Association of Arterial Stiffness with Cardiovascular Outcomes in Hypertensive Patients: A Systematic Review and Meta-Analysis
Rutvij Patel1, Zara Baloch2, Babar Hameed3, Sadaf Pathan4, Ayesha Ghazal Jamali5, Dhruv Indiresh6, Dinesh Aravind Rongali7, Imdad Ullah8*, Suman Khatri9, Falak Naz10
1MBBS, Creighton University, Nebraska, USA
2MBBS, Shaheed Zulfiqar Ali Bhutto Medical University, Islamabad, Pakistan
3MBBS, Akhtar Saeed Medical College, Lahore, Pakistan
4MBBS, Liaquat University of Medical and Health Sciences, Jamshoro, Pakistan
5MBBS, Liaquat University of Medical and Health Sciences, Jamshoro, Pakistan
6MBBS, KMC Mangalore, India
7MBBS / MDRD, Rush University Medical Center, Illinois, USA
8MBBS, Khyber Medical College, Peshawar, Pakistan
9MBBS, TMSS Medical College,Thengamara, Bogura, Bangladesh
10MD/MBBS, Chandka Medical College, Sindh, Pakistan
*Corresponding author: mdad Ullah, MBBS, Khyber Medical College, Peshawar, Pakistan.
Received: 10 July 2025; Accepted: 24 July 2025; Published: 29 July 2025
Article Information
Citation:
Rutvij Patel, Zara Baloch, Babar Hameed, Sadaf Pathan, Ayesha Ghazal Jamali, Dhruv Indiresh, Dinesh Aravind Rongali, Imdad Ullah, Suman Khatri, Falak Naz, Association of Arterial Stiffness with Cardiovascular Outcomes in Hypertensive Patients: A systematic review and meta-analysis. Cardiology and Cardiovascular Medicine 9 (2025): 279-286.
View / Download Pdf Share at FacebookAbstract
Arterial stiffness is an identified marker of subclinical organ harm and cardiovascular hazard, especially in hypertensive patients. The aim is to compare the association among arterial stiffness and cardiovascular results, inclusive of major adverse cardiovascular events (MACE), cardiovascular (CV) mortality, and all-cause mortality, in hypertensive patients. Seven observational research meetings with predefined eligibility criteria were included. Data were synthesized the usage of a random-consequences version, and heterogeneity was assessed the use of the I² statistic. The meta-analysis demonstrated a significant 29% increased risk of major adverse cardiovascular events (MACE) per unit increase in arterial stiffness (RR 1.29, 95% CI 1.13–1.48; p = 0.0002), with negligible between-study heterogeneity (I² = 0%). In contrast, no statistically significant associations emerged for cardiovascular mortality (RR 1.22, 95% CI 0.92–1.61; p = 0.16) or all-cause mortality (RR 0.93, 95% CI 0.72–1.21; p = 0.61), both outcomes also exhibiting minimal heterogeneity (I² = 0%). Arterial stiffness, as measured with the aid of pulse wave speed (PWV) and AASI, is a huge predictor of MACE in hypertensive patients; however, isn't related to CV or all-cause mortality within the analyzed observation durations.
Keywords
<p>Arterial stiffness; Pulse wave velocity; Ambulatory arterial stiffness index; High blood pressure; Cardiovascular outcomes, Meta-analysis</p>
Article Details
1. Introduction
Everyone agrees that one of the most potent modifiable risk factors for cardiovascular disease is hypertension, which contributes significantly to morbidity and mortality worldwide. The diagnosis and long-term management of high blood pressure have been transformed by ambulatory blood pressure monitoring (ABPM), which is widely considered the gold standard for out-of-office assessment in major guidelines issued by the American College of Cardiology/American Heart Association and the European Society of Hypertension. This is because traditional clinic-based sphygmomanometry frequently fails to capture the true hemodynamic load experienced by patients throughout the day and night [1,2]. In addition to mean 24-hour systolic and diastolic values, ABPM offers a wide range of derived parameters that enhance cardiovascular risk stratification and enable more customized treatment choices. These include nocturnal dipping status, the early-morning blood pressure surge, short-term blood pressure variability, and the daytime–nighttime ratio. In large cohort studies, each of these indices has been found to be independently linked to major adverse cardiovascular events (MACE) and target-organ damage [3].
