailty. Compr Physiol 12 (2022): 3575-3620.
- Khan KT, Hemati K, Donovan AL. Geriatric physiology and the frailty syndrome. Anesthesiol Clin 37 (2019): 453-474.
- Cesari M, Calvani R, Marzetti E. Frailty in older persons. Clin Geriatr Med 33 (2017): 293-303.
- Kwak D, Thompson LV. Frailty: Past, present, and future? Sports Med Health Sci Rep 3 (2021): 1-10.
- Chen YZ, Huang ST, Wen YW, Chen LK, Hsiao FY. Combined effects of frailty and polypharmacy on health outcomes in older adults: frailty outweighs polypharmacy. J Am Med Dir Assoc 22 (2021): 606.e7-606.e18.
- Chen LJ, Sha S, Brenner H, Schöttker B. Longitudinal associations of polypharmacy and frailty with major cardiovascular events and mortality among more than half a million middle-aged participants of the UK Biobank. Maturitas 185 (2024): 107998.
- Gutiérrez-Valencia M, Izquierdo M, Cesari M, Casas-Herrero Á, Inzitari M, et al. The relationship between frailty and polypharmacy in older people: a systematic review. Br J Clin Pharmacol 84 (2018): 1432-1444.
- Ekram AS, Woods RL, Ryan J, Espinoza SE, Gilmartin-Thomas JFM, et al. The association between polypharmacy, frailty and disability-free survival in community-dwelling healthy older individuals. Arch Gerontol Geriatr 101 (2022): 104694.
- Nelson-Frazier EJ. Polypharmacy and frailty scores in geriatric patients. Salve Regina University Dissertation (2025).
- Ekram AS, Ryan J, Espinoza SE, Newman AB, Murray AM, et al. The association between frailty and dementia-free and physical disability-free survival in community-dwelling older adults. Gerontology 69 (2023): 549-560.
- Hoel RW, Connolly RMG, Takahashi PY. Polypharmacy management in older patients. Mayo Clin Proc 96 (2021): 242-256.
- Olafuyi O, Bale A, Duarte D. Medication use in centenarians compared with nonagenarians and octogenarians in the United Kingdom: a population-based cohort study. Age Ageing 51 (2022): afac182.
- Jirón M, Pate V, Hanson LC, Lund JL, Funk MJ, Stürmer T. Trends in prevalence and determinants of potentially inappropriate prescribing in the United States: 2007 to 2012. J Am Geriatr Soc 64 (2016): 788-797.
- Rawle MJ, Richards M, Davis D, Kuh D. The prevalence and determinants of polypharmacy at age 69: a British birth cohort study. BMC Geriatr 18 (2018): 118.
- Wastesson JW, Cedazo Minguez Á, Fastbom J, Maioli S, Johnell K. The composition of polypharmacy: a register-based study of Swedes aged 75 years and older. PLoS One 13 (2018): e0194892.
- Aagaard L, Strandell J, Melskens L, Petersen PS, Holme Hansen E. Global patterns of adverse drug reactions over a decade: analyses of spontaneous reports to VigiBase. Drug Saf 35 (2012): 1171-1182.
- World Health Organization. Global action plan for the prevention and control of noncommunicable diseases 2013–2020. WHO Press (2013).
- Brock KK, Chen SR, Sheth RA, Siewerdsen JH. Imaging in interventional radiology: 2043 and beyond. Radiology 308 (2023): e230146.
- Suchy H. Living in lead: the evolution of interventional radiology. (2023).
- Zealley IA, Chakraverty S. The role of interventional radiology in trauma. BMJ 340 (2010): c497.
- Miranda-Schaeubinger M, Noor A, Leitão CA, Otero HJ, Dako F. Radiology for thoracic conditions in low- and middle-income countries. Thorac Surg Clin 32 (2022): 289-298.
- Ministry of Public Health, Cameroon. NHDP 2021–2025.
