Carotid Artery Stenosis in Syncope and in Carotid Sinus Syndrome: A Few Concepts for the General Practitioner to Know
Emran El-Alali1*, Shadi Abu-Halimah2, Laith Maali3, Yaman Alali4
1Department of Internal Medicine, Anne Arundel Medical Center, Maryland, United States
2Associate Professor, Department of Surgery, Vascular and Endovascular Surgery Division, West Virginia University, WV, United States
3Assistant Professor, Department of Neurology, University of Kansas Medical Center, Kansas City, United States
4Internal Medicine Resident, Department of Internal Medicine, Creighton University, Omaha, United States
*Corresponding author: Emran El-Alali, Department of Medicine, Anne Arundel Medical Center, 2001 Medical Pkwy, Annapolis, MD 21401, United States
Received: 07 January 2021; Accepted: 20 January 2021; Published: 25 January 2021
Article Information
Citation: Emran El-Alali, Shadi Abu-Halimah, Laith Maali, Yaman Alali. Carotid Artery Stenosis in Syncope and in Carotid Sinus Syndrome: A Few Concepts for the General Practitioner to Know. Cardiology and Cardiovascular Medicine 5 (2021): 106-122.
View / Download Pdf Share at FacebookAbstract
Background: In clinical practice, some investigations can have low diagnostic yield or little impact on treatment. Objectives: This article reviews the impact of asymptomatic carotid artery stenosis on management of syncope, and the role of carotid Doppler ultrasound in carotid sinus syndrome and in syncope. Unclear concepts in syncope workup and management are identified.
Methods: We conducted a clinical survey of 206 internal medicine providers, to explore how many would consider unilateral carotid revascularization to treat isolated syncope, and how many would consider carotid ultrasound in evaluating carotid sinus syndrome. We searched the literature to identify cases in which carotid revascularization improved syncope, and whether or not carotid ultrasound was used to evaluate carotid sinus syndrome. Literature was reviewed for carotid ultrasound use in syncope.
Results: 34% of medical providers surveyed considered carotid endarterectomy for isolated syncope treatment for unilateral high-grade carotid stenosis and 45% of surveyed providers considered carotid ultrasound in evaluating carotid sinus syndrome. The literature revealed older studies of syncope resolution following carotid endarterectomy in patients with specific characteristics; their detailed features are identified here, and revealed that evaluation of carotid sinus syndrome did not require carotid ultrasound.
Conclusions: Carotid revascularization is not recommended for unilateral asymptomatic carotid artery stenosis to treat isolated syncope, and carotid ultrasound is not needed in the evaluation of carotid sinus syndrome and rates of neurological complications following carotid sinus massage were low.
Keywords
<p>Syncope; Carotid Stenosis; Carotid Hypersensitivity; Carotid Endarterectomy</p>
Article Details
1. Introduction
Syncope is defined as a transient loss of consciousness (TLOC) caused by cerebral hypoperfusion and is associated with an inability to maintain postural tone. It is characterized by a rapid onset, short duration (rarely lasting more than a minute or two), and spontaneous complete recovery [1, 2]. It should be distinguished from nonsyncopal causes of TLOC that are not attributed to cerebral hypoperfusion, which include conditions such as seizures [3], traumatic brain injury (eg, concussion), accidental falls, drug or alcohol intoxication, metabolic disturbances (eg, hypoglycemia), sleep disorders such as narcolepsy and cataplexy, and conversion disorders including pseudoseizures and pseudosyncope.
Causes of syncope are generally grouped into three major categories:
- 1 Reflex (neurally mediated) syncope: includes vasovagal syncope, situational syncope and carotid sinus syndrome.
- 2 Syncope due to orthostatic hypotension (OH): drug-induced OH, volume depletion or autonomic failure (neurogenic OH).
- 3 Cardiovascular syncope: caused by arrhythmias, structural cardiac disease (eg, aortic stenosis, hypertrophic cardiomyopathy) or cardiopulmonary disease (eg, pulmonary embolism [4] or pulmonary hypertension).
1.1 Cerebrovascular disease and syncope
Syncope is caused by a global and transient cerebral hypoperfusion as a result of a decrease in cardiac output. If isolated, it is very unusual for syncope to be related to atherosclerotic cerebrovascular disease, which typically manifests with focal neurologic deficits (ie, transient ischemic attack [TIA] or stroke), because the brain has a redundant blood supply. The brain is supplied by two internal carotid arteries (ie, anterior circulation) and two vertebral arteries (ie, vertebrobasilar or posterior circulation).
The circle of Willis connects the anterior and posterior circulations, with some anatomic variations among individuals [5]. In general, TIA or ischemic stroke is associated with focal neurological deficits without loss of consciousness (LOC), whereas syncope is the opposite. If the posterior circulation is compromised (eg, vertebrobasilar artery insufficiency [VBI] or vertebrobasilar TIA), LOC may occur and usually lasts longer than the TLOC in syncope, but there are always focal signs, such as limb weakness, gait and limb ataxia, vertigo, diplopia, nystagmus, dysarthria, and oropharyngeal dysfunction. Fewer than 1% of cases of VBI have a single presenting symptom [6]. If the anterior circulation is impaired, a focal neurological deficit or retinal ischemia rather than a global decrease in consciousness will occur. A few exceptions will be discussed in this review. In this review, “simple” or “isolated” syncope refers to the absence of focal neurological or retinal ischemia symptoms, which, if present, should prompt a different diagnostic evaluation (ie, for stroke or TIA instead of syncope).
1.2 Carotid artery stenosis versus carotid sinus syndrome (CSS)
Carotid sinus hypersensitivity (CSH) is a heart rate pause, or asystole, lasting ≥3 seconds (cardioinhibitory response) and/or a decrease in systolic blood pressure (SBP) ≥50 mmHg (vasodepressor response) that occurs upon stimulation of carotid baroreceptors (like carotid sinus massage or spontaneous). It may or may not be associated with symptoms [1, 2]. Carotid sinus syndrome (CSS) is diagnosed by the reproduction of symptoms (eg, syncope) as a result of CSH during carotid sinus massage (CSM), with asystole ≥3 seconds or atrioventricular block and/or a decrease in SBP ≥50 mmHg in a patient with syncope of unknown origin compatible with a reflex mechanism. Symptoms of CSS include syncope, lightheadedness (presyncope), or unexplained falls in older patients [1, 2].
