Development of In-Hospital Outcomes in Patients undergoing Transcatheter Aortic Valve Implantation (TAVI) at an Interdisciplinary Heart Center: A Single-Center Experience of 489 Consecutive Cases
Mukaram Rana1, Margit Niethammer1, Christian Sellin2, Hilmar Dörge2, Holger Eggebrecht1,3, and Volker Schächinger1*
1Herz-Thorax-Zentrum Fulda, Medizinische Klinik I (Kardiologie, Angiologie, Intensivmedizin), Klinikum Fulda, Universitätsmedizin Marburg - Campus Fulda, Fulda
2Herz-Thorax-Zentrum Fulda, Klinik für Herz- und Thoraxchirurgie, Klinikum Fulda, Universitätsmedizin Marburg - Campus Fulda, Fulda
3Cardiologisches Centrum Bethanien (CCB), Frankfurt a. M.
*Corresponding author: Volker Schächinger, Herz-Thorax-Zentrum Fulda, Medizinische Klinik I (Kardiologie, Angiologie, Intensivmedizin), Klinikum Fulda, Universitätsmedizin Marburg - Campus Fulda, Fulda.
Received: 23 December 2022; Accepted: 30 December 2022; Published: 13 March 2023
Article Information
Citation:
Mukaram Rana, Margit Niethammer, Christian Sellin, Hilmar Dörge, Holger Eggebrecht, and Volker Schächinger. Development of In-Hospital Outcomes in Patients undergoing Transcatheter Aortic Valve Implantation (TAVI) at an Interdisciplinary Heart Center: A Single-Center Experience of 489 Consecutive Cases. Cardiology and Cardiovascular Medicine 7 (2023): 52-68.
View / Download Pdf Share at FacebookAbstract
Background: Transcatheter Aortic Valve Implantation (TAVI) has emerged over time, reflected in appropriate adjustments in the European Society of Cardiology (ESC) guidelines in 2007, 2012 and 2017.
Objective: The aim of this study was to analyze in-hospital outcomes after TAVI in the development within a single heart center over a period of 10 years depending on adjustments in the guidelines, infrastructural and procedural determinants
Methods: 489 consecutive patients who underwent TAVI from 2010 and 2019 at our center were analyzed retrospectively. Patients were divided into 3 groups of different treatment circumstances depending on guidelines adjustments and local infrastructural progress (group 1: 2010-2015 (n = 132), group 2: 2016-2017 (n = 155), group 3: 2018-2019 (n = 202). The primary endpoint was defined as all-cause in-hospital mortality. Secondary endpoints were selected according to the Valve Academic Research Consortium (VARC)-2 definitions. Multivariate logistic regression analysis was performed to determine predictors of in-hospital mortality. Statistical significance was assumed for p < 0.05.
Results: 489 patients (346 (70.8 %) transfemoral and 143 (29.2 %) transapical) underwent TAVI. Comparing periods (group 1 vs. 2 vs. 3) age (82.1 ± 6.2 vs. 82.5 ± 4.8 vs. 81.1 ± 5.1 years, p = 0.012) and EuroSCORE II (8.4 ± 6.0 vs. 5.8 ± 4.9 vs. 5.5 ± 5.0 %, p < 0.001) declined over time. Rates of in-hospital mortality decreased significantly (9.1 % vs. 5.8 % vs. 2.5 %, p = 0.029), especially with observed-to-expected mortality ratios indicating a disproportionate decline of in-hospital mortality (1.08 vs. 1.00 vs. 0.45). Furthermore, post-procedural complications, such as acute kidney injury stage 3 (10.6 % vs
Keywords
<p>Aortic stenosis (AS); In-hospital outcomes; Transcatheter Aortic Valve Implantation (TAVI)</p>
Article Details
Graphical Abstract:
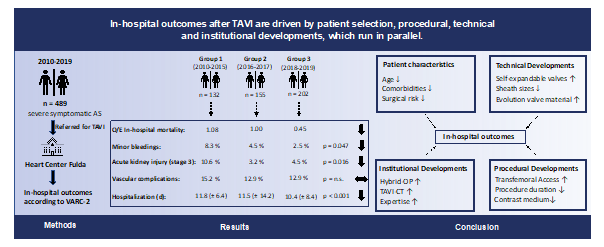
1. Introduction
Aortic stenosis (AS) is the third most common cardiovascular disease after coronary artery disease and arterial hypertension and the leading primary valvular heart disease in Europe and North America [1]. As the onset of symptoms is associated with a shortage of life expectancy and a poor outcome, both appropriate diagnosis and treatment are of major importance [2,3]. Since the first Transcatheter Aortic Valve Implantation (TAVI) has been performed by Alain Cribier in 2002, this interventional approach has experienced rapid dissemination and thus has been accepted as viable alternative in the treatment of patients at high and intermediate surgical risk [4,5]. Although remarkable improvements in outcomes after TAVI could be observed over the last years, there is still a paucity of real-world data analyzing the developments, which have influenced clinical results after TAVI. In the Heart-Thorax Center Fulda (Herz-Thorax Zentrum Fulda) the two disciplines performing TAVI, the cardiology department and the cardiac surgery department are closely connected, defining patient care from a single source. In addition, there is a staff continuity over years, developing the TAVI program together. The main objective of this study was to analyze under these conditions the impact of changing guideline recommendations, technical, institutional and procedural developments on in-hospital outcomes after TAVI.
2. Material and Methods
2.1 Study Design and Population
In this single-center study we retrospectively analyzed data of patients undergoing TAVI at our heart center. From 01.01.2010 until 31.12.2019 a total of 489 consecutive patients with severe symptomatic aortic stenosis were enrolled in this study. Three groups (group I: 2010 – 2015 (n = 132), group II: 2016 – 2017 (n= 155) and group III: 2018 – 2019 (n= 202) were created according to changing ESC guidelines (2007, 2012 and 2017) and institutional developments to compare outcomes between different time periods [5-7]. Patients with symptomatic aortic stenosis requiring a TAVI as decided by the local heart team were eligible for inclusion in this study. Patients with absolute contraindications as defined in the current European Society of Cardiology guidelines were excluded. This retrospective study was approved by the ethics committee at the University of Marburg (ek_mr_010720_rana).
