Assesment of Sequestration Potential and Biomass Accumulation of Managed Mangrove Plantations of Mida Creek, Kilifi County Kenya
Ogola Kevin Omollo1*, Najma Dharani1, Benard’s Okeyo2
1Department of Plant Sciences, Kenyatta University, Nairobi, Kenya
2Department of Environmental Sciences, Pwani University, Mombasa-Malindi Highway: Kilifi county, Kenya
*Corresponding Author: Ogola Kevin Omollo Department of Plant Sciences, Kenyatta University, P.O BOX 34844-00100, Nairobi, Kenya
Received: 24 March 2021; Accepted: 06 April 2021; Published: 15 April 2021
Article Information
Citation: Ogola Kevin Omollo, Najma Dharani, Benard’s Okeyo. Assesment of Sequestration Potential and Biomass Accumulation of Managed Mangrove Plantations of Mida Creek, Kilifi County Kenya. International Journal of Plant, Animal and Environmental Sciences 11 (2021): 233-250.
View / Download Pdf Share at FacebookAbstract
Mangrove forests play a very significant role along the coastal environment throughout the tropical coast. They provide ecosystem services that are able to sustain both flora and faunal organisms found in such ecosystems. They are store large quantities of carbon in their biomass hence referred to as carbon sinks. This carbon can be emitted into the atmosphere when mangrove forests are degraded through unsustainable utility. The main objective of this study is to quantify the amount of carbon sequestered by the managed mangrove forest of the Mida Creek, Kenya. Three sites were selected for this study; Kibusa Plantation, Green Island Plantation, and a Natural Stand. Plots of (10 x10) m2 were selected in each study site. Three carbon pools were investigated; aboveground carbon, belowground carbon, and soil organic carbon. Biomass for carbon determination in Kibusa and Natural Stands was estimated using a general equation. Mean total carbon stocks in Kibusa and Green Island Plantations was 424.52±11.68 Mg C/ha and 958.57±50.01 Mg C/ha while the natural stand contained significantly higher total Carbon stocks of 2159.77±31.09 Mg C/ha (ANOVA, F0.05(1),2,6 = 262.91, P < 0.05). There was no significant difference in the amount of soil organic carbon among the three different sites (F0.05(1),2,15=0.35, p>0.05). This study indicates that reforestation enhances structural development of replanted mangroves and that replanted mangroves are significant carbon stores. From these results, we can deduce that awareness should be raised among the community members on the need for conservation and management which will increase the amount of carbon sequestered since more mangroves increase the rate of carbon (IV) oxide capture. This will help in mitigating the issue of global warming at local levels.
Keywords
<p>Carbon sequestration; Global warming; Carbon sinks</p>
Article Details
Introduction
Background
Estimating carbon sequestration is important for quantifying the roles of mangrove forest as carbon sinks and for supporting sustainable forest management. The knowledge of carbon stocks and fluxes is essential to understand current states and future courses of the carbon cycle in response to changing land uses and climatic conditions [1]. The increase in growth of carbon trade and the need to resolve climate change has spawned a number of legal actions, policies and programs [2]. For example, part of the total carbon accounted for under the United Nations Framework Convention on Climate Change is reported to be contributed by forest carbon stocks for many developing and developed nations. In addition, section 1605 (b) of the Energy Policy Act for the USA has also allowed a voluntary greenhouse gas reporting program. Under this program, a report must be given by the organizations based on their sequestration rates and overall emission budgets [3].
The significance of mangrove forest biomass inventories is further mainstreamed by the necessity of raising our understanding of carbon fluxes within the ecosystems and the atmosphere. To that end, the total biomass found as living vegetation and debris in mangrove forest system is an important factor in definitely ascertaining how forestry helps to control atmospheric carbon dioxide levels at balance with oxygen levels [4]. To be specific, accurate forest biomass estimates are essential for the rising number of emissions of CO2 and other greenhouse gases [5]. Since these systems provide offsets for carbon sequestration, the desire for accuracy in biomass estimation methods is greater than ever before. This desire is further linked by the growing number of climate change agreements and action plans at variable scales. For instance, the House Bill 3543 passed in the legislative session of 2007 in the Oregon State Legislature declared that it is the state’s policy to reduce greenhouse gas to 10% below the 1990 levels by 2020 and to further reduce greenhouse gas to 25% below the 1990 levels by 2050.
Tree biomass estimates are the basis for US forest carbon inventories and most international negotiations [6]. The emergence of biomass as a critical variable in assessing sequestration of atmospheric carbon and in providing an important information to forest resource management and policy decision making has focused attention on its accuracy [7]. Local prediction accuracy of biomass and carbon maps developed for regional analyses (>4,000,000 ha) has been questioned by research scientists, land managers, and decision makers [8]. It is reasonable to seek information so as to add confidence in biomass estimates and map products. In this application, studies on improving the predictive ability of biomass estimation methods are crucial. Studies on mangrove carbon stocks are still limited in Kenya and are mostly focused on natural mangroves with no studies on restored mangroves.
The purpose of this paper is to establish the sequestration potential of the re-planted mangrove, focusing particularly on estimation of above and belowground biomass accumulation. Different methods are highlighted on how to determine the sequestration rates of replanted mangroves in the Mida Creek. In addition, I discuss on the variation in the amount of carbon stock in the replanted R. mucronata and Avicenia marina. Since the methods discussed here cover a broader aspect of biomass estimation rather than comparison of performance of certain techniques, I believe that the ideas are applicable to different species and across many different regions.