1.2.1 In summary: A positive CSM without a history of syncope defines CSH, whereas a history of syncope and its reproduction by CSM defines CSS. CDU only assesses carotid artery stenosis (CAS) caused by atherosclerosis and assesses its degree, this is not diagnostic of CSH or CSS. Although both carotid atherosclerosis and CSH increase with age and may coexist, they are separate entities and require different diagnostic and therapeutic approaches.
2. Materials and Methods
The authors conducted a two-question clinical survey, in the form of clinical case scenarios [7]. The survey was randomly distributed to internal medicine physicians and practitioners, mainly hospital medicine providers. The goals of the survey were clearly explained to survey respondents, which included improving clinical practice by unveiling a few misconceptions in the management of asymptomatic carotid artery disease and syncope, and suggesting cost-effective approaches in such settings. Physicians in training, such as medical residents, interns or students, were excluded from taking the survey. The first question asked about options of management in a 60-year-old man with recurrent unexplained syncope with no neurological deficits, whose workup is negative except for a right-sided high-grade internal carotid stenosis (CAS). The options included: right-sided endarterectomy (CEA) to treat syncope, right-sided CEA to prevent stroke, or medical management for CAS. The second question asked about the most appropriate test for the diagnosis of cause of syncope in a 65-year-old man who collapses when he wears a tight neck collar or when a pressure is applied to his neck. The options included: Carotid ultrasound, carotid sinus massage, or both of these tests. Answers were exported into charts as seen in figures 1 and 2.
3. Results
In total, two hundred and six internal medicine providers responded to the clinical survey [7].
Question 1: One third of medical providers surveyed considered CEA for the new onset of unexplained syncopal episodes in a person with high-grade unilateral CAS (60-79%) who had no transient ischemic attack (TIA) or stroke symptoms. The results are shown in Figure 1.
As can be seen, 34% of respondents (the sum of the first and third bars in Figure-1) would consider CEA for syncope only (4.4%) or for both syncope and stroke prevention (29.6%; “all of above” bar) in a patient with a unilateral high-grade CAS but no TIA or stroke symptoms.
Question 2: Approximately one half of medical providers surveyed considered carotid ultrasound to diagnose the cause of syncope in a patient who collapses when wearing a tight neck collar; when carotid sinus hypersensitivity (CSH) is suspected. The results are shown in Figure 2.
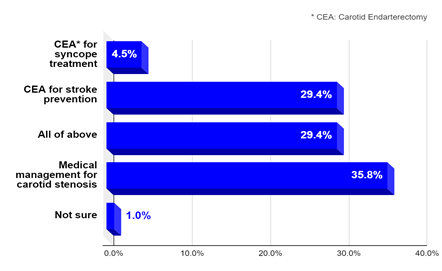
Figure 1: What would you do for unexplained isolated syncope and unilateral high-grade CAS?.
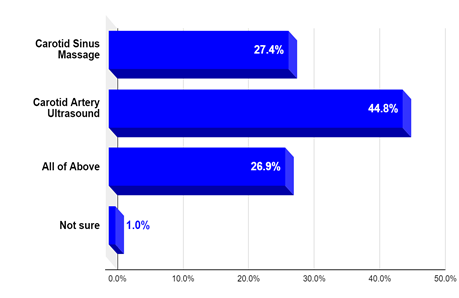
Figure 2: What test is required when carotid sinus hypersensitivity is suspected?.
As we see in a scenario that can be typical for carotid sinus syndrome (CSS), 45% chose CDU as the only needed test for diagnosis. The first option (carotid sinus massage [CSM] only; the most appropriate answer) was chosen by 28% of respondents. 26% considered both tests (CDU and CSM) required for diagnosis.
4. Discussion
4.1 Carotid artery imaging in syncope
Although syncope and, by extension, presyncope are not typical manifestations of carotid artery disease [8, 9], carotid Doppler ultrasound (CDU) has been commonly used in the evaluation of syncope, at a frequency ranging from 10% to around 28% in this setting among different studies from 1970 to 2015 [10-15]. A slight reduction in its use over time is noted (lower in 2000s compared with 1990s). The 2017 American College of Cardiology/American Heart Association/Heart Rhythm Society (ACC/AHA/HRS) Guideline for the Evaluation and Management of Patients With Syncope has clearly recommended against carotid artery imaging in the routine evaluation of syncope in the absence of focal neurological findings (Class III: No benefit) [1]. The 2018 European Society of Cardiology (ESC) Guidelines for the diagnosis and management of syncope state: “EEG, ultrasound of neck arteries, and computed tomography or magnetic resonance imaging of the brain are not indicated in patients with syncope” (Class III: No benefit) [2]. The joint 2011 guidelines from multiple US societies (including the American College of Cardiology, American Heart Association, American Stroke Association, American College of Radiology, and the Society for Vascular Surgery) have recommended against the use of carotid duplex ultrasonography in the routine evaluation of neurological disorders unrelated to focal cerebral ischemia (Class III: No benefit) [16]. The American Academy of Neurology’s Top Five Choosing Wisely recommendations states in recommendation number 2: “Do not perform imaging of the carotid arteries for simple syncope without other neurologic symptoms” [17].
In addition to the society guidelines and recommendations above, several studies have clearly demonstrated a low diagnostic yield or a “low value” of CDU in syncope [12-15, 18-21], and a 2016 article (with a comprehensive literature review) by Dittmar and Feldman [22] has concluded that CDU should not be performed for isolated syncope. However, in this review we emphasize the “no value” of carotid artery imaging in simple or isolated syncope because a positive result in this setting does not add to the diagnostic process for syncope or change the management of an incidentally detected asymptomatic carotid disease in syncope. We discuss the results of a clinical survey that highlights a few misconceptions in isolated syncope workup and in carotid artery disease management, and we suggest interventions to improve our practice.