2.2 Demographics and Clinical Features
Data were retrieved from our local TAVI registry, discharge reports and internal electronical database. Demographic and clinical data included age, gender, body mass index (BMI), body surface area (BSA), cardiovascular risk factors (hypertension, diabetes mellitus, renal insufficiency, hyperlipidemia, obesity, and smoking) and a history of cardiac decompensation. Severity of symptoms was assessed using the New York Heart Association (NYHA) and the Canadian Cardiovascular Society (CCS) classification. Data of relevant cardiac comorbidities (history of ST-elevation myocardial infarction (STEMI) or Non-ST-elevation myocardial infarction (NSTEMI), coronary heart disease with 1-, 2-, 3- vessel disease, left main artery disease) and non-cardiac comorbidities (peripheral and cerebrovascular disease, chronic obstructive pulmonary disease (COPD), stroke, transient ischemic attack (TIA), history of cancer and anemia) were collected from medical reports and our database, if available. Furthermore, patients were screened for prior cardiac interventions, such as bypass and valve operations, balloon valvuloplasty, percutaneous coronary intervention (PCI) and others (e.g. permanent pacemaker (PPM) implantation). Levels of cardiovascular biomarkers (Troponin T and N-terminal pro-B-type natriuretic peptide, creatinine, hemoglobin, and platelets were measured in blood samples, which were obtained from patients before the procedure.
2.3 Preoperative Echocardiography, CT scan and ECG
Preoperative echocardiography was conducted to obtain the left ventricular ejection fraction (LVEF), the mean pressured gradient (MPG), the aortic valve orifice area (AVA) and the aortic annulus diameter. CT scans, which became a mandatory part of the preprocedural planning in 2016, were used to assess the access route (diameters of iliofemoral arteries, presence of kinking, aortic aneurysm, and porcelain aorta), to calculate perimeter- and area-derived diameters of the aortic annulus and the distance of the left and right coronary artery to the aortic annulus. Electrocardiograms (ECGs) were recorded and analyzed on admission and postprocedure. ECGs were screened for rhythm (sinus rhythm or atrial fibrillation (AF)), atrioventricular blocks (degree 1 to 3), left bundle branch block (LBBB), right bundle branch block (RBBB), left anterior fascicular block (LAFB).
2.4 Procedural Characteristics
General procedural characteristics of interest comprised procedure duration, contrast medium consumption, fluoroscopy time and radiation dose. Specific procedure-related characteristics were access route (transfemoral or transapical), valve types (balloon-expandable or self-expandable), valve and sheath sizes, predilatation, rapid pacing, postdilatation, conversion to transapical access or surgery, use of cardiopulmonary bypass (CBP) and valve-in-valve procedures.
2.5 Postprocedural Outcomes
The primary endpoint was defined as all-cause in-hospital mortality. Following secondary endpoints were selected using the Valve Academic Research Consortium (VARC)-2 definitions [8]: Myocardial infarction, stroke, TIA, bleeding (life-threatening bleeding, major and minor bleeding), acute kidney injury (AKIN stage 1-3 and the need of dialysis), access site and access-related complications (major vascular complication, minor vascular complication and percutaneous closure device failure), conduction disturbances and arrhythmias (new atrioventricular (AV) blocks (degree 1-3), RBBB, LBBB, LAFB, new permanent pacemaker (PPM) implantation, new onset of atrial fibrillation (AF), any new arrhythmia resulting in hemodynamic instability or requiring therapy). Furthermore, valve malposition, ventricle injury, pericardial tamponade, endocarditis, sepsis, paravalvular insufficiency, length of hospitalization and stay at intermediate care unit (IMC) and intensive care unit (ICU) were assessed.
2.6 Statistical Analysis
Categorial data are presented as absolute numbers and percentages. The Shapiro-Wilk was used to test the normal distribution for all continuous variables. Continuous variables are expressed as mean (± standard deviation [SD]) and median (interquartile range [IQR]: 25th to 75th percentiles). Categorial variables were compared between the three groups performing pairwise chi-square test. Inter-group comparisons for continuous variables were performed using Kruskal Wallis test or ANOVA as appropriate. Multivariate logistic regression analysis was performed using the enter method to determine independent predictors of in-hospital mortality. Statistical significance was assumed at a p-value less than 0.05. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 26.0 (IBM Corp., Armonk, N.Y., USA).
3. Results
3.1 Patient Characteristics at Baseline
Demographics, clinical features, and comorbidities at baseline per time interval are provided in Table 1 and 2. From January 2010 to December 2019, a total of 489 consecutive patients with severe AS were enrolled in this study. Patients in group 3 (years 2018-2019) were significantly younger, included more men and presented a significant lower predicted surgical risk as assessed by the EuroSCORE 2. Comorbidities, such as peripheral vascular disease (PVD), cerebrovascular disease (CVD), anemia and a history of cancer were significantly more prevalent in group 1 (years 2010-2015). Patients in group 3 displayed significant lower rates of previous myocardial infarction, prior percutaneous coronary interventions and bypass operations.
3.2 Preprocedural Imaging and ECG
The results of preprocedural imaging (echocardiography and CT scan) and ECG are shown in Supplementary Tables 2,3 and 4. Patients in group 2 and 3 had a better left ventricular ejection fraction (LVEF) than patients in group 1. There were significant higher rates of patients with aortic aneurysm and porcelain aorta in group 1.
3.3 Preprocedural Blood Examinations
Blood examinations prior to TAVI revealed a significant better renal function (1.6 ± 1.1 vs. 1.2 ± 0.4 vs. 1.3 ± 0.9 mg/dl, p = 0.005) and higher hemoglobin values (12.1 ± 1.9 vs. 12.5 ± 1.7 vs. 12.6 ± 1.8 g/dl, p = 0.009) in group 3. NT-pro-BNP levels were significantly lower in group 3. Results of preprocedural blood examinations can be seen in Supplementary Table 1.
3.4 Procedural Characteristics
Procedural outcomes are summarized in Supplementary Table 5. The annual number of TAVI procedures has increased from n = 13 (2010) to n = 112 (2019) (Supplementary Figure 1). All 489 TAVI procedures were performed under general anesthesia (100 %). Over time, a total of 345 (70.6 %) transfemoral TAVI procedures were performed with an increasing frequency from the first to the third period. The use of self-expandable transcatheter heart valves increased significantly during the observation period requiring smaller sheath sizes. Procedure duration and laboratory time decreased over time and were significantly lower in group 3. Likewise, contrast volume consumption could be reduced significantly (136.0 ± 66.7 vs. 167.6 ± 84.6 vs. 119.6 ± 56.7 ml, p < 0.001). Rates of conversion to open surgery during TAVI were rare and showed a downward trend from 1.5 % (group 1) to 0.5 % (group 3).