In the following sections, I first outlined different types of biomass estimation methods presently in use and classify the various carbon pools involved in determination of the sequestration potential. I then identified the various parameters that were used in estimating both aboveground and belowground carbon. The fourth section presents an overview of how selected strategies can be integrated with existing databases and knowledge and includes examples and comments on how local carbon conversion helps in local climate regulation and air purification. The final section summarizes my main points and provides the concluding remarks.
Materials and methods
Experimental design: The study was conducted in Mida Creek located in Kilifi County (03o21’S, 39o59’E). Mida Creek is located 88km north of Mombasa and 25 km south of Malindi [5]. Data collection exercise was done in two phases; dry season (January) and wet season (October). The samples were collected in three different sites, namely; the Green Island plantation, the Kibusa Plantation and the Natural forest stand. Quadrats of (10×10) m2 were made in the Natural stand for high density areas and (20×20) m2 for low density areas were randomly established basing on vegetation zone stratification. This was then followed by identification of individual trees whose diameter is greater than 2.5 cm. The parameters that were sampled for the two seasons on both the replanted vegetation stand and the natural stand were diameter at breast height (DBH); which was measured for each tree with a tape measure at 1.5 m above the ground [9], soil organic carbon (SOC), and root carbon biomass. The equation developed by Komiyama [15] was used in determination of the aboveground biomass (AGB) content (AGB =0.251ρD2.46).
Where; AGB is the aboveground biomass in kg, ρ= is the wood density in gcm-3, D= is the tree diameter at breast height in cm.
AGB Carbon content was calculated through multiplication of biomass content of the mangrove tree species by its specific carbon concentration using a default value of 0.5 [10]. Specific wood density values as developed by Bosire [5] were used for computing tree biomass. These values are as shown in the table 1 below:
Table 1: Specific densitiy for different mangrove species [5]
|
Mangrove species |
Wood density (gcm-3) |
|
Rhizophora mucronata |
1.1 |
|
Avicenia marina |
0.9 |
|
Bruguiera gymnorrhiza |
1.3 |
|
Ceriops tagal |
1.1 |
|
Xylocarpus granatum |
0.8 |
Below ground biomass was sampled using the improvised coring method according to Saintilan [11]. This method involves random selection of trees within the 10m by 10 m plots for root coring. Cores (60cm length and 14 cm diameter) were made in three 20cm vertical root profile; 0 to 20cm, 20 to 40cm, and 40 to 60cm at each of the parent root base. Each sample was then carried to the seashore and washed using a 1mm mesh sieve. Fresh live roots (brown in color) were then be put in a labeled carrier bag and kept in a refrigerator until processed. Roots were then separated into different diameter classes; i.e., 5mm, between 5-10mm, 10-20mm, 20-30mm, 30-40mm, 40-50mm, and larger than 50mm. The screened roots were then stored and dried at 70oC in an oven; to obtain the dry weight [12].
Soil samples were collected at varying depth profiles of 0-20 cm, 20-40 cm and 40-60 cm using a soil corer. The soil samples were then put in a carrier bag and stored in cool boxes for transfer in the laboratory for analysis.
Data analysis
Analysis was carried out using Microsoft Excel spreadsheet 2010 and Statistica 8. All data were tested for normality and homogeneity of variance and normalized where necessary for parametric tests. Mean values of biomass and carbon data sets that were collected from various representative sites of the Creek were subjected to significance tests using one-way ANOVA to compare the total variation in the above and below ground mean biomass and carbon accumulation. Descriptive and simple statistical calculations were used to determine root densities and vertical distribution of each species encountered.
Soil nutrient analysis was done using the procedure outlined by Okalebo [13] as discussed below:
An amount of 0.30g of ground soil was weighed into a clean, labelled 100-digestion tube and the weight recorded. 2ml of distilled water was added followed by 10ml 5% potassium dichromate solution. The above mixture was carefully titrated with 5ml sulphuric acid and digested at 150oC for 30 minutes. After cooling, 50ml of 0.4% barium chloride was added to the mixture; swirl and the volume topped up to 100 ml with distilled water. This was then left to stand overnight for a clear supernatant solution to form. An aliquot of the supernatant was then transferred into a colorimeter cuvette and the absorbance measured at 600nm.
The percentage of total organic carbon in the dry soil was calculated as follows:
% organic carbon ={(a-b) * 0.10}/w
Where, a=concentration of Cr3+ in the sample; b =concentration of chromic (III) ion in the blank; w= weight of soil taken for analysis [13].
Results
Carbon pools
Aboveground biomass
The data obtained during dry seasons showed that the total aboveground biomass in Kibusa Plantation, Green Island Plantation and the Natural stand was 246.938 t/ha, 325.4018 t/ha, and 3020.28 t/ha respectively. On the other hand, the data obtained during the wet season had a total aboveground biomass of 452.47125 t/ha, 450.416 t/ha, and 3666.63 t/ha in Kibusa Plantation, Green Island Plantation, and Natural Stands respectively. The wet season had a higher aboveground biomass compared to that of the dry season in all the study sites. There was variation in the percentage biomass contribution among the different mangrove species in all the three study sites for both dry and rainy season as shown in Table 2 below.