4.2 Symptomatic versus asymptomatic carotid stenosis
Symptomatic carotid artery disease is the occurrence of neurologic symptoms consistent with TIA or ischemic stroke, characterized by focal neurologic deficit or amaurosis fugax (ie, transient monocular loss of vision), attributed to a significant carotid artery atherosclerotic lesion in the appropriate distribution (eg, right hemispheric symptoms of left-sided weakness attributed to right-sided carotid disease). Carotid symptoms within the previous six months are included in this definition [23, 24]. The term “carotid artery stenosis (CAS)” in this review refers only to the atherosclerotic narrowing of the carotid bifurcation. Carotid endarterectomy (CEA) has been established as an effective treatment in reducing the risk of ischemic stroke and death in patients with symptomatic high-grade (≥70%) CAS in three major randomized clinical trials: the North American Symptomatic Carotid Endarterectomy Trial (NASCET) [23], the European Carotid Surgery trial (ECST ) [24, 25], and the Veterans Affairs Cooperative Study Program (VACS) [26]. Some healthcare providers may consider syncope an indication to treat CAS with CEA or stenting. This was illustrated by the results of a clinical survey (June 2020) of 206 internal medicine physicians and practitioners, showing that one third would consider CEA for new onset of unexplained syncopal episodes in a person with high-grade unilateral CAS (60-79%) who had no TIA or stroke symptoms [7]. As can be seen in figure 1 above (in results section), 34% of respondents; the sum of the first and third bars in Figure-1, would consider CEA for syncope only (4.4%) or for both syncope and stroke prevention (29.6%; “all of above” bar) in a patient with a unilateral carotid stenosis but no TIA or stroke symptoms.
First, regarding the indications for CEA, patients enrolled in three of the largest trials that have shown the effectiveness of CEA in symptomatic CAS [23-26] had symptoms related only to ischemia of the internal carotid artery territory (ie, anterior circulation TIA or stroke) as a result of carotid atherosclerotic stenosis. Symptoms included unilateral motor or sensory disturbance, speech deficit (ie, hemispheric symptoms), or amaurosis fugax. Syncope or dizziness (ie, non-hemispheric symptoms) were not among the carotid stenosis symptomatology, nor were such symptoms included in these trials. Moreover, patients with symptoms related to vertebrobasilar insufficiency only, including syncope or dizziness, were excluded from these major CEA trials. Second, the benefit of CEA is to reduce the risk of stroke and death in patients with symptomatic CAS, because carotid atherosclerosis is a risk factor for both stroke and vascular death [27-30]. Internal carotid artery revascularization (eg, CEA) should typically not resolve or improve non-carotid, or non-hemispheric symptoms like syncope, in a patient with a unilateral high-grade carotid stenosis because the brain still has adequate perfusion from the three remaining intact major arteries (ie, the contralateral carotid and the two vertebral arteries).
We attempted to identify cases in which carotid revascularization may resolve or improve syncope or VBI symptoms. We conducted a literature search using the MEDLINE databases from 1965 to 2020, and the following is a summary of those cases and situations:
- Patients in whom the global cerebral hypoperfusion causing their syncope resulted directly from an obstructive 4-vessel cerebrovascular disease, rather than from a reduction in cardiac output as would occur in simple syncope (ie, cardiogenic, orthostatic or reflex syncope), can benefit from carotid revascularization (ie, CEA) to relieve the global hypoperfusion. Six reported cases were reviewed [31-33], and all experienced syncope, dizziness, and/or orthostatic neurological symptoms (ie, orthostatic TIAs). Cranial and cervical arterial imaging showed 4-vessel occlusion/stenosis (ie, both carotid and both vertebral arteries) in all cases. Carotid endarterectomy resolved their non-carotid symptoms (syncope and dizziness) by increasing global cerebral perfusion.
- Patients with specific anomalies of the cerebral vasculature in which the carotid arteries directly supply the vertebrobasilar (VB) territory, forming a carotid-basilar anastomosis, like with the persistent trigeminal artery or primitive hypoglossal artery, can benefit from carotid revascularization for VBI because CAS is directly detrimental to both the carotid and VB territories. Several reported cases have demonstrated the resolution of syncope or VBI symptoms following carotid endarterectomy or stenting in such conditions [34-37]. The fetal origin of the posterior cerebral artery is the most common variant causing carotid-basilar anastomosis (20 to 40% of individuals) [38, 39]; however, only symptoms of occipital lobe insufficiency occur as a result of carotid disease, without alteration in consciousness.
- Variations in the connection between the anterior and posterior circulation play a role in outcomes of CEA in syncope or VBI. Cardon et al. [40] found that a patent or functioning circle of Willis yielded 90% good outcomes in cases of VBI following CEA, even in patients with associated vertebro-subclavian lesions, compared with 60% in patients with a non-functioning circle of Willis. In two studies [41, 42], the angiographic visualization of the posterior communicating artery (PCoA) after internal carotid injection accurately predicted improvement of VBI in 92 to 93% of patients following CEA compared with 67% [41] or 47% [42] with a non-visualized PCoA, meaning that carotid-to-vertebrobasilar blood flow through the PCoA was critical for the VB territory.
- Rosenthal et al. [43] reported resolution of VBI after CEA in 80% of their series of 114 patients in whom syncope and dizziness were common symptoms. A low intraoperative carotid bifurcation pressure gradient (less than 25%; ie, a high stump pressure) in 59 of 80 patients whose VBI resolved made it difficult to state that the benefit of CEA was due entirely to elimination of the stenotic focus and increased cerebral blood flow; an embolic mechanism from carotid bifurcation has been postulated, causing transient decrease in cerebral perfusion and symptoms of VBI.
- The concept of “steal VBI” as a result of carotid stenosis/occlusion has been demonstrated in several studies [44-49], in which an intracranial vertebrobasilar-carotid steal occurs; that is, blood flow from the posterior to anterior circulation because of insufficient collaterals from anterior communicating or external carotid arteries. This can lead to symptoms of VBI, so restoring the carotid flow will indirectly relieve VBI, including syncope [47-49].