3.5 Postprocedural Outcomes
In-hospital outcomes are depicted in Table 3. Rates of In-hospital mortality showed a significant decrease during the observation period (9.1 % vs. 5.8 % vs. 2.5 %, p = 0.029) with a disproportionate decline of the risk-adjusted mortality (observed-to-expected (O/E) mortality: 1.08 vs. 1.00 vs. 0.45; Figure 1). Overall bleeding complications showed a downward trend including significantly lower rates of minor bleedings (8.3 % vs. 4.5 % vs. 2.5 %, p = 0.047) in group 3. The prevalence of acute kidney injury (AKIN) (all stages) sank between group 1 and 3 (53.8 % vs. 34.2 % vs. 29.2 %, p < 0.001). Furthermore, vascular access site and access-related complications decreased numerically without reaching statistical significance. Conduction disturbances including higher rates of new AV block degree 3 and new permanent pacemaker implantations were more common in group 3. Rates of hemodynamic relevant arrhythmias were significantly lower in group 3. Both length of hospitalization and stay at intensive care unit (ICU) and intermediate care unit (IMC) could be reduced significantly between group 1 and 3.
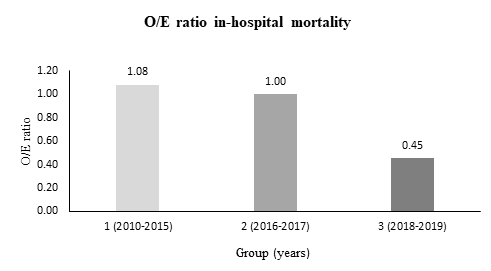
Figure 1: Development of observed-to-expected (O/E) ratio of in-hospital mortality after TAVI. Observed = In-hospital mortality. Expected = EuroScore II.
|
All (2010-2019) n = 489 |
Group 1 (2010-2015) n = 132 |
Group 2 (2016-2017) n = 155 |
Group 3 (2018-2019) n = 202 |
p value |
||
|
Demographic characteristics |
||||||
|
Age (years) |
||||||
|
- Mean (± SD) |
81.8 (± 5.4) |
82.1 (± 6.2) |
82.5 (± 4.8) |
81.1 (± 5.1) |
0.012 |
|
|
- Median (IQR) |
82.0 (6.0) |
83.0 (8.0) |
83.0 (6.0) |
81.0 (5.0) |
||
|
Male - n (%) |
239 (48.9 %) |
62 (47.0 %) |
68 (43.9 %) |
109 (54.0 %) |
0.147 |
|
|
BMI (kg/m2) |
||||||
|
- Mean (± SD) |
27.7 (± 5.1) |
26.9 (± 4.4) |
27.6 (± 5.8) |
28.3 (± 4.9) |
0.030 |
|
|
- Median (IQR) |
27.1 (5.9) |
27.0 (5.7) |
26.7 (5.0) |
27.6 (6.8) |
||
|
BSA (m2) |
||||||
|
- Mean (± SD) |
1.8 (± 0.2) |
1.8 (± 0.2) |
1.8 (± 0.2) |
1.9 (± 0.2) |
0.002 |
|
|
- Median (IQR) |
1.8 (0.3) |
1.8 (0.2) |
1.8 (0.2) |
1.9 (0.3) |
||
|
Logistic |
||||||
|
- Mean (± SD) |
20.2 (± 14.2) |
26.7 (± 14.8) |
18.3 (± 12.8) |
17.4 (± 13.6) |
< 0.001 |
|
|
- Median (IQR) |
15.7 (16.5) |
24 (19.4) |
14.4 (13.3) |
12.7 (10.9) |
||
|
EuroSCORE II (%) |
||||||
|
- Mean (± SD) |
6.4 (± 5.4) |
8.4 (± 6.0) |
5.8 (± 4.9) |
5.5 (± 5.0) |
< 0.001 |
|
|
- Median (IQR) |
4.7 (5.3) |
6.6 (6.5) |
4.4 (4.6) |
3.7 (3.6) |
||
|
Cardiovascular Risk factors |
||||||
|
Hypertension - n (%) |
452 (92.4 %) |
124 (93.9 %) |
145 (93.5 %) |
183 (90.6 %) |
0.432 |
|
|
Diabetes mellitus - n (%) |
171 (35.0 %) |
52 (39.4 %) |
46 (29.7 %) |
73 (36.1 %) |
0.205 |
|
|
Hyperlipidemia - n (%) |
287 (58.7 %) |
82 (62.1 %) |
91 (58.7 %) |
114 (56.4 %) |
0.587 |
|
|
Renal insufficiency - n (%) |
268 (54.8 %) |
81 (61.4 %) |
78 (50.3 %) |
109 (54.0 %) |
0.165 |
|
|
- Dialysis - n (%) |
13 (2.7 %) |
6 (4.5 %) |
1 (0.6 %) |
6 (3.0 %) |
0.115 |
|
|
Obesity - Obesity grade 1 - Obesity grade 2 - Obesity grade 3 |
94 (19.2 %) 30 (6.1 %) 6 (1.2 %) |
25 (18.9 %) 3 (2.3 %) 1 (0.8 %) |
22 (14.2 %) 7 (4.5 %) 4 (2.6 %) |
47 (23.3 %) 20 (9.9 %) 1 (0.5 %) |
0.006 |
|
|
Smoking - n (%) |
25 (5.1 %) |
2 (1.5 %) |
8 (5.2 %) |
15 (7.4 %) |
0.056 |
|
|
Family history of cardiovascular disease – n (%) |
110 (22.5 %) |
36 (27.3 %) |
29 (18.7 %) |
45 (22.3 %) |
0.222 |
|
Table 1: Patient characteristics: Demography and cardiovascular risk factors. Abbreviations: BMI = Body Mass Index; BSA = Body Surface Area; IQR = interquartile range; SD = standard deviation.