Table 2: Aboveground biomass contributions by different species encountered in the three different sites for both dry and wet season in Mida Creek, Kenya
|
Site |
Species encountered |
ABG t C/ha Dry season (January) |
% contribution |
ABG t C/ha Wet season (October) |
% contribution |
|
Natural stand |
A. marina |
1737.96 |
25.99 |
2175.79 |
32.54 |
|
B. gymnorrhiza |
679.16 |
10.16 |
819.71 |
12.26 |
|
|
C. tagal |
100.65 |
1.51 |
118.27 |
1.77 |
|
|
X. granatum |
46.57 |
0.70 |
52.78 |
0.79 |
|
|
R. mucronata |
455.95 |
6.82 |
500.08 |
7.49 |
|
|
Total ABG t C/ha |
3020.28 |
45.15 |
3666.63 |
54.85 |
|
|
Kibusa plantation |
A. marina |
37.04 |
5.30 |
45.62 |
6.52 |
|
B. gymnorrhiza |
36.93 |
5.28 |
70.83 |
10.13 |
|
|
C. tagal |
54.47 |
7.79 |
70.89 |
10.14 |
|
|
X. granatum |
4.39 |
0.70 |
5.23 |
0.77 |
|
|
R. mucronata |
114.10 |
16.31 |
259.91 |
37.16 |
|
|
Total t C/ha |
246.94 |
35.28 |
452.47 |
64.72 |
|
|
Green Island |
A. marina |
192.44 |
24.80 |
220.36 |
28.40 |
|
B. gymnorrhiza |
58.02 |
7.48 |
132.04 |
17.02 |
|
|
C. tagal |
37.17 |
4.79 |
38.59 |
4.97 |
|
|
R. mucronata |
37.78 |
4.87 |
59.43 |
7.66 |
|
|
Total t C/ha |
|
325.40 |
41.95 |
450.42 |
58.05 |
Avicenia marina had the highest biomass contribution in the natural stand followed by B. gymnorrhiza in both dry and wet season. However, in comparison to the dry season, biomass accumulation by the A. marina and B. gymnorrhiza was higher in the wet season. In Kibusa Plantation, biomass contribution by R. mucronata was highest in both dry and wet season. B. gymnorrhiza and C. tagal had almost similar biomass contribution in both dry and wet season. In the Green Island, A. marina had the highest aboveground biomass followed by B. gymnorrhiza in both dry and wet season. However aboveground biomass contribution for both A. marina and B. gymnorrhiza was higher in the wet season than in the dry season.
When 50% of the aboveground biomass is assumed to be carbon, then average aboveground carbon for Kibusa Plantation was computed and found to be 0.4783 t C/ha in the dry season and had a range between 0.01381 and 9.2731 t C/ha. In the wet season the average aboveground carbon was 0.6856 t C/ha and had a range of 0.003929 to 10.9778 t C/ha. Green Island Plantation had an average aboveground biomass of 1.162 t C/ha and had a range of 0.00797 to 21.413 t C/ha in the dry season. In the wet season, Green Island Plantation had an average carbon of 1.6499 t C/ha and had a range of 0.0047 to 26.8851 t C/ha. The Natural Stand had the highest aboveground biomass with an average carbon of 1.162 t C/ha and 13.048 in dry and wet season respectively. Table 3 below shows the amount of carbon sequestered between the two seasons from January to October.
Table 3: Sequestration potential of different mangrove species encountered in the three different sites within a period of 10 months in Mida Creek, Kenya
|
Site |
Species encountered |
ABG g C/ha Dry season (January) |
ABG g C/ha Wet season (October) |
Amount of carbon sequestered |
% sequestration potential |
|
Natural stand |
A. marina |
868.979 |
1087.896 |
218.917 |
67.740 |
|
B. gymnorrhiza |
339.579 |
409.853 |
70.275 |
21.745 |
|
|
C. tagal |
50.324 |
51.135 |
8.810 |
2.726 |
|
|
X. granatum |
23.283 |
26.388 |
3.105 |
0.961 |
|
|
R. mucronata |
50.324 |
250.040 |
22.067 |
6.828 |
|
|
Total ABG g C/ha |
1510.139 |
1833.313 |
323.174 |
100% |
|
|
Average |
10.710 |
13.048 |
|||
|
Range |
117.808 |
145.299 |
|||
|
Kibusa Plantation |
B. gymnorrhiza |
18.465 |
35.415 |
16.95 |
16.494% |
|
C. tagal |
27.236 |
35.444 |
8.207 |
7.986% |
|
|
X. granatum |
2.195 |
0.419 |
0.419 |
0.408% |
|
|
R. mucronata |
57.052 |
129.954 |
72.902 |
70.940% |
|
|
A. marina |
18.521 |
22.808 |
4.287 |
4.172% |
|
|
Total g C/ha |
123.469 |
226.236 |
102.76 |
100% |
|
|
Average |
0.6856 |
0.7483 |
|||
|
Range |
9.259 |
10.973 |
|||
|
Green Island Plantation |
B. gymnorrhiza |
29.007 |
66.020 |
37.012 |
56.423% |
|
C. tagal |
15.110 |
19.296 |
4.186 |
6.382% |
|
|
R. mucronata |
18.888 |
29.715 |
10.826 |
16.504% |
|
|
A. marina |
96.219 |
110.178 |
13.958 |
21.279% |
|
|
Total g C/ha |
|
159.225 |
225.208 |
65.983 |
100% |
|
Average |
|
1.162 |
1.650 |
||
|
Range |
|
21.405 |
26.885 |
.