- Concomitant vertebral artery disease can be a source of embolic versus hemodynamic VBI. Ford et al. [50] reported successful treatment of VBI following CEA in 95% of their 46 patients, in whom syncope and dizziness were common symptoms. Because 17 of 18 patients with concomitant uncorrected vertebral/subclavian disease had resolution of VBI, the authors hypothesized that increased total perfusion was the likely mechanism of this outcome. Rosenthal et al. [43] similarly explained why five patients with concomitant vertebral artery disease attained full relief of persistent VBI following a second-side CEA but none required a vertebral artery surgery. Miran et al. [51] reported the resolution of unexplained syncope in a patient with bilateral internal carotid artery high-grade stenosis and one hypoplastic vertebral artery following carotid revascularization, which likely means the problem in their patient was a hemodynamic VBI (ie, posterior circulation symptom) or a near-global cerebral hypoperfusion from 3-vessel disease, as explained in (1) above. In contrast, Toursarkissian et al. [52] reported the persistence of VBI in 3 of 4 patients following an isolated CEA, making an embolic VBI from the concomitant vertebral disease the likely etiology in those cases.
- Definition of VBI and whether syncope and presyncope were included can affect the outcomes of carotid revascularization in VBI. DeWeese et al. [53] and Ouriel et al. [54] showed a low value for CEA in treating syncope, which was considered a “non-classical symptom of VBI” compared with better results for classical VBI symptoms (eg, non-hemispheric motor, sensory, visual symptoms or ataxia). Ricotta et al. [55] used the same definition for non-hemispheric symptoms from the Advisory Council of the National Institute of Neurological and Communicative Disorders and Stroke [56], which did not include syncope as a VBI symptom.
Finally, Fields et al. [57] showed similar outcomes following CEA versus medical treatment for the subgroup with only VBI symptoms and Kashiwazaki et al. [58] reported the resolution of syncope in nine patients with unilateral or bilateral internal carotid artery disease although only four were surgically treated for their carotid disease and five were only treated medically, which makes it difficult to correlate syncope relief with surgical carotid revascularization. McNamanra et al. [59] suggested that CEA has little or no therapeutic value in patients with VBI.
4.2.1 Our conclusion: We did not find sufficient evidence to support carotid revascularization for unexplained syncope in patients with unilateral high-grade carotid stenosis for several reasons, as follows:
- The studies that showed resolution of syncope with CEA are old (ie, 20 years or older) and have not been repeated since the landmark CEA trials were published [23-26].
- Reported VBI symptoms in these studies were heterogeneous, and syncope was not always clearly mentioned or included among VBI symptoms. Other neurological symptoms were variably associated with syncope in most reported cases.
- The reviewed studies included variable coexistence of vertebro-basilar, subclavian, and bilateral carotid artery disease.
- Complete angiographic studies were not obtained in all cases, and such an invasive test cannot be a universal or a practical option in general syncope evaluation.
- Advances in cardiac diagnostic modalities over time have increased detection of the more common causes of syncope, which has likely obviated the need for cerebrovascular interventions.
4.2.2 What could be the proper answer for this patient?: Going back to the patient’s scenario given in the survey above, the appropriate selections for that case scenario with “asymptomatic” high-grade carotid stenosis would be CEA for stroke prevention, which is a class IIa recommendation for asymptomatic stenosis ≥70% [16], or intensive medical therapy alone, with growing evidence that modern intensive medical therapy has reduced the risk of stroke for patients with asymptomatic carotid disease since the landmark CEA trials of the 1990s and early 2000s, and has probably narrowed the gap between the medical and surgical treatment for carotid artery disease [60-65]. Moreover, the American Academy of Neurology’s Top Five Choosing Wisely recommendations, states in recommendation number 5: “Don't recommend carotid endarterectomy for asymptomatic carotid stenosis unless the complication rate is low (<3%)” [17].
4.2.3 The take-home message: The onset of isolated syncope in a patient with a unilateral carotid artery stenosis, without neurological deficit in the last six months does not change the diagnosis to symptomatic CAS, so management strategies should only follow the available evidences and guidelines for “asymptomatic” CAS [64-66], and should not switch to a surgical or an interventional strategy.
4.2.4 Screening for asymptomatic carotid artery stenosis: In the patient’s scenario above (given in the survey), CDU performed for isolated syncope resulted in an unrelated and incidental finding of asymptomatic carotid artery stenosis. Although such a finding can be helpful to guide and influence measures for stroke and cardiovascular disease prevention, the 2011 guidelines on the management of carotid artery disease, from multiple medical societies [16], state that screening asymptomatic individuals (ie, without hemispheric or retinal ischemic symptoms) for carotid stenosis is reasonable only when carotid bruit is heard (Class IIa recommendation), and that screening “may also be considered” when symptomatic atherosclerosis presents in another vascular bed (eg, peripheral arterial disease, coronary artery disease, or aortic aneurysm) or when two or more risk factors for atherosclerosis are present (Class IIb recommendation). It should be noted that the 2014 US Preventive Services Task Force (USPSTF) recommendation statement [67, 68], the 2014 American Heart Association/American Stroke Association (AHA/ASA) guidelines for the primary prevention of stroke [69], and the 2011 updated Society of Vascular Surgery guidelines for management of extracranial carotid disease [8] have recommended against routine screening for asymptomatic carotid stenosis in the general population [8, 67, 68] or in low-risk populations [69].
In conclusion, in simple terms, syncope is not considered an indication to screen for carotid stenosis. Finally, if screening is considered, it should ideally be deferred to the outpatient setting for better resources utilization and to avoid further unnecessary procedures, consultations and a longer hospital stay [70].
4.3 Carotid artery imaging in carotid sinus syndrome
Healthcare practitioners may commonly order CDU in the evaluation of potential CSH as the likely carotid-related disorder causing syncope, although CDU can only assess for carotid stenosis, whereas CSH is diagnosed by carotid sinus massage (CSM). This misconception was unveiled by the clinical survey (June 2020) of 206 internal medicine physicians and practitioners, which showed that 45% would mainly choose carotid ultrasound when CSS is suspected. Results are in Figure 2. As we see in a scenario that can be typical for CSS, 45% chose CDU as the only needed test for diagnosis. The first choice (CSM only) is the most appropriate answer and was chosen by 27% of respondents.
