Cardiac Surgery and Follow-up Diagnosis of the Left Ventricular Pseudoaneurysm Patient
Giulio Benincasa1, Alessia Parascandolo2, Raffaella Bonavita2, Ilaria Bellino1, Michele Altiero1, Vincenzo Schiavone1, Mikko O. Laukkanen1*, Gennaro Alfano1
1Pineta Grande Hospital, 81030, Caserta, Italy
2Experimental Institute of Endocrinology and Oncology G. Salvatore, IEOS CNR, 80014, Naples, Italy
*Corresponding Author: Mikko O. Laukkanen, PhD, Pineta Grande Hospital, Via Domitiana 30, 81030, Caserta, Italy
Received: 13 August 2020; Accepted: 20 August 2020; Published: 26 October 2020
Article Information
Citation: Giulio Benincasa, Alessia Parascandolo, Raffaella Bonavita, Ilaria Bellino, Michele Altiero, Vincenzo Schiavone, Mikko O. Laukkanen, Gennaro Alfano. Cardiac Surgery and Follow-up Diagnosis of the Left Ventricular Pseudoaneurysm Patient. Cardiology and Cardiovascular Medicine 4 (2020): 623-629.
View / Download Pdf Share at FacebookAbstract
Left ventricular pseudoaneurysms (LVP) are false aneurysms caused by the rupture of left ventricular wall as a complication of myocardial infarction, cardiac surgery, congenital heart disease, and, more rarely endocarditis. It is a rare condition that initiates 3-14 days are myocardial infarction affecting 0.5% of the patients. The known risk factors to develop LVP are age, history of hypertension, deficiency of collateral circulation after myocardial infarction, and female gender. The current case report describes LVP in a 58-year old male patient with frontal acute myocardial infarction history. The patient was diagnosed using ECG, CINE-MRI, DE-IR analysis, and thorax-CT followed by urgent cardiac surgery. The diagnosis was an apical pseudoaneurysm caused by previous myocardial necrosis. The Immunohistochemistry suggested cardiac fibrosis. The patient was discharged asymptomatic for angina pectoris, dyspnea and palpitations, and prescribed warfarin against clotting, β blocker/vasodilator, furosemide diuretic, angiotensin receptor blocker, and proton pump inhibitor medication. After six months of follow up, the patient demonstrated the absence of preoperative symptoms.
Keywords
Pseudoaneurysm; Cardiac Surgery
Cardiac Surgery articles
Article Details
Introduction
Left ventricular pseudoaneurysms (LVP) are rare perforations of the ventricular wall located at the pericardium, apical, and lateral regions of the heart. They are commonly caused by a delayed complication of myocardial infarction (four to seven days post-MI) or as a result of surgery, trauma, and endocarditis [1]. In contrast to a true aneurysm, which is caused by a weakness of the myocardial wall and affects all three layers of the myocardial wall, LVP is a result of a rupture of the wall forming a cavity with a narrow neck instead of a broad base frequently observed in true aneurysm [2]. The symptoms of LVP are unspecific presenting chest pain, dyspnea, or heart failure, therefore making the diagnosis challenging. Although in some cases LVP patients may be asymptomatic for years, the damage tends to progress rapidly, thus emphasizing the need for early diagnosis to avoid more severe clinical consequences, such as tamponade, shock, or sudden death of the patient [3].
Currently, the imaging techniques, e.g. echocardiography is used for the diagnosis. However, because none of the echocardiographic criteria is specific enough to accurately diagnose LVP, or to distinguish a real aneurysm from LVP, angiography, cardiac CT, and cardiac MRI are recommended in cases in which the condition of the patient is adequately stable [4]. LVPs appear in echocardiography anechoic or demonstrate accessory chambers close to the cardiac chambers and as a continuation of the left ventricle. Additionally, cardiac MRI or angiography with contrast medium of the laceration area and the extravasation in the pericardium space may help to distinguish LVP from a true aneurysm [5].
Case report
A 58-year old male patient with chronic ischemic cardiopathy was registered to the emergency department at the hospital because of dyspnea. The patient had had four months earlier a frontal acute myocardial infarction that showed sub-occlusive stenosis of the medial and apical intraventricular artery with small-caliber vessel and heterogeneous coronary collateral circulation. As an immediate therapy, the patient received a maximum dose of b-blocker bisoprololo 2.5 mg (Cardicor, Merck), furosemide diuretiuc 25 mg (Lasix, Pfizer), and a combination of cardioaspirin 100 mg (Bayer) and clopidogrel 75 mg (Clopidogrel, Bristol-Myers Squibb) to inhibit platelet aggregation. The patient had been using valproic acid 50 mg (Depakin, Deafarma) for epilepsy that caused dyspnea and palpitation despite given cardiac support medication.