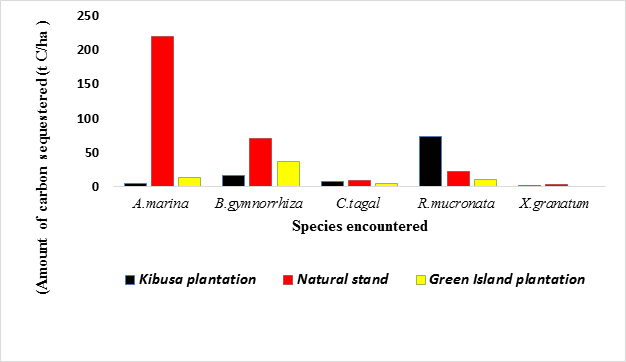
Within the period of ten months, the amount of carbon sequestered by A. marina in the Green Island Plantation, Kibusa Plantation and the Natural Stand was estimated to be 13.958 t C/ha, 4.287 t C/ha, and 218.917 t C/ha respectively. In contrast, the amount of carbon sequestered by R. mucronata within the ten-month period was estimated to be 22.067, 72.902, and 10.826 t C/ha in Green Island Plantation, Kibusa Plantation and Natural Stand respectively. The total amount of carbon sequestered by A. marina in all the three sites within the ten-month period was 237.162893 t C/ha while that sequestered by R. mucronata was 105.795908 t C/ha. The variation in sequestration potential among the mangrove species encountered in all the sites is summarized in Figure 1 below.
From the above figure, it is evident that R. mucronata had the highest amount of carbon sequestered within the ten-months period in Kibusa plantation followed by B. gymnorrhiza. In both Natural Stand and Green Island Plantation, A. marina had the highest amount of carbon sequestered within the ten-months period. In Kibusa Plantation, R. mucronata had the highest amount of carbon sequestered. X. granatum had the lowest sequestration potential in all the three sites.
Based on the ANOVA test done for the wet season there was a significant difference in the aboveground biomass among the three different plantations since the calculated F (12.76373) is greater than the critical F (F0.05(1),2,831=3.00065). This was also true for the dry season, where the calculated F (35.57665) was greater than the critical F (F0.05(1),2,882=3.00595). There was no significant difference in the aboveground biomass among the four different mangrove species (F0.05(1),3,195=2.65) in the Natural stand. On the other hand, in Kibusa plantation, there was a significant difference in the aboveground biomass among the four different mangrove species (F0.05(1),3,103=2.69).
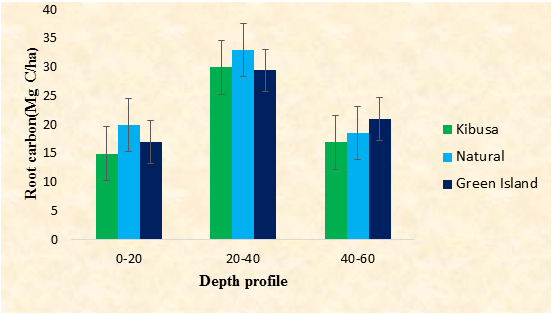
Belowground biomass
The natural stand had the highest root carbon concentration of 78.36 Mg C/ha, followed by the Green Island Plantation of 53.43 Mg C/ha. Kibusa Plantation had the lowest root carbon concentration of 47.34 Mg C/ha (Figure 2). There was no significant difference in root carbon concentration among the three study sites (F0.05(1),2,67=3.05, P<0.05). There was a significant difference in belowground biomass between the natural stand and the two plantations (P<0.05, Tukey test). There was a significant difference in belowground biomass within the different depth profiles among the sites (F0.05(1), 2, 67=5.14). There was no significant difference in root carbon concentration between Green Island Plantation and the Natural Stand (P<0.05, Turkey test). There was a significant difference in root carbon concentration between the depth profiles of 20-40cm and 40-60cm of the Kibusa Plantation and the other study sites (P<0.05, Tukey test). From the three study sites, the Kibusa Plantation had the lowest amount of root carbon concentration in all the three-depth profiles sampled i.e. 15 Mg C/ha, 30 Mg C/ha, and 17 Mg C/ha in 0-20 cm, 20-40cm, and 40-60cm respectively. In all the three study sites, root carbon concentration was highest at the depth of 20-40 cm, where Kibusa Plantation, Natural Stand, and the Green Island Plantation recorded 30 Mg C/ha, 33 Mg C/ha, and 29.5 Mg C/ha respectively.
Soil organic carbon (SOC)
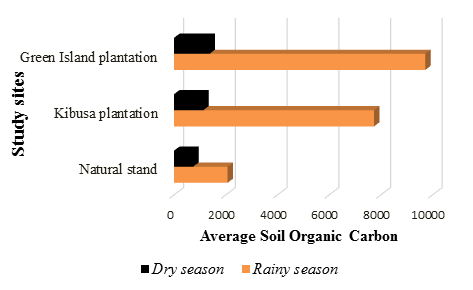
The Green Island Plantation had the highest amount of SOC in the rainy season with a mean of 9714.79±4732.56. This was followed by the Natural Stand, which had an average of 3544.25±1149.37. The Kibusa Plantation had the least SOC with a mean of 2156.27±736.50. There is a significant difference between the means of the samples from the two seasons since the calculated t (t=1.527) was less than the critical t (t=4.30) (t0.05(2),3=1.527). Figure 3 below summarizes the variation in SOC between the dry and rainy season.