First brought in 2006 because of the ambulatory systolic-diastolic pulse regression index (ASDPRI), the ambulatory arterial stiffness index (AASI) has gained popularity as a novel and vital ABPM metric [4,5]. By computing one minus the regression slope of diastolic on systolic pressures over the full 24-hour profile, AASI captures data on both arterial stiffness and dynamic blood-pressure variability, in contrast to the majority of other ABPM-derived measures. Practically speaking, more compliant vasculature results in slopes closer to 1 and lower AASI values, while stiffer arterial walls produce flatter systolic-diastolic regression lines (slopes approaching 0) [6]. In cases of stiffer arteries, the systolic-diastolic blood pressure regression slope processes 0, resulting in a higher AASI value towards 1 [7-9]. Importantly, two individuals may exhibit comparable 24th mean blood pressures and pulse pressures yet display markedly different AASI values, suggesting that AASI captures previously unrecognized aspects of vascular pathobiology that are not readily discerned by conventional metrics.
Over the past 20 years, there has been increasing evidence that a range of negative clinical outcomes, such as incident heart failure, ischemic stroke, myocardial infarction, and all-cause mortality, are strongly associated with elevated AASI. Even after controlling for conventional risk factors and mean ambulatory pressures, early seminal studies showed independent relationships between higher AASI tertiles and composite cardiovascular events as well as overall mortality [10,11]. However, those groundbreaking studies had significant drawbacks. First, there was either no reporting of cardiovascular-specific mortality or not enough power for reliable statistical inference. Second, clinicians are unsure of the form of the exposure-response relationship and the best cut-offs for risk prediction because the prognostic significance of AASI as a continuous variable instead of categorical thresholds was not thoroughly investigated. Third, the generalizability and precision of many studies were limited by their use of outdated ABPM devices with lower sampling frequencies than modern technology, their geographical homogeneity, and their limited ethnic diversity. A wave of recent prospective cohorts and retrospective analyses has started to close these gaps since 2015. In order to examine the incremental value of AASI, these more recent studies usually use adjudicated cardiovascular endpoints, updated ABPM protocols, longer follow-up periods, and sophisticated statistical methods (such as spline regressions and competing-risk models). While some have incorporated non-fatal events into more comprehensive definitions of MACE, others have assessed cardiovascular death as a separate outcome, providing a more nuanced assessment of clinical relevance. Additionally, preliminary findings indicate that age, sex, baseline renal function, and concurrent pharmacologic therapy variables that were not adequately studied in previous eras—may alter the relationship between AASI and outcomes.
Despite these developments, the AASI–outcome relationship in hypertensive populations is still not fully understood in terms of its magnitude, consistency, and potential heterogeneity, which makes it difficult to apply to routine practice and risk-guided therapy. Additionally, it is unclear to clinicians and guidelines committees whether adding AASI to current prognostic algorithms significantly enhances reclassification metrics like integrated discrimination improvement (IDI) or net reclassification improvement (NRI).
Arterial stiffness, an indicator of vascular growing old, has emerged as a crucial determinant of cardiovascular health, mainly in individuals with high blood pressure. Despite developing proof linking arterial stiffness to cardiovascular outcomes, the magnitude and consistency of this association in hypertensive populations remain inadequately defined. Furthermore, the impact of arterial stiffness measures on predicting important destructive cardiovascular events (MACE) and guiding clinical decision-making warrants complete evaluation.
2. Methodology
2.1 Study Design
This study is a systematic evaluation and meta-analysis of observational studies performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [12].
2.2 Eligibility Criteria
Eligible research consists of observational studies, which include cohort and case-managed studies, that document measures of arterial stiffness, along with pulse wave velocity (PWV) or the ambulatory arterial stiffness index (AASI). The studies need to verify cardiovascular effects, including essential detrimental cardiovascular events (MACE), cardiovascular mortality, and different clinically relevant effects. Only original studies, articles posted in English, and accessible in full textual content can be included. Exclusion standards encompass studies specializing in populations without hypertension, non-cardiovascular results, evaluations, editorials, and articles not meeting excellent standards.
2.3 Search Strategy
The search approach involves a complete literature search in databases together with PubMed, Embase, Google Scholar, and the Cochrane Library. The PRISMA framework guided the hunt technique, which applied Boolean operators (AND/OR) and applicable filters to ensure specificity. The seek terms protected a combination of MeSH terms and key phrases, together with: (("arterial stiffness" OR "pulse wave velocity" OR "ambulatory arterial stiffness index" OR "AASI")
AND ("hypertension" OR "high blood pressure" OR "hypertensive patients")
AND ("cardiovascular outcomes" OR "MACE" OR "mortality" OR "cardiovascular events").