|
All (2010-2019) n = 489 |
Group 1 (2010-2015) n = 132 |
Group 2 (2016-2017) n = 155 |
Group 3 (2018-2019) n = 202 |
p value |
|
|
Clinical features |
|||||
|
NYHA class |
0.164 |
||||
|
NYHA I - n (%) |
2 (0.4 %) |
2 (1.5 %) |
0 (0.0 %) |
0 (0.0 %) |
|
|
NYHA II - n (%) |
61 (12.5 %) |
17 (12.9 %) |
22 (14.2 %) |
22 (10.9 %) |
|
|
NYHA III - n (%) |
370 (75.7 %) |
94 (71.2 %) |
114 (73.5 %) |
162 (80.2 %) |
|
|
NYHA IV - n (%) |
52 (10.6 %) |
17 (12.9 %) |
19 (12.3 %) |
16 (7.9 %) |
|
|
CCS class |
< 0.001 |
||||
|
CCS I - n (%) |
15 (3.1 %) |
3 (2.3 %) |
1 (0.6 %) |
11 (5.4 %) |
|
|
CCS II - n (%) |
233 (47.6 %) |
34 (25.8 %) |
114 (73.5 %) |
85 (42.1 %) |
|
|
CCS III - n (%) |
62 (12.7 %) |
47 (35.6 %) |
10 (6.5 %) |
5 (2.5 %) |
|
|
CCS IV - n (%) |
1 (0.2 %) |
1 (0.8 %) |
0 (0.0 %) |
0 (0.0 %) |
|
|
Cardiac decompensation - n (%) |
177 (36.2 %) |
60 (45.5 %) |
54 (34.8 %) |
63 (31.2 %) |
0.027 |
|
Comorbidities |
|||||
|
Coronary artery disease - n (%) |
0.433 |
||||
|
- 1-vessel disease - n (%) - 2-vessel disease - n (%) - 3-vessel disease - n (%) - left main artery stenosis - n (%) |
100 (20.4 %) 70 (14.3 %) 112 (22.9 %) 21 (4.3 %) |
27 (20.5 %) 15 (11.4 %) 38 (28.8 %) 8 (6.1 %) |
31 (20.0 %) 23 (14.8 %) 29 (18.7 %) 9 (5.8 %) |
42 (20.8 %) 32 (15.8 %) 45 (22.3 %) 4 (2.0 %) |
|
|
Previous myocardial infarction - |
0.018 |
||||
|
- no myocardial infarction |
68 (13.9 %) |
28 (21.2 %) |
17 (11.0 %) |
23 (11.4 %) |
|
|
- STEMI – n (%) - |
32 (6.5 %) |
17 (12.9 %) |
6 (3.9 %) |
9 (4.5 %) |
|
|
- NSTEMI – n (%) |
34 (7.0 %) |
13 (9.8 %) |
10 (6.5 %) |
11 (5.4 %) |
|
|
Previous bypass operation- n (%) |
79 (16.2 %) |
33 (25.0 %) |
17 (11.0 %) |
29 (14.4 %) |
0.004 |
|
Previous valve operation - n (%) |
14 (2.9 %) |
4 (3.0 %) |
3 (1.9 %) |
7 (3.5 %) |
0.685 |
|
Previous balloon valvuloplasty - n (%) |
37 (7.6 %) |
1 (0.8 %) |
16 (10.3 %) |
20 (9.9 %) |
0.002 |
|
Previous PCI - n (%) |
199 (40.7 %) |
57 (43.2 %) |
60 (38.7 %) |
82 (40.6 %) |
0.744 |
|
PVD - n (%) |
118 (24.1 %) |
51 (38.6 %) |
37 (23.9 %) |
30 (14.9 %) |
< 0.001 |
|
CVD - n (%) |
62 (12.7 %) |
30 (22.7 %) |
15 (9.7 %) |
17 (8.4 %) |
< 0.001 |
|
COPD - n (%) |
57 (11.7 %) |
18 (13.6 %) |
14 (9.0 %) |
25 (12.4 %) |
0.440 |
|
Previous stroke - n (%) |
51 (10.4 %) |
19 (14.4 %) |
15 (9.7 %) |
17 (8.4 %) |
0.203 |
|
History of cancer - n (%) |
123 (25.2 %) |
28 (21.2 %) |
51 (32.9 %) |
44 (21.8 %) |
0.027 |
|
Anemia – n (%) |
230 (47.1 %) |
77 (58.3 %) |
67 (43.5 %) |
86 (42.6 %) |
0.010 |
Table 2: Patient characteristics: Clinical features and comorbidities. Abbreviations: CCS = Canadian Cardiovascular Society; COPD = chronic obstructive pulmonary disease; CVD = cerebrovascular disease; NSTEMI = Non-ST-elevation myocardial infarction; NYHA = New York Heart Association; PCI = percutaneous coronary intervention; PVD = peripheral vascular disease; STEMI = ST-elevation myocardial infarction.
|
All(2010-2019)n = 489 |
Group 1 (2010-2015) |
Group 2 (2016-2017) |
Group 3 |
p value |
|
|
Postprocedural outcomes |
|||||
|
In-hospital mortality - n (%) |
26 (5.3 %) |
12 (9.1 %) |
9 (5.8 %) |
5 (2.5 %) |
0.029 |
|
Myocardial infarction - n (%) |
2 (0.4 %) |
2 (1.5 %) |
0 (0.0 %) |
0 (0.0 %) |
0.066 |
|
Neurological complications (all) - n (%) |
11 (2.2 %) |
2 (1.5 %) |
3 (1.9 %) |
6 (3.0 %) |
0.647 |
|
- Stroke - n (%) |
9 (1.8 %) |
2 (1.5 %) |
2 (1.3 %) |
5 (2.5 %) |
0.675 |
|
- TIA - n (%) |
2 (0.4 %) |
0 (0.0 %) |
1 (0.6 %) |
1 (0.5 %) |
0.673 |
|
Bleeding complications (all) - n (%) |
53 (10.8 %) |
19 (14.4 %) |
18 (11.6 %) |
16 (7.9 %) |
0.165 |
|
- Life-threatening bleeding - n (%) |
18 (3.7 %) |
5 (3.8 %) |
6 (3.9 %) |
7 (3.5 %) |
0.977 |
|
- Major bleeding - n (%) |
12 (2.5 %) |
3 (2.3 %) |
5 (3.2 %) |
4 (2.0 %) |
0.743 |
|
- Minor bleeding - n (%) |
23 (4.7 %) |
11 (8.3 %) |
7 (4.5 %) |
5 (2.5 %) |
0.047 |
|
- Blood transfusion - n (%) |
121 (24.7 %) |
47 (35.6 %) |
40 (25.8 %) |
34 (16.8 %) |
< 0.001 |
|
- Transfusion of thrombocyte concentrates - n (%) |
21 (4.3 %) |
7 (5.3 %) |
7 (4.5 %) |
7 (3.5 %) |
0.711 |
|
- Transfusion of fresh frozen plasma - n (%) |
20 (4.1 %) |
7 (5.3 %) |
8 (5.2 %) |
5 (2.5 %) |
0.318 |
|
Acute Kidney Injury (AKIN) (all) - n (%) |
183 (37.4 %) |
71 (53.8 %) |
53 (34.2 %) |
59 (29.2 %) |
< 0.001 |
|
- AKIN I - n (%) |
152 (31.1 %) |
57 (43.2 %) |
48 (31.0 %) |
47 (23.3 %) |
0.001 |
|
- AKIN II - n (%) |
3 (0.6 %) |
0 (0.0 %) |
0 (0.0 %) |
3 (1.5 %) |
0.117 |
|
- AKIN III - n (%) |
28 (5.7 %) |
14 (10.6 %) |
5 (3.2 %) |
9 (4.5 %) |
0.016 |
|
- Dialysis after TAVI (all) - n (%) |
24 (4.9 %) |
11 (8.3 %) |
4 (2.6 %) |
9 (4.5 %) |
0.074 |
|
- Acute dialysis after TAVI - n (%) |