In the dry season, there was no significance difference in the amount of SOC between the three different sites (F0.05(1),2,15=0.35, p>0.05). This was also true for the rainy season where there was no significance difference in the amount of SOC among the different sites (F0.05(1),2,15=3.55, p>0.05). Based on soil profile, SOC increased with increase in depth in both the Plantations and the Natural Stand. At a depth of 50-100cm, there was the highest amount of SOC while the depth of 0-15 had lowest amount of SOC in both dry and rainy season.
In Kibusa Plantation, 56.3% of the total SOC in the upper 100 cm was contributed by that obtained at 50-100 cm depth while that of the Green Island Plantation and the natural stand were 83.5% and 44.3% respectively. There was a more significant difference in soil organic carbon concentration between 50-100 cm depth profile and other depth profiles (P < 0.05, Tukey test).
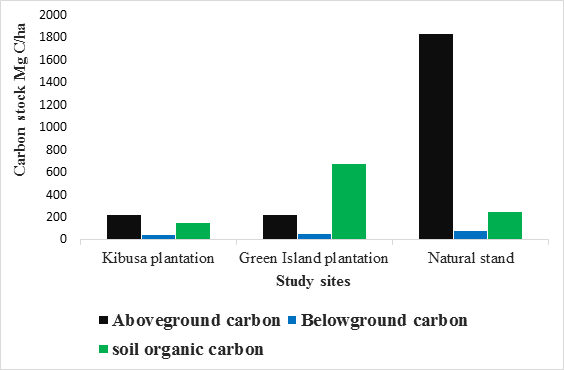
Total carbon stocks
This was done by adding all the carbon components accounted for during the study. Average total carbon stocks were estimated for the whole period of study. Both Kibusa and Green Island Plantation had an average of 424.52±11.68 and 958.57±50.01 respectively, while the Natural Stand had 2159.77±31.09. The total carbon stocks differed significantly between the sites (F0.05(1),2,6 = 262.91, P < 0.05). There was a significant difference between the two Plantations and Natural Stand (P < 0.05, Tukey test). Plantations had a significant amount of soil organic carbon compared to the Natural Stand (Table 4).
Table 4: Carbon stocks in Mg C ha-1 for various carbon pools of Natural Stand and Plantations of Mida Creek, Kenya
|
Carbon stock Mg C/ha |
Kibusa Plantation |
Green Island Plantation |
Natural Stand |
|
Aboveground carbon |
226.24±1.62 |
225.1±0.179 |
1833.31±1.04 |
|
Belowground carbon |
47.34±2.7 |
53.43±25 |
78.36±18.56 |
|
Soil organic carbon |
150.94±7.36 |
680.04±47.33 |
248.1±11.49 |
|
Total Carbon Stock |
424.52±11.68 |
958.57±50.01 |
2159.77±31.09 |
This information is summarized in Figure 4 below
Discussion
Carbon pools
Aboveground biomass (AGB) carbon
In this study, there was a significant difference in aboveground carbon among the three sites. The mangrove species in the Natural Stand had a high aboveground carbon compared to the Plantations. This can be due to the difference in the age, since they were older [14], difference in soil nutrient regime [15], differences in topography, light conditions, natural disturbances and their interactions [4]. The Kibusa and the Green Island Plantations had younger mangrove trees compared to the Natural Stand, which had trees for more than 51 years old [16]. This explains why the Natural Stand had a higher foliage and consequently higher primary productivity compared to the Plantations. In addition to this, the high species richness in the Natural Stand can also explain why there was higher aboveground carbon since it was a mixed stand compared to the Plantations [17]. Maintenance practices done on the Plantations such as pruning could also be a plausible reason to the low amount of aboveground carbon in Plantations since this leads to loss of biomass [18]. Other factors that might have contributed to the variation in the aboveground biomass include global positioning of the mangrove forest. In this case, mangroves located near the sea shore may differ from those in the offshore in terms of height and stem diameter due to the differences in the nutrient statuses, soil type, and wave effects in these zones [9]. Ecological differences between different mangrove positions may also contribute to the variations in the aboveground biomass in the mangrove species. This is due to the differences in the level of microbial activities that cause decomposition that leads to nutrient availability within a given plantation [3]. Plant age may also lead to differences in the AGB that is noticed within the three sites since in each year, in dicotyledonous [6].
Belowground biomass
The results obtained from the above study indicates that the Natural Stand had the highest belowground root carbon (78.36 Mg C/ha), followed by the Green Island Plantation (53.43 Mg C/ha). The Kibusa Plantation had the least concentration of the belowground carbon of 47.34 Mg C/ha (Figure 2). The high root carbon concentration in the Natural Stand compared to the two Plantations could be due to the species richness there in [19]. This could also be explained by the proper root development of the mature trees in the Natural Stand [20]. The low root carbon concentration in the Kibusa Plantation could be attributed to by their small age since a young forest has incomplete root development [21]. The trees were not yet mature hence their root systems were not well developed. This low root carbon content in the Kibusa Plantation could also be due to reduced species diversity there in [19].
Root carbon concentration was abundant at the depth of 20-40 cm in all the three study sites. A plausible reason to this could be due to the presence of low nutrient concentration at this depth profile, requiring plants to invest more carbon in roots to adequately capture available nutrients [22]. The higher belowground biomass at this depth (20-40 cm) may also be associated with relatively slow carbohydrate depletion from roots, resulting from low respiration rates underneath [23]. In addition to this, since the mangroves grow in a soft substrate, their roots grow to a deep profile to provide anchorage to make them withstand storm effect and tide inundation effect [24]. This depth is also appropriate for the root development since it allows for aeration in the soil [25]. This result agrees with that found in a study conducted by Castaneda-Moya [3], who reported a high root carbon concentration at a depth of 0-40 cm compared to deeper root zones where there were no free air circulations. The deeper horizons had low root carbon concentration due to lack oxygen coupled with low microbial activities in these zones [12].