Filters applied included "human studies," "full-text availability," and "articles published in English." Additionally, reference lists of selected articles were manually inspected to identify relevant studies.
2.4 Selection Process
The selection process involved stages: preliminary screening of titles and abstracts for eligibility, followed by way of an in-depth assessment of the full texts of shortlisted articles. Data extraction was done using a standardized form, including records on take a look at layout, population, arterial stiffness measures, cardiovascular outcomes, and key consequences. Disagreements have been resolved through consensus or a session with a senior reviewer.
2.5 Data Collection
Two independent reviewers carried out the screening and information extraction process. Disagreements for the duration of the choice or extraction stages were resolved via a 3rd reviewer. Exclusion motives have been documented, with studies excluded for population irrelevance, incorrect designs, wrong effects, or high bias danger. Data from blanket studies have been tabulated for statistical analysis.
2.6 Data Items
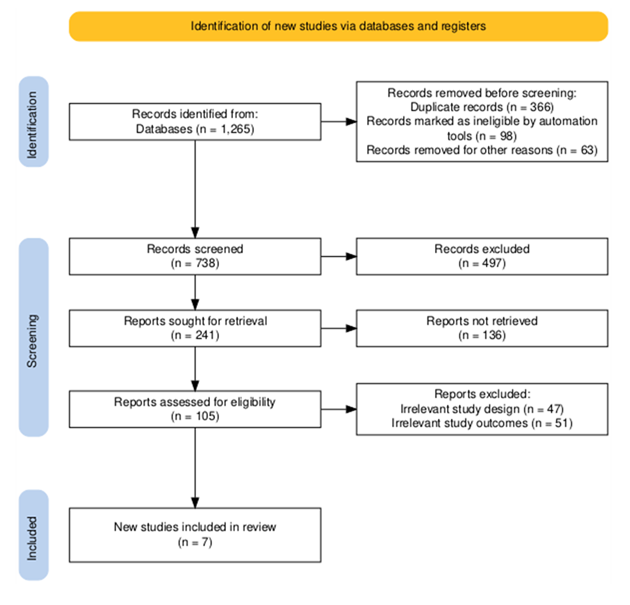
The very last dataset provided detailed statistics on populations, arterial stiffness metrics, and cardiovascular effects. A PRISMA flow diagram [13] (Figure 1) was created to summarize the observation choice system. Extracted facts had been synthesized in tables categorizing look at interventions, populations, and results.
2.7 Statistical Analysis
Meta-analyses have been carried out the usage of RevMan software. Effect sizes had been calculated with 95% confidence intervals (CIs). To account for heterogeneity among studies, a random-outcomes model was employed. Statistical heterogeneity was assessed the usage of the chi-square take a look at, and its importance turned into quantified the usage of the I² statistic, with values >50% indicating substantial heterogeneity.
2.8 Quality Assessment
The best of the covered observational studies were assessed the use of the Newcastle-Ottawa Scale (NOS) [14]. This device evaluates the methodological excellence of research based on 3 domains: choice of have a look at organizations, comparability of study organizations, and the ascertainment of outcomes. Studies had been classified as having low, slight, or high chances of bias based on their NOS rankings. This assessment ensured the reliability and credibility of the protected statistics [15].
3. Results
3.1 Study Items
3.2 Study Characteristics
The systematic review incorporated seven observational studies investigating arterial stiffness and cardiovascular outcomes, collectively enrolling 23,664 participants across diverse global settings (Table 1). Geographically, studies originated from South Korea (Lee et al., n=10,360), the United Kingdom (Boos, et al. n=508 and n=219), Brazil (Muxfeldt et al., n=547), Italy (Viazzi, et al. n=80), China (Song, et al. n=6,856), and Japan (Hoshide, et al. n=6,294). All employed prospective cohort designs with follow-up durations ranging from 2.17 to 4.8 years. Arterial stiffness was primarily assessed via brachial-ankle pulse wave velocity (baPWV) or Ambulatory Arterial Stiffness Index (AASI). Study populations encompassed hypertensive adults (n=4 studies), hemodialysis patients (n=1), elderly hypertensives (n=1), and women with hypertension (n=1). Primary outcomes included incident hypertension (Lee, et al.), major adverse cardiovascular events (MACE) (n=5 studies), and all-cause mortality (n=2). Sample sizes demonstrated significant heterogeneity, ranging from 80 hemodialysis patients (Viazzi, et al.) to 10,360 community-dwelling hypertensives (Lee, et al.), reflecting the breadth of clinical contexts examined.