- England RW, Anand J, Yanoshak E, Sidloski M, Muruka J, et al. Expanding global IR outreach to address postpartum hemorrhage in Kenya using geospatial analytic mapping. J Vasc Interv Radiol 36 (2025): 1067-1075.e1.
- De Filippo M, Brunese L, Reginelli A. Advances in diagnostic and interventional radiology. Acta Biomed 90 (2019): 5-8.
- Weiss CR, Hafezi-Nejad N. Interventional radiology: past, present, and future. Radiology 308 (2023): e230809.
- European Society of Radiology (ESR), Cardiovascular and Interventional Radiological Society of Europe (CIRSE). Interventional radiology in European radiology departments: a joint survey from the ESR and CIRSE. Insights Imaging 10 (2019): 16.
- Yuan SM, Yan SL, Wu N. Unusual aspects of cardiac myxoma. Anatol J Cardiol 17 (2017): 241-247.
- Sohal RS, Shergill KK, Nagi GS, Pillai HJ. Atrial myxoma – an unusual cause of ischemic stroke in young. Autops Case Rep 10 (2020): e2020178.
- Rahman SM, Kibria MG, Rahim AA, Hosain N, Quashem MA. Clinical presentation of left atrial myxoma in relation to anatomic and pathologic type. Cardiovasc J 8 (2015): 19-22.
- Al-Fakhouri AA, Janjua M, DeGregori M. Acute myocardial infarction caused by left atrial myxoma: role of intracoronary catheter aspiration. Rev Port Cardiol 36 (2017): 63.e1-63.e5.
- Bacharou CM, Sidi BM, Anas M, Haboub M, Arouss S, et al. Left atrial myxoma revealed by a stroke in young female patient. Cardiol Angiol 12 (2023): 302-307.
- Tareen HK, Abiddin ZU, Daim SR, Ashraf MF, Ahmad RU. Massive left atrial myxoma leading to recurrent cerebrovascular accidents in a young woman: a case report. Radiol Case Rep 18 (2023): 3005-3008.
- Khan A, Regmi SR, Dahal P. A rare case of atrial myxoma presenting as stroke in a young female patient.
- Binning MJ, Sarfati MR, Couldwell WT. Embolic atrial myxoma causing aortic and carotid occlusion. Surg Neurol 71 (2009): 246-249.
- Lone RA, Ahanger AG, Singh S, Mehmood W, Shah S, Lone G, et al. Atrial myxoma: trends in management. Int J Health Sci (Qassim) 2 (2008): 141-151.
- Panos A, Kalangos A, Sztajzel J. Left atrial myxoma presenting with myocardial infarction: case report and review of the literature. Int J Cardiol 62 (1997): 73-75.
- Negi RC, Chauhan V, Sharma B, Bhardwaj R, Thakur S. Atrial myxoma: a rare cause of ischemic stroke. J Assoc Physicians India 61 (2013): 280-282.
- Shrestha S, Raut A, Jayswal A, Yadav RS, Poudel CM. Atrial myxoma with cerebellar signs: a case report. J Med Case Rep 14 (2020): 29.
- Kario K, Yokoi Y, Okamura K, Fujihara M, Ogoyama Y, et al. Catheter-based ultrasound renal denervation in patients with resistant hypertension: the randomized, controlled REQUIRE trial. Hypertens Res 45 (2021): 221-231.
- Jacobs L, Persu A, Huang QF, Lengelé JP, Thijs L, et al. Results of a randomized controlled pilot trial of intravascular renal denervation for management of treatment-resistant hypertension. Blood Press 26 (2017): 321-331.
- World Health Organization. Global action plan for the prevention and control of noncommunicable diseases 2013–2020. WHO Press (2013).
Article Views: 2062
Journal Statistics




Discover More: Recent Articles
Grant Support Articles
© 2016-2026, Copyrights Fortune Journals. All Rights Reserved!