Diagnosis and treatment
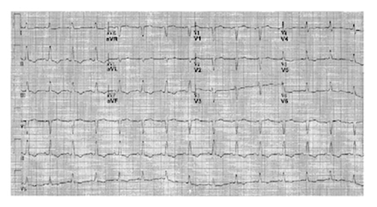
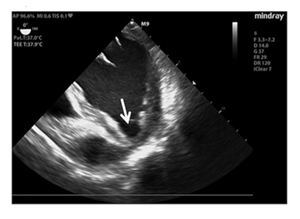
Electrocardiogram (ECG) showed negative T-waves in the sinus rhythm in the inferolateral location (Figure 1), whereas the echocardiographic examination suggested in the left ventricle reduced systolic function (ejection fraction 0.45%) and LVP formation, which extended to lower wall apical collar (Figure 2).
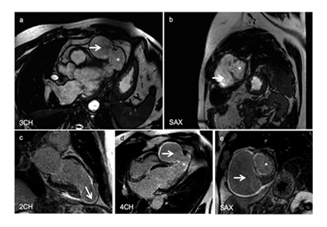
CINE cardiac magnetic resonance imaging (CINE-MRI) with gadolinium contrast agent to study myocardial contractility suggested markedly reduced thickness of the cardiac wall, akinesia of the apex in infarct region, and aneurysmal remodeling of the apex that correlated with a large pseudo-aneurysm cavity (Figure 3a,b). Delayed enhancement inversion recovery (DE-IR) late sequences indicated a transmural infarction in the entire apex that was associated with aneurysmal remodeling and a pseudo-aneurysmal pouch (Figure 3c-e). As a secondary observation thorax computed tomography (CT) scan demonstrated pulmonary nodulation at the antero-basal segment of the right lower lobe of the lungs. Blood test results were at normal range (data not shown). The diagnosis was an apical pseudoaneurysm caused by previous myocardial necrosis.
Figure 3: a. CINE-MRI at the level of 3CH showing a pseudo-aneurysmal pouch (white arrow) and aneurysmal remodeling (*), b. CINE-MRI at the level SAX showing the pseudo-aneurysmal pouch (white arrow) and aneurysmal remodeling (*), c. DE-IR at the level of 2CH suggesting previous myocardial infarction (white arrow), d. DE-IR at the level of 4CH suggesting pseudo-aneurysmal pouch (white arrow) and aneurysmal remodeling (*), e. DE-IR at the level of SAX suggesting pseudo-aneurysmal pouch (white arrow) and aneurysmal remodeling (*).
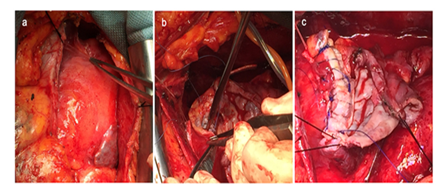
For the treatment of the LVP, the patient was subjected to surgery to remove the apical pseudo-aneurysm and surrounding scar tissue at the crest of the left ventricle (Figure 4).
Follow-up of the patient
After surgery, the patient was discharged in reasonable clinical conditions being asymptomatic for angina pectoris, dyspnea, and palpitations. The patient was prescribed warfarin 5 mg once a day (Coumadin, Bristol-Myers Squibb) against clotting, a “third-generation” β-blocking agent carvedilol 6.25 mg twice a day (Dilatrend, Roche) that has also vasodilator properties, furosemide (diuretic) 25 mg once a day (Lasix, Pfizer), angiotensin receptor blocker valsartan 160 mg once a day (Tareg, Deafarma), and proton pump inhibitor pantoprazole 20 mg once day (Peptazol, Recordati). The patient was encouraged to perform cardiorespiratory rehabilitation. Currently, at a six-month follow-up time point, the patient demonstrates a stable hemodynamic condition and compensation. The preoperative symptoms have reduced markedly.
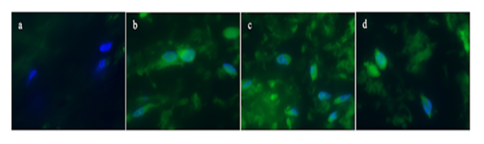
The post-operative immunohistochemistry suggested cardiac fibrosis in the myocardial tissue (Figure 5) demonstrating increased collagen 1A expression in the surgically isolated cardiac tissue.
Figure 5: Immunohistochemistry showing increased collagen formation. a. Staining from the isolated tissue showing no collagen expression, b-d. Staining showing increased collagen expression, c. Staining showing increased collagen expression, d. Staining showing increased collagen expression. Primary antibody: Collagen 1A1 (Cell Signaling Catalog number 39952), Secondary antibody Goat anti-mouse IgG Alexa Fluor 488 (Thermo Fisher , Catalog number A28175). Magnification 200x. Nuclei of the cells were counterstained with HOECST.