Tamooh [26], did a similar research in Gazi Bay, Kenya and they found a lower root carbon concentration in a twelve-year-old plantation dominated by R. mucronata. In their study, they estimated belowground biomass for the Natural Stand to be 18.1 Mg C/ha and that for the twelve-year old R. mucronata to be 17.9 Mg C/ha. In Gabon and DRC Congo, belowground root carbon was estimated to be 75.5 Mg C/ha and 61 Mg C/ha respectively [2]. These results were equivalent to that obtained in this study where in the Natural Stand, belowground biomass content was 78.36 Mg C/ha. However, in a similar research done in Cameroon by Ajonina [2], the root carbon content for Rhizophora. racemosa was higher (153 Mg C/ha) compared to the root carbon content for R. mucronata in the Green Island Plantation (53.4 Mg C/ha). In Thailand, the belowground root carbon for mangroves of Sawi Bay in the natural stand dominated by R. mucronata was reported to range between 70.3-176.3 Mg C/ha [9]. Studies by Lovelock, [27] in the Cuban mangroves, the total belowground biomass for the R. mucronata forest at a sampling depth of 40 cm was reported to be 16.3 Mg C/ha. This is lower compared to that obtained in the Kibusa Plantation of the Mida Creek for the R. mucronata (47.34 Mg C/ha). Studies by Fujimoto [28] and Kridiborworn [29] reported that belowground root carbon for Rhizophora species ranges between 19.5-142 Mg C/ha.
As an adaptive feature for living in soft and wet sediment in the mangrove ecosystem, mangrove trees have a higher amount of belowground biomass. This is due to their inability to support too much weight on the aboveground without a heavy root system [12].
Soil organic carbon (SOC)
In this study, soil organic carbon content was low in the Natural Stand compared to that in both Kibusa and Green Island Plantations. The high soil organic carbon in the plantations is due to previous deposition by the pre-existing stands [12]. Another reason could be due to deposition of sediment from borderland. The higher soil organic carbon stocks in the Green Island Plantation shows that there was incomplete oxidation of carbon from the dead debris after the destruction of the pre-existing stand [6]. The soil organic content also increased during the rainy season due to increased deposition by soil erosion from the hinterland [6]. The higher SOC in the young plantations can also be due to high root turnover rate. The result from this study therefore indicates that restoration of mangroves forest leads to increased SOC accumulation in the sediments.
The efficacy of carbon conversion in soil sediments increases with age of mangroves forests. For instance, carbon sequestration efficiency improves from 16% for a five-year-old forest to 27% for and eighty-five-year-old forest stand [25].
The sustained anoxic conditions in the Plantations than in the Natural stand could also provide a plausible reason for the high soil organic content therein [28]. This is because, unlike the Plantations, the Natural Stand had been degraded and exposed to the scorching sun effects. During the study, an observation was made for the periodic inundation of the Plantations, especially during the neap tide [28]. This led to anoxic conditions in the Plantations and some parts of the Natural Stand at such times. Consequently, there as a slower rate of decomposition of organic matter in the sediment in the Plantations and the affected parts of the Natural Stand [3]. This therefore led to high accumulation of the organic debris in the Plantations compared to the Natural Stands where microbial decomposition was not severely affected by inundation. Furthermore, the Natural systems were degraded and exposed to the sun hence provided better conditions for microbial decomposition, resulting to low organic carbon content in the soil [22].
This result is analogous to the findings by Pandey [21] who found that mangrove ecosystems exposed to adequate inundation had higher soil organic content due to a reduced rate of microbial decomposition.
Accumulation of soil organic carbon in the mangrove sediment can be due to inputs of organic carbon in form of litter, tide and root turnover while depletion of carbon from the mangrove sediment may be due to mineralization, dilution by inorganic material and export by tide [30]. The high soil organic carbon content in the Green Island Plantation can also be explained by the fact that it was dominated by the R. mucronata. This species has unique features responsible for sediment trapping [5]. Some of these features include stilt roots, which is a complex root system. The complex root system is responsible for trapping and providing a suitable environment for accumulation of detritus materials such as litter and fallen dead wood, hence sequestered in the soil sediment. The stilt root system in the Plantations dominated by R. mucronata also facilitated high sedimentation due to reduced speed of water flow, hence deposition of organic matter in the sediment [5]. This also explains why there was a high amount of SOC in the plantations than in the Natural stand, which was a mixed forest. According to the study by Lovelock, [27], she found out that 80% of the soil particles carried by coastal waters were trapped and stagnated under the mangrove root areas. Other studies that accounts for high organic carbon accumulation in the mangrove sediment were done by Fujimoto [28] and Zhang [30], who reported that leaf litter production combines with low rate of microbial decomposition of organic matter in the plantations, leading to high SOC.
