Association of Inpatient Initiation of Sacubitril/Valsartan With Short and Intermediate-Term Readmissions in Patients Hospitalized for Heart Failure: A Retrospective Cohort Study
Shamaiza Waqas¹*, Catherine Raymond¹, Khurram Arshad², Luxhman Gunaseelan²,Taiwo Opaleye¹, Jacob Klein¹, Dua Malik¹, Mehrun Nisa Ahmed³, Benjamin Collins-Hamel¹
1Henry Ford Health, Warren, Michigan, United States
2Corewell Health East, Dearborn, Michigan, United States
3CMH Lahore Medical College, Pakistan
*Corresponding author: Shamaiza Waqas, MD, Henry Ford Health, Warren, Michigan, United States.
Received: 22 December 2025; Accepted: 30 December 2025; Published: 03 January 2025
Article Information
Citation:
Waqas S, Raymond C, Arshad K, Gunaseelan L, Opaleye T, Klein J, Malik D, Nisa Ahmed M, Collins-Hamel B. Association of Inpatient Initiation of Sacubitril/Valsartan With Short and Intermediate-Term Readmissions in Patients Hospitalized for Heart Failure: A Retrospective Cohort Study. Cardiology and Cardiovascular Medicine. 10 (2026): 01-05
View / Download Pdf Share at FacebookAbstract
Background: Sacubitril/valsartan improves outcomes in heart failure with reduced ejection fraction (HFrEF), but real-world data on its effect on hospital readmissions after inpatient initiation are limited. Methods: We conducted a retrospective cohort study of adults hospitalized with HFrEF across multiple Southeast Michigan hospitals between October 2017 and October 2024. Patients initiated on sacubitril/ valsartan during hospitalization and discharged on therapy were compared with contemporaneous controls not treated with sacubitril/valsartan. Readmissions at 30, 60, and 90 days were assessed. Results: A total of 164 patients initiated on sacubitril/valsartan and 16 control patients were included. Baseline ejection fraction distribution was similar between groups. Readmission rates in the sacubitril/valsartan group were 18.9% at 30 days, 15.8% at 60 days, and 12.7% at 90 days. Compared with controls, sacubitril/valsartan initiation was associated with a significantly lower 60-day readmission rate (p = 0.04), while differences at 30 and 90 days were not statistically significant. Conclusion: Inpatient initiation of sacubitril/valsartan was associated with reduced 60-day readmissions in patients hospitalized with HFrEF, with no significant effect at 30 or 90 days. These findings suggest a potential intermediate-term benefit and support early initiation during hospitalization, although confirmation in larger prospective cohorts is needed
Keywords
<p>Sacubitril; Ejection fraction; ARNI therapy; Heart failure; Mortality</p>
Article Details
Introduction
Heart failure (HF) affects about 6.2 million adults in the United States, and its prevalence is expected to increase by 46% by 2030. This rise will add to the burden of illness, death, and healthcare costs [1]. Sacubitril/valsartan, an angiotensin receptor–neprilysin inhibitor (ARNI), has transformed the treatment of heart failure with reduced ejection fraction (HFrEF). The PARADIGM-HF trial demonstrated that sacubitril/valsartan reduces cardiovascular mortality and HF-related hospitalizations as compared to enalapril and helped establish ARNI therapy as a cornerstone of guideline-directed medical therapy (GDMT) [2]. The PIONEER HF trial further confirmed its safety and clinical benefits when initiated during hospitalization for acute decompensated HF [3].
Although these major trials exist, there is little real-world data on how starting sacubitril/valsartan in the hospital affects short- and mid-term readmissions. Our study looks at readmission rates at 30, 60, and 90 days after starting sacubitril/valsartan for the first time in the hospital setting. We hypothesized that inpatient initiation of sacubitril/valsartan would be associated with lower short- and intermediate-term readmission rates compared with usual care.
2. Methods
2.1 Study Design and Setting
This is a retrospective cohort study that was conducted across multiple major hospital networks within Southeast Michigan. The study period extended from October 1, 2017, to October 31, 2024.