13 (2.7 %) |
6 (4.5 %) |
4 (2.6 %) |
3 (1.5 %) |
0.235 |
|
Vascular complications (all) – n (%) |
66 (13.5 %) |
20 (15.2 %) |
20 (12.9 %) |
26 (12.9 %) |
0.809 |
|
- Major vascular complication - n (%) |
23 (4.7 %) |
7 (5.3 %) |
6 (3.9 %) |
10 (5.0 %) |
0.83 |
|
- Minor vascular complication - n (%) |
40 (8.2 %) |
12 (9.1 %) |
12 (7.7 %) |
16 (7.9 %) |
0.903 |
|
- Percutaneous closure device failure- n (%) |
4 (0.8 %) |
1 (0.8 %) |
2 (1.3 %) |
1 (0.5 %) |
0.708 |
|
Conduction disturbances andarrhythmias (all) - n (%) |
242 (49.5 %) |
64 (48.5 %) |
77 (49.7 %) |
101 (50.0 %) |
0.962 |
|
- New AV block 1 - n (%) |
48 (9.8 %) |
11 (8.3 %) |
16 (10.3 %) |
21 (10.4 %) |
0.799 |
|
- New AV block 2 - n (%) |
7 (1.4 %) |
1 (0.8 %) |
2 (1.3 %) |
4 (2.0 %) |
0.645 |
|
- New AV block 3 - n (%) |
35 (7.2 %) |
5 (3.8 %) |
6 (3.9 %) |
24 (11.9 %) |
0.003 |
|
- New LBBB - n (%) |
109 (22.3 %) |
24 (18.2 %) |
36 (23.2 %) |
49 (24.3 %) |
0.403 |
|
- New RBBB - n (%) |
6 (1.2 %) |
2 (1.5 %) |
1 (0.6 %) |
3 (1.5 %) |
0.728 |
|
- New LAFB - n (%) |
34 (7.0 %) |
17 (12.9 %) |
11 (7.1 %) |
6 (3.0 %) |
0.002 |
|
- New onset atrial fibrillation - n (%) |
24 (4.9 %) |
4 (3.0 %) |
7 (4.5 %) |
13 (6.4 %) |
0.357 |
|
- Hemodynamic relevant arrhythmia-n (%) |
29 (5.9 %) |
6 (4.5 %) |
16 (10.3 %) |
7 (3.5 %) |
0.018 |
|
- New permanent pacemaker implantation - n (%) |
73 (14.9 %) |
10 (7.6 %) |
17 (11.0 %) |
46 (22.8 %) |
< 0.001 |
|
Valve malposition (all) - n (%) |
5 (1.0 %) |
0 (0.0 %) |
3 (1.9 %) |
2 (1.0 %) |
0.267 |
|
- Valve migration - n (%) |
1 (0.2 %) |
0 (0.0 %) |
1 (0.6 %) |
0 (0.0 %) |
0.34 |
|
- Valve embolization - n (%) |
4 (0.8 %) |
0 (0.0 %) |
2 (1.3 %) |
2 (1.0 %) |
0.452 |
|
- Ectopic valve deployment - n (%) |
0 (0.0 %) |
0 (0.0 %) |
0 (0.0 %) |
0 (0.0 %) |
|
|
Other Complications |
|||||
|
- Ventricle injury - n (%) |
6 (1.2 %) |
2 (1.5 %) |
2 (1.3 %) |
2 (1.0 %) |
0.909 |
|
- Pericardial tamponade - n (%) |
8 (1.6 %) |
2 (1.5 %) |
4 (2.6 %) |
2 (1.0 %) |
0.492 |
|
- Sepsis - n (%) |
5 (1.0 %) |
3 (2.3 %) |
1 (0.7 %) |
1 (0.5 %) |
0.252 |
|
- Endocarditis - n (%) |
1 (0.2 %) |
0 (0.0 %) |
1 (0.7 %) |
0 (0.0 %) |
0.328 |
|
Paravalvular insufficiency - n (%) |
0.401 |
||||
|
- Grade 1 |
127 (26.0 %) |
30 (22.7 %) |
38 (24.5 %) |
59 (29.2 %) |
|
|
- Grade 2 |
23 (4.7 %) |
4 (3.0 %) |
7 (4.5 %) |
12 (5.9 %) |
|
|
- Grade 3 |
0 (0.0 %) |
0 (0.0 %) |
0 (0.0 %) |
0 (0.0 %) |
|
|
Hospitalization after TAVI (days) |
|||||
|
- Mean (± SD) |
11.1 (± 10.2) |
11.8 (± 6.4) |
11.5 (± 14.2) |
10.4 (± 8.4) |
< 0.001 |
|
- Median (IQR) |
8.0 (6.0) |
9.0 (8.0) |
8.0 (6.0) |
8.0 (6.0) |
|
|
Stay at ICU und IMC after TAVI (days) |
|||||
|
- Mean (± SD) |
4.4 (± 6.1) |
4.8 (± 5.0) |
4.6 (± 4.1) |
2.6 (± 7.1) |
0.001 |
|
- Median (IQR) |
3.0 (3.0) |
3.0 (5.0) |
3.0 (4.0) |
2.0 (2.0) |
|
Table 3: Postprocedural outcomes. Abbreviations: AV = Atrioventricular; ICU = Intensive Care Unit; IMC = Intermediate Care Unit; IQR = Interquartile Range; LAFB = Left Anterior Fascicular Block; LBBB = Left Bundle Branch Block; RBBB = Right Bundle Branch Block; SD = Standard Deviation; TIA = Transient Ischemic Attack.
3.6 Predictors of in-hospital Mortality
Variables that were statistically significant in univariate analysis (Supplementary Table 6) were entered into multivariate logistic regression analysis according to their clinical relevance. Following independent predictors of in-hospital mortality could be revealed (Table 4): Age (OR: 1.103; 95 % CI: 1.013 – 1.202; p = 0.025), creatinine level before TAVI (OR: 1.497; 95 % CI: 1.013 - 2.212; p = 0.043), atrial fibrillation (OR: 2.956; 95 % CI: 1.127 - 7.749; p= 0.028) and procedure duration (OR: 1.017; 95 % CI: 1.009 - 1.025; p < 0.001).
|
Multivariate analysis: In-hospital mortality |
|||
|
Variables |
p value |
Odds Ratio (OR) |
95 % Confidence Interval (CI) |
|
Group |
0.405 |
1.723 |
0.479 – 6.197 |
|
Age (years) |
0.025 |
1.103 |
1.013 – 1.202 |
|
Creatinine before TAVI (mg/dl) |
0.043 |
1.497 |
1.013 – 2.212 |
|
Atrial fibrillation before TAVI |
0.028 |
2.956 |
1.127 – 7.749 |
|
PVD |
0.101 |
2.284 |
0.851 – 6.134 |
|
CT scan before TAVI |
0.527 |
0.631 |
0.151 – 2.632 |
|
Hybrid OR |
0.703 |
0.732 |
0.148 – 3.625 |
|
Edwards THV |
0.523 |
0.656 |
0.180 – 2.394 |
|
Procedure duration (min) |
< 0.001 |
1.017 |
1.009 – 1.025 |
|
R2 |
0.269 |
||
|
Significance (Chi2) |
< 0.001 |
||
Table 4: Multivariate analysis: In-hospital mortality. Abbreviations: CT = Computer Tomography; OR = Operating Room; PVD = Peripheral Vascular Disease; R2 = Nagelkerke R Square; TAVI = Transcatheter Aortic Valve Implantation; THV = Transcatheter Heart Valve.