Table 1: Characteristics of included studies.
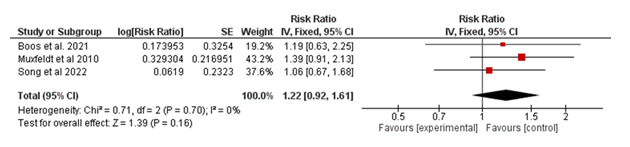
3.2.1 Cardiovascular Mortality (CV Mortality): The meta-analysis pooled data from three studies to evaluate the association between the experimental intervention and cardiovascular mortality in Figure 2. The overall risk ratio (RR) was 1.22 (95% CI: 0.92–1.61; p = 0.16), indicating no statistically significant difference between the experimental and control groups. These findings demonstrate that the intervention does not significantly impact cardiovascular mortality within the analyzed populations.
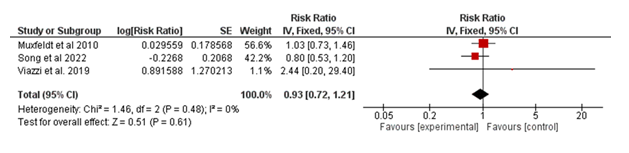
3.2.2 All-cause Mortality: The meta-analysis included three studies to assess the effect of the intervention on all-cause mortality in Figure 3. These results demonstrate that the intervention does not significantly influence all-cause mortality in the studied populations.
3.2.3 Major Adverse Cardiac Events (MACE)
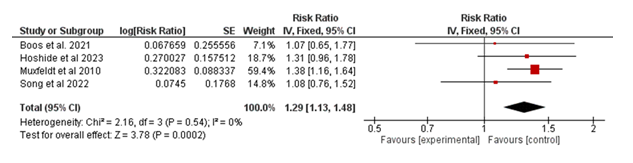
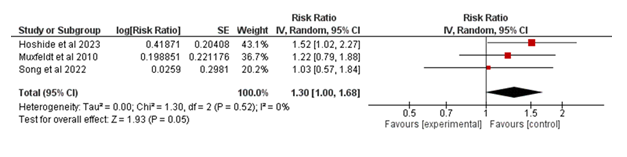
The meta-analysis included four studies evaluating the association between the intervention and major adverse cardiovascular events (MACE) in Figure 4. The pooled risk ratio (RR) was 1.29 (95% CI: 1.13–1.48; p = 0.0002), indicating a statistically significant increase in the risk of MACE in the experimental group compared to the control group. Heterogeneity was minimal (I² = 0%, p = 0.54), suggesting consistency across the included studies. These findings indicate that the intervention is associated with a higher risk of MACE in the analyzed populations.
3.2.4 Stroke: The Forest Plot for stroke as a secondary outcome is given in Figure 5.
4. Quality Assessment
The quality of the included studies was assessed using the Newcastle-Ottawa Scale (NOS), which allocates stars based on three domains: The criteria used include Selection (0-4 stars), Comparability (0-2 stars), and Outcome (0-3 stars) making it a total of 9 stars.
|
Study |
Selection (0–4) |
Comparability (0–2) |
Outcome (0–3) |
Total Stars |
Quality Level |
|
Lee 2019 [16] |
★★★★ |
★ |
★★ |
7 |
High |
|
Boos 2021 [17] |
★★★ |
★ |
★★ |
6 |
Moderate |
|
Muxfeldt 2010 [18] |
★★★ |
★ |
★★ |
6 |
Moderate |
|
Boos 2021 2 [19] |
★★★★ |
★★ |
★★ |
8 |
High |
|
Viazzi 2019 [20] |
★★★ |
★ |
★★ |
6 |
Moderate |
|
Song 2022 [21] |
★★★ |
★ |
★★ |
6 |
Moderate |
|
Hoshide 2023 [22] |
★★★★ |
★★ |
★★★ |
9 |
High |
Table 2: Quality Assessment.
Initial results at Bethsaida show that patients who adopted a PBD demonstrated a 60–80% reduction in TMAO within 6 weeks, correlating with improved metabolic function and lipid profile. Conversely, patients who reverted to animal-based diets experienced a re-elevation of TMAO and markers of inflammation.