The results obtained in this study, together with that reviewed form the existing literature shows that the amount of carbon sequestered in the sediment increases when mangrove forests are restored [5]. This is because mangrove restoration locks the previous carbon left in the soil after destruction of the pre-existing mangrove stand [5]. More so, forest plantation with dissimilar rates of carbon sequestration results to differences in carbon conversion rates in the sediment [24]. The high carbon content within the mangrove sediment could also be due to faster growth rates in these ecosystems as they try to keep pace with sea-level rise and trap debris and residues from tidal movements and alluvial deposits [6,31]. Research by Sakho [32]shows that mangrove ecosystem can keep on accumulating sediment over millennia (a period of a thousand years), thus making them more critical carbon sinks compared to terrestrial ecosystems that reach maximum soil carbon content over decades (a period of ten years).
Total carbon stocks
The total carbon stocks among the three sites studied in mangroves of Mida Creek differed significantly. The variation in the carbon stock among the sites differed due to the difference in structure of the above ground vegetation and the species composition, tree density, age, management regime, and soil depth sampled in each study site [7]. Homogeneity in appearance was evident in the managed mangroves with uniform diameter stems while in the Natural Stands, there was heterogeneity in both structure and diameter. The high amount of carbon stocks in the Natural forest could be due to their old age compared to the Plantations [5]. The increase in the carbon stocks during the rainy season could be attributed to increase in secondary growth since xylem vessels and tracheids were formed in large numbers [6]. These cells are large, have thin walls and the wood has a light texture. High carbon stocks in Green Island Plantation could be due to restoration and good management practices carried out there [5]. Therefore, to maintain a maximum carbon stocks in both aboveground and in sediments, it is important to manage the mangrove plantations without any form of disturbance [5].
The results obtained for total carbon stocks in the three sites in Mida Creek with a value of 1180.95±67 can be compared to similar studies done in other places around the world. For example, in the Indo pacific region, a study by Donate [10] for mangroves gave a report of total carbon stock to be 1023 ±88 Mg C/ha. The total carbon stock of mangroves in the Mexican Caribbean was 987±338 Mg C/ha [33] while that for Indonesian mangrove was estimated to be 986 Mg C/ha. In the West and Central Africa had higher values of carbon stocks of 1520±164 Mg C/ha [2]. In the Gazi Bay, Kenya, the total carbon stock for the twelve- year old Kinondo was 65.8 Mg C/ha. This was lower compared to the one obtained in the Kibusa Plantation (424.52±11.68) of Mida Creek, Kenya. As a tree matures, its biomass production also [7]. Other factors such as availability of nutrients, climatic conditions and edaphic factors also affect biomass accumulation. However, the level of interactions between these factors and the mangrove accretion makes it difficult to identify the main factors contributing to biomass production in any given [25].
Unlike other major forests, the total carbon storage reported in mangroves is exceptionally high [10]. For instance, in Kenya, the total carbon stock for Arabuko Sokoke forest which is an indigenous coastal forest is reported to range between 53-80 Mg C/ha [34]. In Riverine forests of Tana River County, the aboveground carbon pool was 257±43 Mg C/ha in levee forests, while that in evergreen forests was 170±13 Mg C/ha and that for woodland areas was 163±15 [34]. According to a research by Lung [18] in Kakamega forest, the total carbon stock was estimated at 218±17.7 Mg C/ha. This is lower compared to those reported for the mangrove forests; thus, mangroves have a greater potential for sequestering carbon thereby good for global climate change regulation [5]. In this study, soil carbon stock was higher in the plantations compared to that in the Natural mangrove stand. Differences in carbon stocks between the Natural mangrove stand and the Plantations could be due differences in species composition, elemental carbon concentration in trees, forest structure, tree density, age, and level of management and the depth of soil sampled for soil carbon analysis [4].
References
- Piao S, Ciais P, Friedlingstein P, et al. Net carbon dioxide losses of northern ecosystems in response to autumn warming. Nature 451 (2008): 49-52.
- Ajonina GN, Kairo J, Grimsditch G, et al. Assessment of mangrove carbon stocks in Cameroon, Gabon, the Republic of Congo (RoC) and the Democratic Republic of Congo (DRC) including their potential for reducing emissions from deforestation and forest degradation (REDD+). InThe Land/Ocean Interactions in the Coastal Zone of West and Central Africa. Springer Cham (2014): 177-189.
- Castañeda-Moya E, Twilley RR, Rivera-Monroy VH, et al. Patterns of root dynamics in mangrove forests along environmental gradients in the Florida Coastal Everglades, USA. Ecosystems 14 (2011): 1178-1195.
- Lin D, Lai J, Muller-Landau HC, et al. Topographic variation in aboveground biomass in a subtropical evergreen broad-leaved forest in China. PloS One 7 (2012): e48244.
- Kairo JG, Bosire J, Langat J, et al. Allometry and biomass distribution in replanted mangrove plantations at Gazi Bay, Kenya. Aquatic Conservation: Marine and Freshwater Ecosystems 19 (2009): S63-S69.
- Krauss KW, Lovelock CE, McKee KL, et al. Environmental drivers in mangrove establishment and early development: a review. Aquatic Botany 89 (2008): 105-127.
- Joshi HG, Ghose M. Community structure, species diversity, and aboveground biomass of the Sundarbans mangrove swamps. Tropical Ecology 55 (2014): 283-303.
- Ver Hoef JM, Temesgen H. A comparison of the spatial linear model to nearest neighbor (k-NN) methods for forestry applications. PloS One 8 (2013): e59129.
- Thompson BS. The political ecology of mangrove forest restoration in Thailand: Institutional arrangements and power dynamics. Land Use Policy 78 (2018): 503-514.