4. Discussion
In this single-center analysis of 489 consecutive patients who underwent TAVI procedures at the Heart-Thorax Center Fulda from January 2010 to December 2019, we compared in-hospital outcomes between three cohorts according to ESC guideline adjustments, infrastructural, procedural and technical developments. The primary study findings were as follows: (1) The number of TAVI procedures performed annually has increased significantly;
(2) Changes in patient characteristics with a clear shift from high-risk towards lower surgical risk and less comorbidities could be observed; (3) Procedural characteristics including procedure duration and contrast medium consumption could be reduced significantly; (4) Postprocedural complications decreased overall with a remarkable disproportionate decline in risk-adjusted mortality (observed-to-expected mortality); (5) The length of hospitalization as well as the stay at intensive care unit (ICU) and intermediate care unit (IMC) after TAVI could be reduced significantly.
4.1 In-hospital Mortality
Our results demonstrated that in-hospital mortality rates were declining significantly from 2010 to 2019 (9.1 % vs. 5.8 % vs. 2.5 %, p = 0.029). These results are substantially lower than mortality rates reported in a previous study by Akinseye et al. (5.0 %) and comparable with recently published data by the German Institute for Quality Assurance and Transparency in Healthcare (IQTIG), who reported a hospital mortality rate of 2.3 % for Germany in 2020 [9,10]. Especially the observed-to-expected mortality ratio (O/E ratio) showed a disproportionate decline with an O/E ratio of 0.45 in group 3 (years 2018-2019), which indicates that this favorable development was not only driven by selecting patients at lower surgical risk with less comorbidities, but also by improvements in procedure planning, catheter material and technique.
4.1.1 Patient-related Risk Factors: As far as patient-related factors are concerned, a clear shift from patients at high surgical risk to lower surgical risk could be observed. This is primarily due to changing indications in the European guidelines over time. Referring to the first European guideline on the management of valvular heart disease from 2007, it must be stated that TAVI was not suggested as an alternative to surgery due to limited data [6]. The first recommendation in favor of TAVI was adopted in the ESC guideline from 2012 for patients with severe symptomatic aortic stenosis who were deemed unsuitable for surgery after an individual assessment by the heart team [11]. The introduction of the ESC guideline in 2017 further specified the indications for patients with a symptomatic aortic stenosis recommending TAVI for patients 75 years, STS/ EuroSCORE II 4 % or logistic EuroSCORE I 10 % and further risk factors, which are not included in the risk calculators [5]. In this regard, we could observe that patients in the initial period of this study were not only older with a higher calculated surgical risk, but also had higher rates of pre-existing comorbidities, which may have influenced the mortality rates. Apart from age our study revealed renal function at baseline as a further patient-related independent predictor of in-hospital mortality. The role of renal function has already been underlined in previously used scores, such as the ACEF score (age, creatinine, ejection fraction), which was developed and validated for patients undergoing cardiac surgery [12]. Although the systolic left ventricular ejection fraction was not an independent predictor of in-hospital mortality in our multivariate analysis, we assume that a further simplification of conventional risk predicting scores by using less variables might be helpful in clinical routine. Atrial fibrillation was another patient-related predictor of hospital mortality that could be identified in multivariate regression analysis. Although the underlying mechanism for in-hospital mortality remains unclear in this study, the relevance of this comorbidity is indisputable, as there is evidence that patients with atrial fibrillation are at higher risk of rehospitalization due to heart failure after TAVI [13]. Given the fact that there was no follow-up of the patients in this study, we cannot provide further information about rehospitalizations due to heart failure.
4.1.2 Infrastructural and Procedural Risk Factors: As far as infrastructural developments are concerned, it can be stated that the hybrid operating room at our heart center was launched in 2016. Although prior studies showed no significant difference regarding midterm mortality rates in patients who underwent TAVI in hybrid operating rooms versus cardiac catheterization laboratories, we assume that patients treated in hybrid operating rooms may have benefited from various predefined logistic standards [14,15]. Technical equipment and complementary imaging methods allow instantaneous interventions in case of severe complications avoiding any considerable delay. In this context, our data could emphasize the prognostic relevance of procedure duration itself, as it was identified as an independent predictor of hospital mortality. This implies that the implementation of the hybrid operating room with all its advantages led to shorter procedure durations, which may have positively affected our hospital mortality rates.
Another infrastructural aspect that needs to be discussed in this regard is the team of operators, which showed a high consistency during the observation period, apart from one cardiologist joining the team in 2016. This is directly in line with previous findings that were reported by Salemi et al. [16]. They proved that there is an inverse relationship between personal experience and the composite endpoint of in-hospital mortality, stroke and/or myocardial infarction [16]. From this it can be deduced that a constant composition of the TAVI team leads to continually growing individual and team experience, which might have had a positive impact on outcome, although procedural hospital volume itself does not play a role for outcome [17].