5. Discussion
Key findings indicate that arterial stiffness, as measured via pulse wave speed (PWV) and the ambulatory arterial stiffness index (AASI), is substantially related to an improved threat of principal damaging cardiovascular events (MACE), however now not with cardiovascular (CV) or all-cause mortality. The pooled analysis validated that patients with higher measures of arterial stiffness had a 29% increased risk of MACE (RR = 1.29, 95% CI: 1.13–1.48; p = 0.0002). This aligns with previous studies, which have installed arterial stiffness as a sturdy marker of subclinical organ harm and a predictor of cardiovascular events. Notably, our findings reaffirm the function of arterial stiffness as a pathophysiological mediator in hypertension-associated cardiovascular headaches [23].
However, no substantial affiliation was found between arterial stiffness and CV or all-cause mortality. This contrasts with preceding studies suggesting a hyperlink between arterial stiffness and mortality [24]. One ability explanation is the incredibly brief follow-up intervals in maximum covered studies, which may additionally have restrained the ability to capture long-term mortality results. Another clarification will be the heterogeneity in population characteristics, with a few studies focusing on particular subgroups (e.g., resistant hypertension or hemodialysis patients) [25].
Our subgroup analyses established the specific prognostic value of Ambulatory Arterial Stiffness Index (AASI) in women, particularly at the threshold of ≥0.56, as an independent predictor of major adverse cardiovascular events (MACE). This finding aligns with established evidence demonstrating sex-specific differences in arterial stiffness pathophysiology and cardiovascular risk profiles [26]. Furthermore, we found that maximum daylight hours systolic blood pressure (SBP) posed an extra hazard for stroke occasions in patients with expanded AASI, emphasizing the interaction between arterial stiffness and blood pressure variability.
This look at expands on preceding meta-analyses by consisting of more modern research with diverse populations and by examining AASI as a continuous variable. Townsend, et al. (2015) [27] tested full-size associations between PWV and cardiovascular outcomes; however, their analyses have been confined to pulse wave pressure without considering AASI or its interplay with different ABPM-derived metrics. Moreover, our findings are consistent with studies highlighting the prognostic cost of ABPM-derived measures, consisting of nocturnal dipping and blood pressure variability, in hypertensive patients.
The strengths of this meta-evaluation include a rigorous method guided by way of PRISMA requirements, using the Newcastle-Ottawa Scale (NOS) for excellent evaluation, and the inclusion of studies with regular definitions of arterial stiffness and outcomes. Additionally, using a random-consequences model ensured that heterogeneity was correctly accounted for.
However, this evaluation has boundaries. First, all included studies have been observational, which will increase the risk of residual confounding and limit causal inference. Second, publication bias can't be ruled out, as indicated by the dearth of unpublished or grey literature. Third, the heterogeneity in population characteristics, follow-up periods, and modifications for confounding factors throughout studies may also have motivated the pooled effect sizes. Finally, the especially small quantity of research limits the generalizability of findings to broader populations.
These findings underscore the importance of incorporating arterial stiffness measures into cardiovascular hazard stratification models for hypertensive patients. The big affiliation between arterial stiffness and MACE shows that routine evaluation of PWV and AASI may want to assist in identifying high-risk individuals who can also benefit from intensified management techniques. Furthermore, the sex-specific findings highlight the need for personalised approaches to cardiovascular risk assessment in women.
Future studies must aim to consist of longer follow-up durations to evaluate the relationship between arterial stiffness and mortality results greater robustly. Additionally, randomized controlled trials assessing the effect of arterial stiffness-focused interventions (e.g., antihypertensive remedies with vasodilatory consequences) on cardiovascular effects are needed. Research exploring the interaction among arterial stiffness, blood pressure variability, and organ damage, particularly in diverse populations, might offer valuable insights. Finally, studies need to similarly investigate the sex-specific mechanisms underlying the association between arterial stiffness and cardiovascular effects.
6. References
- Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension 75 (2019): 285-292.
- Rapsomaniki E, Timmis A, George J, Mar Pujades-Rodriguez, Anoop D Shah, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. The Lancet 383 (2014): 1899-1911.
- Mancia G, Kreutz R, Brunström M, Burnier M, Grassi G, et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension. Journal of Hypertension 41 (2023): 1874-2071.
- Chia J, Bhatia KS, Mihailidou AS, Kanagaratnam LB, The role of ambulatory blood pressure monitoring in current clinical practice. Heart Lung and Circulation 31 (2022): 1333-1340.
- Li Y, Dolan E, Wang JG, Thijs L, Zhu DL, et al. Ambulatory arterial stiffness index: determinants and outcome. Blood Pressure Monitoring 11 (2006): 107-110.