- Donato DC, Kauffman JB, Mackenzie RA, et al. Whole-island carbon stocks in the tropical Pacific: Implications for mangrove conservation and upland restoration. Journal of Environmental Management 97 (2012): 89-96.
- Saintilan N, Rogers K. Woody plant encroachment of grasslands: a comparison of terrestrial and wetland settings. New Phytologist 205 (2015): 1062-1070.
- Komiyama A, Ong JE, Poungparn S. Allometry, biomass, and productivity of mangrove forests: A review. Aquatic Botany 89 (2008): 128-137.
- Okalebo JR, Gathua KW, Woomer PL. Laboratory methods of soil and plant analysis: a working manual second edition. Sacred Africa, Nairobi 21 (2002).
- Guo Q. The diversity–biomass–productivity relationships in grassland management and restoration. Basic and Applied Ecology 8 (2007): 199-208.
- Warren JM, Meinzer FC, Brooks JR, et al. Hydraulic redistribution of soil water in two old-growth coniferous forests: Quantifying patterns and controls. New Phytologist 173 (2007): 753-765.
- Abuodha PA, Kairo JG. Human-induced stresses on mangrove swamps along the Kenyan coast. Hydrobiologia 458 (2001): 255-265.
- Cohen R, Kaino J, Okello JA, et al. Propagating uncertainty to estimates of above-ground biomass for Kenyan mangroves: A scaling procedure from tree to landscape level. Forest Ecology and Management 310 (2013): 968-982.
- Lung M, Espira A. The influence of stand variables and human use on biomass and carbon stocks of a transitional African forest: Implications for forest carbon projects. Forest Ecology and Management. 2015 Sep 1;351:36-46.
- Mommer L, Cotton TA, Raaijmakers JM, et al. Lost in diversity: the interactions between soil-borne fungi, biodiversity and plant productivity. New Phytologist 218 (2018): 542-553.
- Paul KI, Larmour J, Specht A, et al. Testing the generality of below-ground biomass allometry across plant functional types. Forest Ecology and Management 432 (2019): 102-114.
- Sanquetta CR, Corte AP, da Silva F. Biomass expansion factor and root-to-shoot ratio for Pinus in Brazil. Carbon Balance and Management 6 (2011): 1-8.
- Chen S, Wang W, Xu W, et al. Plant diversity enhances productivity and soil carbon storage. Proceedings of the National Academy of Sciences 115 (2018): 4027-4032.
- Yang Y, Fang J, Ji C, et al. Above-and belowground biomass allocation in Tibetan grasslands. Journal of Vegetation Science 20 (2009): 177-184.
- Ren C, Wang T, Xu Y, et al. Differential soil microbial community responses to the linkage of soil organic carbon fractions with respiration across land-use changes. Forest Ecology and Management 409 (2018): 170-178.
- Alongi DM. Carbon sequestration in mangrove forests. Carbon Management 3 (2012): 313-322.
- Tamooh F, Huxham M, Karachi M, et al. Below-ground root yield and distribution in natural and replanted mangrove forests at Gazi bay, Kenya. Forest Ecology and Management 256 (2008): 1290-1297.
- Lovelock CE. Soil respiration and belowground carbon allocation in mangrove forests. Ecosystems 11 (2008): 342-354.
- Fujimoto K, Imaya A, Tabuchi R, et al. Belowground carbon storage of Micronesian mangrove forests. Ecological Research 14 (1999): 409-413.
- Kridiborworn P, Chidthaisong A, Yuttitham M, et al. Carbon sequestration by mangrove forest planted specifically for charcoal production in Yeesarn, Samut Songkram. J. Sustain. Energy Environ 3 (2012): 87-92.
- Zhang JP, Cheng-De SH, Hai R, et al. Estimating change in sedimentary organic carbon content during mangrove restoration in southern China using carbon isotopic measurements. Pedosphere 22 (2012): 58-66.
- McKee KL, Cahoon DR, Feller IC. Caribbean mangroves adjust to rising sea level through biotic controls on change in soil elevation. Global Ecology and Biogeography 16 (2007): 545-556.
- Sakho I, Mesnage V, Deloffre J, et al. The influence of natural and anthropogenic factors on mangrove dynamics over 60 years: The Somone Estuary, Senegal. Estuarine, Coastal and Shelf Science 94 (2011): 93-101.
- Adame MF, Kauffman JB, Medina I, et al. Carbon stocks of tropical coastal wetlands within the karstic landscape of the Mexican Caribbean. PloS One 8 (2013): e56569.
- Glenday J. Carbon storage and emissions offset potential in an East African tropical rainforest. Forest Ecology and Management 235 (2006): 72-83.
- Pandey CN, Pandey R. Carbon sequestration in mangroves of Gujarat, India. International Journal of Botany and Research 3 (2013): 57-70.
- Piao S, Ciais P, Friedlingstein P, et al. Net carbon dioxide losses of northern ecosystems in response to autumn warming. Nature 451 (2008): 49-52.
- Temesgen H, Affleck D, Poudel K, et al. A review of the challenges and opportunities in estimating above ground forest biomass using tree-level models. Scandinavian Journal of Forest Research 30 (2015): 326-335.
- Zhang Y, Chen HY, Taylor AR. Positive species diversity and above-ground biomass relationships are ubiquitous across forest strata despite interference from overstorey trees. Functional Ecology 31 (2017): 419-426.


 Impact Factor: * 4.1
Impact Factor: * 4.1 Acceptance Rate: 75.32%
Acceptance Rate: 75.32%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 