Regarding the third option (all of above: both CDU and CSM are required for diagnosis), the Newcastle protocol for CSM [71] warrants CDU prior to CSM only if carotid bruits are present. If CDU shows >70% stenosis, then CSM should be avoided; for 50 to 70% stenosis, only the supine test will be performed. The usual protocol includes a supine CSM and, if negative, a repeat CSM in a 70° head-up tilt position. First, CDU only assesses carotid stenosis and its degree, which is not diagnostic of CSH or CSS. Second, although CSM should be avoided if a high-grade carotid stenosis (>70%) is present [71], syncope guidelines (ie, the 2018 ESC and 2017 ACC/AHA/HRS guidelines for syncope diagnosis and management) have not considered CDU to be required imaging prior to CSM and have only considered the auscultation of a carotid bruit as a contraindication to CSM [1, 2].
Moreover, when data from nine studies [72-80] that evaluated the safety and cerebrovascular complications of CSM were pooled (a total of 13,145 patients), CDU was not routinely performed prior to CSM, and auscultation of carotid bruits was the sole finding used to exclude patients with potential carotid disease from performing CSM in the majority of these studies. In their prospective study of 1401 patients, Ungar et al. [80] performed CDU only in the presence of carotid bruits, but not for all subjects, to exclude those with critical carotid stenosis prior to CSM whereas Walsh et al. [78] and Lacerda et al. [79] excluded patients with known carotid stenosis >50% documented only on previous CDU if available (ie, prior to the study) or in those with carotid bruits. The main complications of CSM are neurological. Although auscultation of carotids, rather than routine CDU, was the main method required to evaluate for and exclude potential carotid disease prior to CSM in those nine studies [72-80], neurologic complications (TIA or stroke) following CSM were rare, occurred in 37 patients out of 13,145 (0.28%), and only eight developed persistent deficits (0.06%; 6 per 10,000 patients).
Among patients with neurologic complications following CSM (a total of 37), 15 had undergone CDU after the development of TIA or stroke symptoms [72-74, 78, 79]. Interestingly, only six of these 15 showed significant carotid stenosis that would have prevented, or excluded, them from CSM. This means that CDU, if done prior to CSM, would not have prevented neurologic complications in nine of those 15 patients. Interestingly, in one of these studies, by Puggioni et al. [75], patients with carotid bruits were not excluded and carotid massage was performed for 10 seconds rather than the standard 5 seconds recommended by the Newcastle protocol [71]; nevertheless, none of their 1219 patients developed persistent neurologic complications after CSM. Compared with the nine studies [72-80] in which carotid bruits were used to exclude patients from CSM, Richardson et al. [81] evaluated CSM only in patients with carotid bruits. Among their 121 patients with unexplained or recurrent falls and carotid bruit, 18 had moderate carotid stenosis (50-69%), and none had any serious complications after CSM. In addition, four other studies [82-85] evaluated CSM and its complications, but no details were provided in regard to exclusion of patients or how carotid stenosis was evaluated.
4.3.1 In summary: Combining all patient data, we analyzed in this review a total of 15,121 patients from 14 studies [72-85] in whom CSM was performed and its complications were reported. The overall rate of neurologic complications (TIA or stroke) was 0.26% (40 patients) and persistent deficits were reported in only 0.07%. Of note, other contraindications to CSM should be respected; myocardial infarction, TIA, or stroke in the past three months are absolute contraindications. Previous ventricular fibrillation or tachycardia are relative contraindications [71].
4.3.2 Our conclusion: Although the Newcastle protocol warrants CDU if carotid bruit is heard [71] and although carotid bruit is a poor indicator of the presence or severity of carotid artery stenosis [86], we do not find sufficient evidence to support the routine use of CDU prior to performing CSM if the massage is otherwise not contraindicated (as above). We suggest that it is safe to not use CDU before CSM because of the following two reasons:
- The rates of persistent neurologic complications associated with CSM are low, even when CDU is not routinely done to exclude significant carotid stenosis or even when carotid bruit does not exclude patients from CSM,
- The absence of large prospective studies on the predictive value of carotid Doppler ultrasound in this setting.
4.4 How to improve our practice
Unfortunately, according to one study [87], intensive education for internal medicine physicians-in-training on evidence-based guidelines in syncope evaluation did not reduce the use of low-yield neuroimaging. In another study conducted at a major university hospital in the United States, there was an underutilization of orthostatic testing, carotid sinus massage, and prolonged cardiac monitoring with overutilization of imaging studies and neurologic consultation for the evaluation of patients with “faint” [88]. Based on these two studies and on the details in this review, we suggest the use of “electronic clinical decision support systems” (CDSS), which have shown improved adherence to guidelines in general in one review [89], along with “hard-stop alerts”, which resulted in 79% improvement in health outcomes in a systematic review [90].
The following is our suggestion for an in-hospital electronic medical system, using both a hard-stop alert and a CDSS. A progress report of the results and outcomes of this approach after its implementation in our facility will follow in a separate review:
- If the provider uses “dizziness”, “syncope”, “presyncope or lightheadedness” or “carotid sinus hypersensitivity or carotid sinus syndrome” as an indication for carotid ultrasound the system will not allow the order to proceed (hard-stop alert), then,
- A message appears explaining why the above conditions are not indications for carotid ultrasound (CDSS), showing the guidelines for asymptomatic CAS screening, asking the provider to defer screening to the outpatient setting, and reminding the provider of carotid massage for suspected carotid hypersensitivity and of the actual inpatient indication for this test: focal neurological deficits, stroke, or amaurosis fugax.
Acknowledgment
Joyce C Miller, MLS
Medical Librarian, Anne Arundel Medical Center, Annapolis, Maryland, E-mail: jmiller@aahs.org ; Tel: +1(443) 481-4877
References
- Shen W-K, Sheldon RS, Benditt DG, Cohen MI, Forman DE, Goldberger ZD, et al. 2017 ACC/AHA/HRS Guideline for the Evaluation and Management of Patients With Syncope: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 136 (2017): e60-e122.