4.2 Adverse Events
Vascular complications, which are commonly associated with bleeding complications, have a major influence on mortality [18,19]. Contrary to the findings of the PARTNER study we found lower rates of major (4.7 %) and minor (8.2 %) vascular complications with a decreasing trend between group 1 (years 2010-2015) and group 3 (years 2018-2019) [18]. Several factors are known to play a role in determining vascular complications. The development of valve profiles and the use of smaller sheath sizes may have played the most substantial role in reducing vascular complications. Vascular calcification and female sex with smaller vessel diameters were described as further risk factors in previous studies [20]. However, the implementation of the planning software “3mensio” since 2016 as a mandatory part of the preprocedural planning along with advanced valve and catheter material were contributing factors for safer procedures with a lower prevalence of vascular complications in the last observation period. As far as bleeding complications are concerned, we report a slight downward trend for life-threatening and major bleedings between group 1 (years 2010-2015) and group 3 (2018-2019), while minor bleeding complications decreased significantly. There is some evidence that bleeding complications may affect the survival after TAVI [21,22]. Wang et al. could show that the occurrence of life-threatening and major bleedings after TAVI was linked to higher rates of 30-days mortality [23]. Another relevant finding of our study was the significant decrease of acute kidney injury rates, especially for stage 1 (AKIN 1) and stage 3 (AKIN 3). The association between acute kidney injury after interventional and surgical procedures and mortality has been described in numerous studies [24-27]. The reported incidence of AKIN after TAVI is heterogenous varying between 3.4 % and 57 %, which is comparable with our local results [28-30]. The etiology of an acute kidney injury following TAVI is multifactorial. Peripheral vascular disease could be identified as an important predictor for AKIN [31]. A possible explanation is that numerous procedural steps, including vascular puncture, catheter passage through an atherosclerotic vascular system or valve deployment, may increase the risk of generating emboli from atherosclerotic plaques and thus effecting the renal perfusion with a subsequent impairment of renal function. However, our analysis could show that the number of patients with peripheral vascular disease decreased over time, which might explain the declining rates of AKIN during this period. In addition, our results impressively demonstrated a significant decrease of contrast medium consumption between group 1 and 3. As far as procedure-related predictors are concerned blood transfusion has been identified as another risk factor for AKIN in several studies [32,33]. Our findings are directly in line with these studies showing a significant decrease of blood transfusions during the observed period, which can be explained by a decrease of overall bleeding complications by nearly 50 %. New permanent pacemaker (PPM) implantations due to conductance disturbances are among the most frequent complications after TAVI [34]. The rates of PPM implantations after TAVI range from 9 % to 26 % and are comparable with our local prevalence (22.8 %) [35-38]. Numerous risk factors have been discussed in previous studies. However, transcatheter heart valves still play an eminent role, although newer generation transcatheter heart valves have undergone an impressive technical progress with respect to material and valve profiles. Erkapic et al. demonstrated that self-expandable valves compared to balloon-expandable valves are associated with a higher risk of PPM implantation after TAVI [39]. In accordance with findings reported by Erkapic et al., our results showed a significant increase in PPM implantation rates, while numbers of self-expandable valve implantations raised accordingly. Nevertheless, we assume that the risk of new conductance disturbances can be minimized by comprehensive preprocedural planning strategies. In this context, a novel strategy has been introduced by Tang et al. in 2018. The so-called “cusp overlap technique” was developed to facilitate fluoroscopy-guided implantation of self-expandable transcatheter heart valves. However, this technique was introduced at the end of our study period and therefore was not an integral part of our preprocedural planning strategies at that time, which might be one explanation for the high PPI rates.
5. Limitations
Our analysis has some limitations. Firstly, this was a single-center retrospective study with its natural intrinsic limitations, such as selection bias or unknown confounding factors. Secondly, our study was limited by its small sample size. Thirdly, no follow-up was done as the study was designed to only assess in-hospital outcomes. Lastly, due to various developments running in parallel during the observation period, the decline in postprocedural complications needs to be considered as an overall result of the interplay between changing guideline indications, technical, procedural and institutional developments.
6. Conclusion
In this investigation, the aim was to assess determinants that have affected in-hospital outcomes after TAVI. Our study has shown that the patient-related characteristics age, creatinine level before TAVI, the presence of atrial fibrillation and procedure duration are independent predictors for in-hospital mortality. Although these predictors decreased along with other comorbidities during the observation period, the decline in hospital-mortality was disproportionate, which was indicated by an observed-to-expected mortality ratio of 0.45 for the third treatment period. From this, it can be assumed that apart from patient-related risk factors, there were further institutional, technical and procedural developments, which ran in parallel and had a major impact on postprocedural outcomes, especially on hospital mortality rates after TAVI.
Declarations
Funding
No funding was received for conducting this study.
Conflicts of Interest
The authors state that they have no conflicts of interest.
Availability of Data and Material
The dataset can be provided by the corresponding author on demand.
Code Availability
Not applicable.
Ethics Approval
This study was conducted retrospectively from data obtained for clinical purposes. Ethical approval was waived by the local ethics committee of University Marburg (ek_mr_010720_rana). This study was performed in line with the principles of the Declaration of Helsinki.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
References
- Lindman BR, Clavel MA, Mathieu P, et al. Calcific aortic stenosis. Nat Rev Dis Primers 2 (2016): 16006.
- Carabello BA. Introduction to aortic stenosis. Circ Res 113 (2013): 179-185.
- Rosenhek R, Zilberszac R, Schemper M, et al. Natural history of very severe aortic stenosis. Circulation 121 (2010): 151-156.
- Joint Task Force on the Management of Valvular Heart Disease of the European Society of C, European Association for Cardio-Thoracic S, Vahanian A, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 33 (2012): 2451-2496.
- Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 38 (2017): 2739-2791.
- Vahanian A, Baumgartner H, Bax J, et al. Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J 28 (2007): 230-268.
- Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 35 (2014): 2873-2926.
- Kappetein AP, Head SJ, Genereux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J 33 (2012): 2403-2418.
- Akinseye OA, Shahreyar M, Nwagbara CC, et al. Modifiable Predictors of In-Hospital Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement. Am J Med Sci 356 (2018): 135-140.
- Gaede L, Blumenstein J, Eckel C, et al. Transcatheter-based aortic valve replacement vs. isolated surgical aortic valve replacement in 2020. Clin Res Cardiol 111 (2022): 924-933.
- Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 33 (2012): 2451-2496. (In eng).
- Ranucci M, Castelvecchio S, Menicanti L, et al. Risk of assessing mortality risk in elective cardiac operations: age, creatinine, ejection fraction, and the law of parsimony. Circulation 119 (2009): 3053-3061.
- Zahid S, Din MTU, Khan MZ, et al. Trends, Predictors, and Outcomes of 30-Day Readmission With Heart Failure After Transcatheter Aortic Valve Replacement: Insights From the US Nationwide Readmission Database. J Am Heart Assoc 11 (2022): e024890.
- Spaziano M, Lefevre T, Romano M, et al. Transcatheter Aortic Valve Replacement in the Catheterization Laboratory Versus Hybrid Operating Room: Insights From the FRANCE TAVI Registry. JACC Cardiovasc Interv 11 (2018): 2195-2203.
- Schächinger V, Nef H, Achenbach S, et al. Leitlinie zum Einrichten und Betreiben von Herzkatheterlaboren und Hybridoperationssälen/Hybridlaboren. Der Kardiologe 9 (2015): 89-123.
- Salemi A, Sedrakyan A, Mao J, et al. Individual Operator Experience and Outcomes in Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv 12 (2019): 90-97.