3. Patient Identification and Eligibility
Patients were identified via ICD-10 codes for acute or chronic HF. Inclusion required hospitalization with HF, inpatient initiation of sacubitril/valsartan, and discharge home with the medication for the first time.
A control group consisted of HF patients who did not receive sacubitril/valsartan during hospitalization or at discharge.
Readmissions were defined as all cause hospitalizations within the Ascension Southeast Michigan network occurring within 30, 60, or 90 days of index discharge.
4. Data Collection
Electronic medical records were reviewed for demographics, clinical characteristics, including left ventricular ejection fraction (EF), sacubitril/valsartan dose at discharge, 30, 60, and 90-day readmission rates. This study was approved by the institutional review board with a waiver of informed consent.
5. Outcomes
Primary outcomes were readmission rates at 30, 60, and 90 days post-discharge.
6. Statistical Analysis
Chi-square, Student’s t test, and Mann-Whitney U tests were used where appropriate. Significance was set at p < 0.05. Due to the limited size of the control group, multivariable adjustment was not performed.
7. Results
A total of 164 patients who were started on inpatient sacubitril/valsartan and discharged home for the first time were compared to a control group of 16 patients who were neither initiated on nor discharged with sacubitril/valsartan. The average age of patients in the sacubitril/valsartan group was 64.8 ± 15.1 years. The cohort consisted of 33% Caucasian and 67% African American patients. Additionally, 33% of the patients were male, and 67% were female (Table 1).
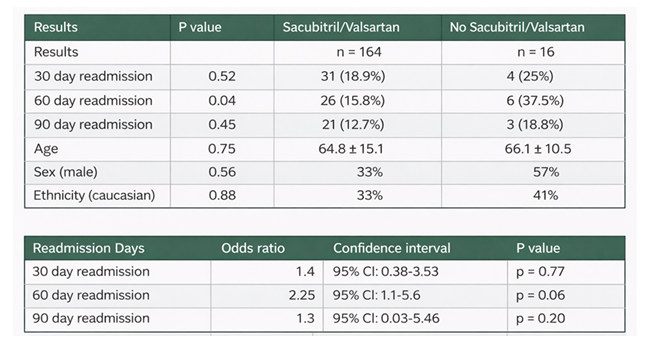
Among the sacubitril/valsartan group, the 30-day readmission rate was 18.9% (31/164) with a p-value of 0.52, the 60-day readmission rate was 15.8% (26/164), and the 90-day readmission rate was 12.7% (21/164) (Table 2). The majority of patients treated with sacubitril/valsartan had an EF of 15-35% (60.4%), followed by EF 10-15% (15.2%). EF categories were similar in the control group (Table 2).
In the study cohort, 145 patients were discharged on a 24-26 mg strength of acubitril/valsartan, 18 patients were discharged on a 49-51 mg strength, and 1 patient was discharged on a 97-103 mg strength (Table 2).
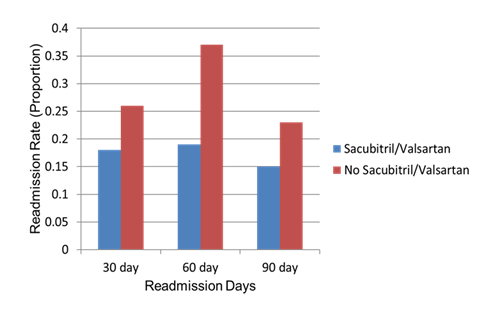
Figure 1: Thirty-day, 60-day, and 90-day hospital readmission rates in patients treated with sacubitril/valsartan compared with those not receiving sacubitril/valsartan.
Table 1: Baseline demographics and clinical characteristics of patients receiving Sacubiril/Valsartan versus no Sacubitril/ Valsartan.