- Brignole M, Moya A, de Lange FJ, Deharo J-C, Elliott PM, Fanciulli A, et al. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J 39 (2018): 1883-1948.
- Sheldon R, Rose S, Ritchie D, Connolly SJ, Koshman M-L, Lee MA, et al. Historical criteria that distinguish syncope from seizures. J Am Coll Cardiol 40 (2002): 142-148.
- Pop C, Ianos R, Matei C, Mercea D, Todea B, Dicu D, et al. Prospective Study of Pulmonary Embolism Presenting as Syncope. Am J Ther 26 (2019): e301-e307.
- Circle of Willis Anatomy (2020).
- Savitz SI, Caplan LR. Vertebrobasilar disease. N Engl J Med 352 (2005): 2618-2626.
- What would you do in the following clinical scenarios? (2020).
- Ricotta JJ, Aburahma A, Ascher E, Eskandari M, Faries P, Lal BK. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vasc Surg 54 (2011): e1-31.
- Clark Gillett R Jr. Should Carotid Artery Stenosis Be Examined as a Cause of Dizziness?. AFP 83 (2011): 879.
- Pires LA, Ganji JR, Jarandila R, Steele R. Diagnostic patterns and temporal trends in the evaluation of adult patients hospitalized with syncope. Arch Intern Med 161 (2001): 1889-1895.
- Keyhani S, Cheng EM, Naseri A, Halm EA, Williams LS, Johanning J, et al. Common Reasons That Asymptomatic Patients Who Are 65 Years and Older Receive Carotid Imaging. JAMA Intern Med 176 (2016): 626-633.
- Mendu ML, McAvay G, Lampert R, Stoehr J, Tinetti ME. Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med 169 (2009): 1299-1305.
- Barvalia M, Silber D, DiVita M, Joshi A, Wasty N, Cohen M. Utility of carotid duplex ultrasonography in a general inner-city hospital. Cardiovasc Ultrasound 12 (2014): 48.
- Scott JW, Schwartz AL, Gates JD, Gerhard-Herman M, Havens JM. Choosing wisely for syncope: low-value carotid ultrasound use. J Am Heart Assoc 3 (2014): e001063.
- Pournazari P, Oqab Z, Sheldon R. Diagnostic Value of Neurological Studies in Diagnosing Syncope: A Systematic Review. Can J Cardiol 33 (2017): 1604-1610.
- Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, et al. 2011 ASA/ACCF/AHA/AANN /AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS Guideline on the Management of Patients With Extracranial Carotid and Vertebral Artery Disease. Stroke 42 (2011): e464-e540.
- Langer-Gould AM, Anderson WE, Armstrong MJ, Cohen AB, Eccher MA, Iverson DJ, et al. The American Academy of Neurology’s top five choosing wisely recommendations. Neurology 81 (2013): 1004-1011.
- Schnipper JL, Ackerman RH, Krier JB, Honour M. Diagnostic yield and utility of neurovascular ultrasonography in the evaluation of patients with syncope. Mayo Clin Proc 80 (2005): 480-488.
- Kadian-Dodov D, Papolos A, Olin JW. Diagnostic utility of carotid artery duplex ultrasonography in the evaluation of syncope: a good test ordered for the wrong reason. Eur Heart J Cardiovasc Imaging 16 (2015): 621-625.
- Johnson PC, Ammar H, Zohdy W, Fouda R, Govindu R. Yield of diagnostic tests and its impact on cost in adult patients with syncope presenting to a community hospital. South Med J 107 (2014): 707-714.
- Linzer M, Yang EH, Estes NA 3rd, Wang P, Vorperian VR, Kapoor WN. Diagnosing syncope. Part 1: Value of history, physical examination, and electrocardiography. Clinical Efficacy Assessment Project of the American College of Physicians. Ann Intern Med 126 (1997): 989-996.
- Dittmar PC, Feldman LS. Carotid artery ultrasound for syncope. J Hosp Med 11 (2016): 117-119.
- Barnett HJM, Taylor DW, Haynes RB, Sackett DL, Peerless SJ, Ferguson GG, et al. Beneficial Effect of Carotid Endarterectomy in Symptomatic Patients With High-Grade Carotid Stenosis. N Engl J Med 325 (1991): 445-453
- MRC European Carotid Surgery Trial: Interim Results for Symptomatic Patients With Severe (70-99%) or With Mild (0-29%) Carotid Stenosis. European Carotid Surgery Trialists’ Collaborative Group. Lancet 337 (1991).
- Randomised Trial of Endarterectomy for Recently Symptomatic Carotid Stenosis: Final Results of the MRC European Carotid Surgery Trial (ECST). Lancet 351 (1998).
- Mayberg MR, Wilson SE, Yatsu F, Weiss DG, Messina L, Hershey LA, et al. Carotid Endarterectomy and Prevention of Cerebral Ischemia in Symptomatic Carotid Stenosis. Veterans Affairs Cooperative Studies Program 309 Trialist Group. JAMA 266 (1991).
- Chimowitz MI, Weiss DG, Cohen SL, Starling MR, Hobson RW. Cardiac prognosis of patients with carotid stenosis and no history of coronary artery disease. Veterans Affairs Cooperative Study Group 167. Stroke 25 (1994).
- Goessens BM, Visseren FL, Kappelle LJ, Algra A, van der Graaf Y. Asymptomatic carotid artery stenosis and the risk of new vascular events in patients with manifest arterial disease: the SMART study. Stroke 38 (2007).
- Flaherty ML, Kissela B, Khoury JC, Alwell K, Moomaw CJ, Woo D, et al. Carotid artery stenosis as a cause of stroke. Neuroepidemiology 40 (2013): 36-41.
- Touzé E. Natural history of asymptomatic carotid artery stenosis. Rev Neurol 164 (2008): 793-800.
- AhChong AK, Law CB, Chiu KM. Carotid endarterectomy for non-hemispheric cerebrovascular symptoms: an unusual indication. Hong Kong Med J 5 (1999).