Discussion
Because LVP has a high risk of rupture causing the death of the patient and the prediction of the progression of the damage remains a challenge the early diagnosis with consequent immediate surgical intervention is crucially important. The surgical intervention is always recommended to the patients, even to those without symptoms [6] to prevent the enlargement of the pseudoaneurysm lesion. These procedures include a simple closure of the aneurysm neck, closure using pericardial tissue or synthetic graft material, or alternatively, complete removal of the pseudoaneurysm sac [6]. The untreated patients have a 35%-45% risk of enlargement and rupture of the lesion with mortal consequences, whereas the mortality rate decreases down to 23% in surgically treated patients [3, 7].
Fibrosis, which characterizes various pathological conditions as a response to injury, such as cardiac fibrosis, frequently arises from activated myofibroblasts. In general, fibrosis refers to pathological wound healing in which the activated myofibroblasts and their paracrine molecules replace the normal tissue subsequently affecting tissue function and inducing remodeling. The fibrotic response, initiated by e.g. inflammatory cytokines, transforming growth factor-beta (TGFb), int/Wingless (WNT1), or sonic hedgehog (SHH) signaling, progresses often in three phases spanning from initiating phase to effective phase and to amplificative phase [8-10]. Therefore, signaling molecules activating the fibrotic tissue development, such as the abovementioned TGFb, are potential drug targets and even tested in clinical trials [11, 12]. In cardiac fibrosis, the accumulation of extracellular matrix proteins may cause cardiac dysfunction by disrupting the collagen network consequently debilitating cardiomyocyte contraction that results in uncoordinated contraction of cardiomyocyte bundles [13].
Conclusions
In the current work, we report the diagnosis and the therapy of an LVP patient that had an acute myocardial infarction history. The diagnosis, the apical pseudoaneurysm, was based on the combination of ECG, CINE-MRI, DE-IR analysis, and thorax-CT. The pseudo-aneurysm and surrounding scar tissue were removed in open chest cardiac surgery. After a 6-month follow-up of the patient demonstrated markedly reduced preoperative symptoms.
Acknowledgements
This work has been supported by the project POR Campania CUP B63D18000210007.
Conflict of interest
The authors declare no conflict of interest.
References
- Ba'Albaki HA, Clements Jr SD. Left ventricular aneurysm: a review. Clinical Cardiology 12 (1989): 5-13.
- Vlodaver Z, Coe JI, Edwards JE. True and false left ventricular aneurysms. Propensity for the altter to rupture. Circulation 51 (1975): 567-572.
- Frances C, Romero A, Grady D. Left ventricular pseudoaneurysm. Journal of the American College of Cardiology 32 (1998): 557-561.
- Cresti A, Cannarile P, Aldi E, et al. Multimodality imaging and clinical significance of congenital ventricular outpouchings: recesses, diverticula, aneurysms, clefts, and crypts. Journal of Cardiovascular Echography 28 (2018): 9.
- Avegliano G, Conde D, Ruiz MI, et al. Lateral left ventricular wall rupture following acute myocardial infarction: pathophysiological interpretation by multimodality imaging approach. Echocardiography 31 (2014): E296-E299.
- Sahan E, Gül M, Sahan S, et al. Pseudoaneurysm of the mitral–aortic intervalvular fibrosa. Herz 40 (2015): 182-189.
- Si D, Shi K, Gao D, et al. Ruptured left ventricular pseudoaneurysm in the mediastinum following acute myocardial infarction: a case report. European Journal of Medical Research 18 (2013): 2.
- Ma ZG, Yuan YP, Wu HM, et al. Cardiac fibrosis: new insights into the pathogenesis. International Journal of Biological Sciences 14 (2018): 1645.
- Castellone MD, Laukkanen MO. TGF-beta1, WNT, and SHH signaling in tumor progression and in fibrotic diseases. Front Biosci (Schol Ed) 9 (2017): 31-45.
- Biernacka A, Cavalera M, Wang J, et al. Smad3 signaling promotes fibrosis while preserving cardiac and aortic geometry in obese diabetic mice. Circulation: Heart Failure 8 (2015): 788-798.
- Fang L, Murphy AJ, Dart AM. A clinical perspective of anti-fibrotic therapies for cardiovascular disease. Frontiers in Pharmacology 8 (2017): 186.
- Goumans MJ, ten Dijke P. TGF-β signaling in control of cardiovascular function. Cold Spring Harbor Perspectives in Biology 10 (2018): a022210.
- Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cellular and Molecular Life Sciences 71 (2014): 549-574.


 Impact Factor: * 5.6
Impact Factor: * 5.6 Acceptance Rate: 74.36%
Acceptance Rate: 74.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 