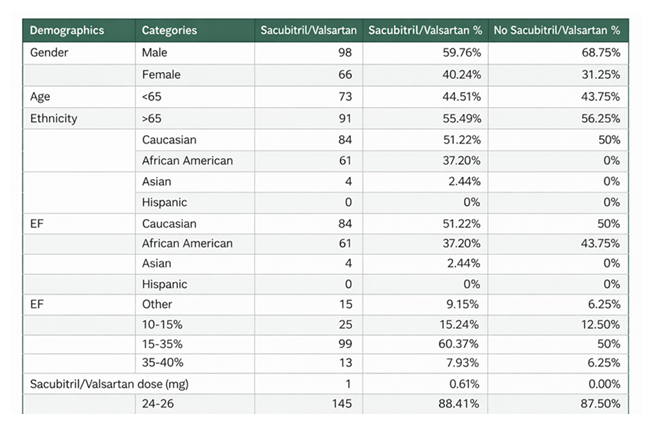
Table 2: Comparison of result between patients receiving Sacubitril/Valsartan versus no Sacubitril/Valsartan.
8. Discussion
Our retrospective study evaluated the impact of first-time starting sacubitril/valsartan during hospitalization on short and intermediate-term readmission rates among patients with heart failure. Our findings showed that while sacubitril/valsartan initiation did not significantly reduce 30 day or 90 day readmissions but there was a statistically significant reduction in 60 day readmissions rate in treated patients. This suggests that the therapeutic benefits of sacubitril/valsartan may emerge during the intermediate recovery period following discharge, potentially reflecting the time required for neurohormonal modulation and ventricular remodeling effects to manifest.
The findings of our study support and add to the evidence from major trials like PARADIGM-HF and PIONEER-HF. In PARADIGM-HF, sacubitril/valsartan was shown to significantly cut heart failure hospitalizations and cardiovascular deaths compared with enalapril, establishing it as a key treatment for HFrEF. These results came from highly controlled trial settings with carefully chosen patients, close monitoring, and optimized dose adjustments [2,3].
PIONEER-HF looked specifically at starting sacubitril/valsartan in the hospital and showed that it was safe and led to meaningful drops in NT-proBNP and other cardiac biomarkers within just a few weeks. However, that study didn’t follow readmissions beyond eight weeks, so its real-world impact on readmission rates remained unclear [3]. Our finding of fewer 60-day readmissions lines up with the 6-8 week biochemical improvements seen in PIONEER-HF, providing a reasonable physiologic explanation for why benefits may emerge in this intermediate time frame.
Blocking neprilysin, the drug increases levels of natriuretic peptides, which help widen blood vessels, remove excess fluid, and reduce fibrosis. At the same time, the ARB component suppresses the RAAS pathway, lowering afterload and slowing harmful cardiac remodeling. Together, these effects reduce wall stress, improve blood flow and volume control, lessen neurohormonal activation, and support early reverse remodeling. These physiologic changes usually take several weeks before they lead to noticeable clinical stability, as suggested by the lack of clinical benefit at 30 days in our study [3].
The lack of a difference at 90 days may stem from real-world challenges, for example, difficulty staying on the medication, inability to increase the dose due to low blood pressure or kidney issues, worsening comorbid conditions, or progression of severe heart failure, especially in patients with very low EF. These factors could offset the early improvements observed around the 60-day mark.
Most patients in our study (60.4%) had a baseline EF of 15-35%, indicating a group with advanced systolic dysfunction. Because the EF distribution was similar between those who received sacubitril/valsartan and those who did not, it’s unlikely that baseline disease severity explains the differences we saw in readmission rates.
Another important real world observation is that most patients were started on the lowest sacubitril/valsartan dose (24-26 mg). In clinical trials, patients were usually titrated to higher doses, which were associated with better outcomes. When titration doesn’t happen or happens too slowly, the medication may not deliver its full benefit, which could help explain why we didn’t see significant improvement at 90 days. In our study, the distribution of dosages across the cohort (with the majority receiving the 24-26 mg strength) suggests a predominance of lower dose prescriptions, but no direct comparison or analysis of how different dosage strengths affect readmission rates, clinical outcomes, or patient responses has been made.
It’s also important to remember that readmission risk is shaped by many non-medication factors like socioeconomic challenges, access to and affordability of prescriptions, quality of post-discharge follow-up, heart failure education and self-management skills, and the availability of transitional care programs. In real-world settings, especially in communities facing high socioeconomic stress, these factors can overshadow the expected benefits of therapy.