- Flanagan CP, Sheth PD, Simons JP. Positional transient loss of consciousness and hemispheric deficits in the setting of severe four-vessel extracranial cerebrovascular disease. Journal of Vascular Surgery Cases and Innovative Techniques 5 (2019): 461.
- Stark RJ, Wodak J. Primary orthostatic cerebral ischaemia. J Neurol Neurosurg Psychiatry 46 (1983).
- Sneha Konda, Samantha Dayawansa, Soren Singel, Jason H Huang. Pseudo subclavian steal syndrome: Case report. Int J Surg Case Rep 16 (2015): 177-180.
- Battista RA, Kwartler JA, Martinez DM. Persistent trigeminal artery as a cause of dizziness. Ear Nose Throat J 76 (1997).
- Katoh M, Kamiyama H, Kobayashi N, Makino K, Takano K, Tokumitsu N, et al. Severe stenosis of the internal carotid artery presenting as loss of consciousness due to the presence of a primitive hypoglossal artery: a case report. Surg Neurol 51 (1999).
- Kanazawa R, Ishihara S, Okawara M, Ishihara H, Kohyama S, Yamane F. A successful treatment with carotid arterial stenting for symptomatic internal carotid artery severe stenosis with ipsilateral persistent primitive hypoglossal artery: case report and review of the literature. Minim Invasive Neurosurg 51 (2008).
- Zampakis P, Panagiotopoulos V, Petsas T, Kalogeropoulou C. Common and uncommon intracranial arterial anatomic variations in multi-detector computed tomography angiography (MDCTA). What radiologists should be aware of. Insights Imaging 6 (2015): 33.
- Kovac JD, Stankovic A, Stankovic D, Kovac B, Šaranovic D. Intracranial arterial variations: a comprehensive evaluation using CT angiography. Med Sci Monit 20 (2014).
- Cardon A, Kerdiles Y, Lucas A, Podeur L, Ferte L, Du J L, et al. Results of isolated carotid surgery in patients with vertebrobasilar insufficiency. Ann Vasc Surg 12 (1998).
- Harward TR, Wickbom IG, Otis SM, Bernstein EF, Dilley RB. Posterior communicating artery visualization in predicting results of carotid endarterectomy for vertebrobasilar insufficiency. Am J Surg 148 (1984).
- Hamann H, Vollmar JF. Carotid endarterectomy in vertebrobasilar insufficiency. Langenbecks Arch Chir 369 (1986).
- Rosenthal D, Cossman D, Ledig CB, Callow AD. Results of carotid endarterectomy for vertebrobasilar insufficiency: an evaluation over ten years. Arch Surg 113 (1978).
- Shichinohe H, Kuroda S, Houkin K, Ushikoshi S, Iwasaki Y, Abe H. [Two cases of “steal VBI” with stenosis of the internal carotid artery]. No Shinkei Geka 29 (2001).
- Schott B, Michel D, Goutelle A. Carotid stenoses and thromboses with vertebral-basilar manifestationa. Basilar-carotid artery steal?. Rev Neurol 121 (1969).
- Bogousslavsky J, Regli F. Transient ischemic attacks before and after occlusion of the internal carotid artery. Rev Neurol 139 (1983).
- Bogousslavsky J, Regli F. Vertebrobasilar transient ischemic attacks in internal carotid artery occlusion or tight stenosis. Arch Neurol 42 (1985).
- Yanagihara T, Klass DW, Piepgras DG, Houser OW. Brief loss of consciousness in bilateral carotid occlusive disease. Arch Neurol 46 (1989).
- Kader I, Jones SM, Harrison C, Miteff F, Kumar S. Common Carotid Artery Occlusion Presenting with Recurrent Syncopal Episodes. Neurol Int 8 (2016).
- Ford JJ, Baker WH, Ehrenhaft JL. Carotid endarterectomy for nonhemispheric transient ischemic attacks. Arch Surg 110 (1975).
- Miran MS, Suri MF, Qureshi MH, Ahmad A, Suri MK, Basreen R, et al. Syncope in Patient with Bilateral Severe Internal Carotid Arteries Stenosis/Near Occlusion: A Case Report and Literature Review. J Vasc Interv Neurol 9 (2016).
- Toursarkissian B, Rubin BG, Reilly JM, Thompson RW, Allen BT, Sicard GA. Surgical treatment of patients with symptomatic vertebrobasilar insufficiency. Ann Vasc Surg 12 (1998).
- DeWeese JA, Rob CG, Satran R, Marsh DO, Joynt RJ, Summers D, et al. Results of carotid endarterectomies for transient ischemic attacks-five years later. Ann Surg 178 (1973): 258.
- Ouriel K, May AG, Ricotta JJ, DeWeese JA, Green RM. Carotid endarterectomy for nonhemispheric symptoms: predictors of success. J Vasc Surg 1 (1984).
- Ricotta JJ, O’Brien MS, DeWeese JA. Carotid endarterectomy for non-hemispheric ischaemia: long-term follow-up. Cardiovasc Surg 2 (1994).
- A classification and outline of cerebrovascular diseases. II. Stroke 6 (1975).
- Fields WS, Maslenikov V, Meyer JS, Hass WK, Remington RD, Macdonald M. Joint study of extracranial arterial occlusion. V. Progress report of prognosis following surgery or nonsurgical treatment for transient cerebral ischemic attacks and cervical carotid artery lesions. JAMA 211 (1970).
- Kashiwazaki D, Kuroda S, Terasaka S, Ishikawa T, Shichinohe H, Aoyama T, et al. [Carotid occlusive disease presenting with loss of consciousness]. No Shinkei Geka 33 (2005).
- McNamara JO, Heyman A, Silver D, Mandel ME. The value of carotid endarterectomy in treating transient cerebral ischemia of the posterior circulation. Neurology 27 (1977).
- Abbott AL. Medical (nonsurgical) intervention alone is now best for prevention of stroke associated with asymptomatic severe carotid stenosis: results of a systematic review and analysis. Stroke 40 (2009).
- Marquardt L, Geraghty OC, Mehta Z, Rothwell PM. Low risk of ipsilateral stroke in patients with asymptomatic carotid stenosis on best medical treatment: a prospective, population-based study. Stroke 41 (2010).