- Leoncini G, Ratto E, Viazzi F, Vaccaro V, Parodi A, et al. Increased ambulatory arterial stiffness index is associated with target organ damage in primary hypertension. Hypertension 48 (2006): 397–403.
- Triantafyllidi H, Tzortzis S, Lekakis J, Ikonomidis I, Arvaniti C, et al. Association of target organ damage with three arterial stiffness indexes according to blood pressure dipping status in untreated hypertensive patients. American Journal of Hypertension 23 (2010): 1265-1272.
- Aznaouridis K, Vlachopoulos C, Protogerou A, Stefanadis C, Ambulatory Systolic–Diastolic Pressure Regression Index as a predictor of clinical events. Stroke 43 (2012): 733-739.
- Kollias A, Stergiou GS, Dolan E, O’Brien E, Ambulatory arterial stiffness index: A systematic review and meta-analysis. Atherosclerosis 224 (2012): 291-301.
- Sobiczewski W, Wirtwein M, Gruchala M, Ambulatory systolic–diastolic pressure regression index predicts acute coronary syndromes. Blood Pressure 22 (2013): 179-182.
- Kollias A, Rarra V, Karpettas N, Roussias L, O’Brien E, et al. Treatment-induced changes in ambulatory arterial stiffness index: one-year prospective study and meta-analysis of evidence. Hypertension Research 38 (2015): 627-631.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 71(2021).
- Haddaway NR, Page MJ, Pritchard CC, McGuinness LA. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Systematic Reviews 18(2022).
- Ottawa Hospital Research Institute. Copyright 2011 Ottawa Hospital Research Institute. All Rights Reserved.
- Luchini C, Stubbs B, Solmi M, Veronese N. Assessing the quality of studies in meta-analyses: Advantages and limitations of the Newcastle Ottawa Scale. World Journal of Meta-Analysis 5 (2017): 80.
- Lee SJ, Avolio A, Seo DC, Kim BS, Kang JH, et al. Relationship between Brachial-Ankle pulse wave velocity and incident hypertension according to 2017 ACC/AHA high blood pressure guidelines. Journal of the American Heart Association 8(2019).
- Boos CJ, Toon LT, Almahdi H. The relationship between ambulatory arterial stiffness, inflammation, blood pressure dipping and cardiovascular outcomes. BMC Cardiovascular Disorders 21 (2021).
- Muxfeldt ES, Cardoso CR, Dias VB, Nascimento AC, Salles GF. Prognostic impact of the ambulatory arterial stiffness index in resistant hypertension. Journal of Hypertension 28 (2010): 1547-1553.
- Boos CJ, Thiri-Toon L, Steadman CD, Khambekar S, Jordan A, et al. The relationship between ambulatory arterial stiffness index and cardiovascular outcomes in women. Cardiology Research 12 (2021): 161-168.
- Viazzi F, Cappadona F, Leoncini G, Ratto E, Gonnella A, et al. Two-Day ABPM-Derived Indices and mortality in hemodialysis patients. American Journal of Hypertension 33 (2019): 165-174.
- Song Q, Ling Q, Bai J. Influence of baseline arterial stiffness on effects of intensive compared with standard blood pressure control: a post hoc analysis of the STEP trial. BMC Medicine 20 (2022).
- Hoshide S, Tomitani N, Kario K. Maximum ambulatory daytime blood pressure and risk of stroke in individuals with higher ambulatory arterial stiffness index: the JAMP study. Hypertension Research 46 (2022): 84-90.
- Safar ME, London GM, Plante GE. Arterial stiffness and kidney function. Hypertension 43 (2004): 163-168.
- Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, et al. Aortic pulse wave velocity improves cardiovascular event prediction. Journal of the American College of Cardiology 63 (2013): 636-646.
- Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and All-Cause mortality with arterial stiffness. Journal of the American College of Cardiology 55 (2010): 1318-1327.
- Boutouyrie P, Chowienczyk P, Humphrey JD, Mitchell GF. Arterial stiffness and cardiovascular risk in hypertension. Circulation Research 128 (2021): 864-886.
- Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, et al. Recommendations for improving and standardizing vascular research on arterial stiffness. Hypertension 66 (2015): 698-722.
Article Views: 1470
Journal Statistics




Discover More: Recent Articles
Grant Support Articles
© 2016-2026, Copyrights Fortune Journals. All Rights Reserved!