Literature has shown mixed results on whether ARNI therapy reduces readmissions. Some observational studies report lower heart failure readmissions and mortality, while others find only modest benefits or no benefit at all in the short term (6-9). These differences can be attributed to when the medication is started, how well doses are titrated, patient adherence, overall illness severity, and the resources available within different healthcare systems.
Our study findings add to this evolving body of evidence by suggesting that starting sacubitril/valsartan in the hospital may help reduce readmissions but also that this benefit may be most noticeable within a specific intermediate timeframe.
The 60-day improvement that was observed in this study highlights the value of initiating therapy during hospitalization, ensuring patients receive organized follow-up within the first month after discharge, and adjusting the medication dose as recommended by guidelines. It also emphasizes the need to address socioeconomic barriers that can interfere with continued use of the medication.
This study has several important strengths. It was conducted across multiple hospitals within a single integrated health system, enhancing the generalizability of the findings while maintaining consistency in clinical practice patterns and data capture. The cohort represents a real-world population with substantial racial diversity, increasing the relevance of the results to everyday clinical care and to populations often underrepresented in randomized clinical trials. Additionally, the analysis evaluated readmissions at three clinically meaningful time points (30, 60, and 90 days), allowing for assessment of both short-term and intermediate-term outcomes and providing insight into the temporal emergence of potential benefits associated with inpatient sacubitril/valsartan initiation.
This study also has several limitations that should be considered when interpreting the results. The small size of the comparison group (n = 16) limits statistical power and may reduce the ability to detect significant differences, particularly at the 30 and 90-day endpoints. The retrospective observational design restricts control over confounding variables and precludes causal inference. Additionally, data on outpatient medication adherence and post-discharge dose titration were not available, which limited the assessment of whether continued therapy or achieving target dosing influenced outcomes. Finally, the analysis did not stratify patients by heart failure phenotype or comorbidity burden, which may have impacted readmission risk and response to therapy. Despite these limitations, the study offers valuable insights into the real-world implementation of ARNI and its intermediate-term outcomes.
9. Conclusion
In this real-world retrospective cohort study, inpatient initiation of sacubitril/valsartan was associated with a significant reduction in 60-day readmissions among patients hospitalized with HFrEF, with no observed difference at 30 or 90 days. These findings suggest that the clinical benefits of ARNI therapy may emerge during the intermediate post-discharge period. Early initiation during hospitalization, coupled with close outpatient follow-up and dose titration, may be critical to optimizing outcomes. Prospective studies with larger comparator cohorts are needed to confirm these results.
References
- Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics—2020 Update. Circulation 141 (2020): e139-e596.
- McMurray JJV, Packer M, Desai AS, et al. Angiotensin-Neprilysin Inhibition versus Enalapril in Heart Failure. N Engl J Med 371 (2014): 993-1004.
- Velazquez EJ, Morrow DA, DeVore AD, et al. Angiotensin-Neprilysin Inhibition in Acute Decompensated Heart Failure. N Engl J Med 380 (2019): 539-548.
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guidelines for the Management of Heart Failure. J Am Coll Cardiol 70 (2017): 776-803.
- Desai AS, Solomon SD, Shah AM, et al. Effect of Sacubitril-Valsartan Versus Enalapril on Aortic Stiffness in Patients With Heart Failure. Circ Heart Fail 12 (2019): e005637.
- Calmingaert S, Lee MMY, Lam CSP, et al. Real-world effectiveness of sacubitril/valsartan in patients with heart failure: A systematic review. ESC Heart Fail 8 (2021): 868-876.
- Koufakis T, Goulis DG, Karras S, et al. Real-world clinical outcomes of sacubitril/valsartan in heart failure: A retrospective cohort study. Cureus 14 (2022): e32752.
- Silverio A, Di Maio M, Polito MV, et al. Real-world use and outcomes of sacubitril/valsartan in heart failure with reduced ejection fraction. Eur Heart J Cardiovasc Pharmacother 9(2023): 232-240.
- Gupta A, Fonarow GC, Liu Y, et al. Association of sacubitril/valsartan with clinical outcomes in routine practice. Int J Cardiol 371 (2023): 247-253.


 Impact Factor: * 5.6
Impact Factor: * 5.6 Acceptance Rate: 74.36%
Acceptance Rate: 74.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 