- Spence JD, Coates V, Li H, Tamayo A, Muñoz C, Hackam DG, et al. Effects of intensive medical therapy on microemboli and cardiovascular risk in asymptomatic carotid stenosis. Arch Neurol 67 (2010).
- Woo K, Garg J, Hye RJ, Dilley RB. Contemporary results of carotid endarterectomy for asymptomatic carotid stenosis. Stroke 41 (2010).
- Raman G, Moorthy D, Hadar N, Dahabreh IJ, O’Donnell TF, Thaler DE, et al. Management strategies for asymptomatic carotid stenosis: a systematic review and meta-analysis. Ann Intern Med 158 (2013).
- Constantinou J, Jayia P, Hamilton G. Best evidence for medical therapy for carotid artery stenosis. J Vasc Surg 58 (2013).
- Chambers BR, Donnan GA. Carotid endarterectomy for asymptomatic carotid stenosis. Cochrane Database Syst Rev 2005 (2005).
- Jonas DE, Feltner C, Amick HR, Sheridan S, Zheng ZJ, Watford DJ, et al. Screening for asymptomatic carotid artery stenosis: a systematic review and meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med 161 (2014).
- LeFevre ML. Screening for asymptomatic carotid artery stenosis: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 161 (2014).
- Meschia JF, Bushnell C, Boden-Albala B, Braun LT, Bravata DM, Chaturvedi S, et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 45 (2014).
- Burns SJ, Orner JD, Lacy ME. Avoidance of Carotid Ultrasound in Syncope-Keeping It Simple: A Teachable Moment. JAMA Intern Med 176 (2016).
- Kenny RA, O’Shea D, Parry SW. The Newcastle protocols for head-up tilt table testing in the diagnosis of vasovagal syncope, carotid sinus hypersensitivity, and related disorders. Heart 83 (2000): 564-569.
- Munro NC, McIntosh S, Lawson J, Morley CA, Sutton R, Kenny RA. Incidence of complications after carotid sinus massage in older patients with syncope. J Am Geriatr Soc 42 (1994): 1248-1251.
- Davies AJ, Kenny RA. Frequency of neurologic complications following carotid sinus massage. Am J Cardiol 81 (1998): 1256-1257.
- Richardson D. Complications of carotid sinus massage - a prospective series of older patients. Age and Ageing (2000): 413-417.
- Puggioni E, Guiducci V, Brignole M, Menozzi C, Oddone D, Donateo P, et al. Results and complications of the carotid sinus massage performed according to the “method of symptoms.” Am J Cardiol 89 (2002): 599-601.
- Kerr SRJ, Pearce MS, Brayne C, Davis RJ, Kenny RA. Carotid Sinus Hypersensitivity in Asymptomatic Older Persons: Implications for Diagnosis of Syncope and Falls. Arch Intern Med 166 (2006): 515-520.
- Benchimol M, Oliveira-Souza R. Diagnostic Relevance of the Carotid Sinus Massage During a Head Up Tilt Table Test (HUTT). Arq Bras Cardiol 90 (2008).
- Walsh T, Clinch D, Costelloe A, Moore A, Sheehy T, Watts M, et al. Carotid sinus massage – How safe is it?. Age Ageing 35 (2006): 518-520.
- LG C, Pedrosa RC, Lacerda RC, Santos MC, Brasil AT, Siqueira-Filho AG. Complications Related to Carotid Sinus Massage in 502 Ambulatory Patients. Arq Bras Cardiol 92 (2009).
- Ungar A, Rivasi G, Rafanelli M, Toffanello G, Mussi C, Ceccofiglio A, et al. Safety and tolerability of Tilt Testing and Carotid Sinus Massage in the octogenarians. Age Ageing 45 (2016): 242-248.
- Richardson DA, Shaw FE, Bexton R, Steen N, Kenny RA. Presence of a Carotid Bruit in Adults With Unexplained or Recurrent Falls: Implications for Carotid Sinus Massage. Age Ageing 31 (2002).
- Kumar NP, Thomas A, Mudd P, Morris RO, Masud T. The Usefulness of Carotid Sinus Massage in Different Patient Groups. Age Ageing 32 (2003).
- Paling D, Vilches-Moraga A, Akram Q, Atkinson O, Staniland J, Paredes-Galán E. Carotid Sinus Syndrome Is Common in Very Elderly Patients Undergoing Tilt Table Testing and Carotid Sinus Massage Because of Syncope or Unexplained Falls. Aging Clin Exp Res 23 (2011).
- Tan MP, Newton JL, Reeve P, Murray A, Chadwick TJ, Parry SW. Results of carotid sinus massage in a tertiary referral unit—is carotid sinus syndrome still relevant? Age Ageing 38 (2009): 680-686.
- Cooke J, Carew S, Costelloe A, Sheehy T, Quinn C, Lyons D. The Changing Face of Orthostatic and Neurocardiogenic Syncope With Age. QJM 104 (2011).
- Hankey GJ, Warlow CP. Symptomatic Carotid Ischaemic Events: Safest and Most Cost Effective Way of Selecting Patients for Angiography, Before Carotid Endarterectomy. BMJ 300 (1990).
- Sclafani JJ, My J, Zacher LL, Eckart RE. Intensive education on evidence-based evaluation of syncope increases sudden death risk stratification but fails to reduce use of neuroimaging. Arch Intern Med 170 (2010).
- Brignole M, Malasana G, Sherwood RP, Daccarett M, Jetter TL, Hamdan MH. Evaluation of patients with “faint” in an American teaching hospital: a dire need for a standardized approach. Pacing Clin Electrophysiol 34 (2011).
- Beeler PE, Bates DW, Hug BL. Clinical decision support systems. Swiss Med Wkly 144 (2014).
- Powers EM, Shiffman RN, Melnick ER, Hickner A, Sharifi M. Efficacy and unintended consequences of hard-stop alerts in electronic health record systems: a systematic review. J Am Med Inform Assoc 25 (2018).


 Impact Factor: * 5.6
Impact Factor: * 5.6 Acceptance Rate: 74.36%
Acceptance Rate: 74.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 