- Bestehorn K, Bestehorn M, Zahn R, et al. Transfemoral aortic valve implantation: procedural hospital volume and mortality in Germany. Eur Heart J (2022).
- Genereux P, Webb JG, Svensson LG, et al. Vascular complications after transcatheter aortic valve replacement: insights from the PARTNER (Placement of AoRTic TraNscathetER Valve) trial. J Am Coll Cardiol 60 (2012): 1043-1052.
- Sherwood MW, Xiang K, Matsouaka R, et al. Incidence, Temporal Trends, and Associated Outcomes of Vascular and Bleeding Complications in Patients Undergoing Transfemoral Transcatheter Aortic Valve Replacement: Insights From the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapies Registry. Circ Cardiovasc Interv 13 (2020): e008227.
- Hayashida K, Lefevre T, Chevalier B, et al. Transfemoral aortic valve implantation new criteria to predict vascular complications. JACC Cardiovasc Interv 4 (2011): 851-858.
- Khan H, Gilani A, Qayum I, et al. An Analysis of the Predictors of Major Bleeding After Transcatheter Aortic Valve Transplantation Using the National Inpatient Sample (2015-2018). Cureus 13 (2021): e16022.
- Ullah W, Jafar M, Zahid S, et al. Predictors of In-Hospital Mortality in Patients With End-Stage Renal Disease Undergoing Transcatheter Aortic Valve Replacement: A Nationwide Inpatient Sample Database Analysis. Cardiovasc Revasc Med 34 (2022): 63-68.
- Wang J, Yu W, Jin Q, et al. Risk Factors for Post-TAVI Bleeding According to the VARC-2 Bleeding Definition and Effect of the Bleeding on Short-Term Mortality: A Meta-analysis. Can J Cardiol 33 (2017): 525-534.
- Lok CE, Austin PC, Wang H, et al. Impact of renal insufficiency on short- and long-term outcomes after cardiac surgery. Am Heart J 148 (2004): 430-438.
- Loef BG, Epema AH, Smilde TD, et al. Immediate postoperative renal function deterioration in cardiac surgical patients predicts in-hospital mortality and long-term survival. J Am Soc Nephrol 16 (2005): 195-200.
- Webb JG, Altwegg L, Boone RH, et al. Transcatheter aortic valve implantation: impact on clinical and valve-related outcomes. Circulation 119 (2009): 3009-3016.
- Bagur R, Webb JG, Nietlispach F, et al. Acute kidney injury following transcatheter aortic valve implantation: predictive factors, prognostic value, and comparison with surgical aortic valve replacement. Eur Heart J 31 (2010): 865-874.
- Saia F, Ciuca C, Taglieri N, et al. Acute kidney injury following transcatheter aortic valve implantation: incidence, predictors and clinical outcome. Int J Cardiol 168 (2013): 1034-1040.
- Strauch JT, Scherner MP, Haldenwang PL, et al. Minimally invasive transapical aortic valve implantation and the risk of acute kidney injury. Ann Thorac Surg 89 (2010): 465-470.
- Julien HM, Stebbins A, Vemulapalli S, et al. Incidence, Predictors, and Outcomes of Acute Kidney Injury in Patients Undergoing Transcatheter Aortic Valve Replacement: Insights From the Society of Thoracic Surgeons/American College of Cardiology National Cardiovascular Data Registry-Transcatheter Valve Therapy Registry.Circ Cardiovasc Interv 14 (2021): e010032
- Sinning JM, Ghanem A, Steinhauser H, et al. Renal function as predictor of mortality in patients after percutaneous transcatheter aortic valve implantation. JACC Cardiovasc Interv 3 (2010): 1141-1149.
- Bove T, Calabro MG, Landoni G, et al. The incidence and risk of acute renal failure after cardiac surgery. J Cardiothorac Vasc Anesth 18 (2004): 442-445.
- Ellenberger C, Schweizer A, Diaper J, et al. Incidence, risk factors and prognosis of changes in serum creatinine early after aortic abdominal surgery. Intensive Care Med 32 (2006): 1808-1816.
- Auffret V, Puri R, Urena M, et al. Conduction Disturbances After Transcatheter Aortic Valve Replacement: Current Status and Future Perspectives. Circulation 136 (2017): 1049-1069.
- Bekeredjian R, Szabo G, Balaban U, et al. Patients at low surgical risk as defined by the Society of Thoracic Surgeons Score undergoing isolated interventional or surgical aortic valve implantation: in-hospital data and 1-year results from the German Aortic Valve Registry (GARY). Eur Heart J 40 (2019): 1323-1330.
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 374 (2016): 1609-1620.
- Popma JJ, Deeb GM, Yakubov SJ, et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N Engl J Med 380 (2019): 1706-1715.
- Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 376 (2017): 1321-1331.
- Erkapic D, De Rosa S, Kelava A, et al. Risk for permanent pacemaker after transcatheter aortic valve implantation: a comprehensive analysis of the literature. J Cardiovasc Electrophysiol 23 (2012): 391-397.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license 4.0
Supplementary Data
Supplementary Table 1: Blood examination before TAVI. Abbreviations: IQR = Interquartile Range; SD = Standard Deviation.
|
All (2010 - 2019) n = 489 |
Group 1 (2010 – 2015)n = 132 |
Group 2 (2016 – 2017)n = 155 |
Group 3 (2018 – 2019) n = 202 |
p value |
|
|
Blood examination |
|||||
|
Creatinine (mg/dl) |
|||||
|
- Mean (± SD) |
1.4 (± 0.9) |
1.6 (± 1.1) |
1.2 (± 0.4) |
1.3 (± 0.9) |
0.005 |
|
- Median (IQR) |
1.2 (0.6) |
1.3 (0.8) |
1.2 (0.5) |
1.1 (0.5) |
|
|
NT-pro-BNP (pg/ml) |
|||||
|
- Mean (± SD) |
3483.7(± 5217.4) |
4928,1(± 6792.7) |
3259.8(± 5202.9) |
2769.1(± 3807.1) |
< 0.001 |
|
- Median (IQR) |
1636 (2845.0) |
2535,5 (4725.0) |
1409 (2482.0) |
1262 (2416.0) |
|
|
Troponin-T (pg/ml) |
|||||
|
- Mean (± SD) |
42.3 (± 124.6) |
64.5 (± 223.4) |
38.0 (± 95.0) |
33.3 (± 34.5) |
0.217 |
|
- Median (IQR) |
25.0 (23.0) |
30.5 (29.0) |
24.0 (18.0) |
25.0 (22.0) |
|
|
Hemoglobin (g/dl) |
|||||
|
- Mean (± SD) |
12.5 (± 1.8) |
12.1 (± 1.9) |
12.5 (± 1.7) |
12.6 (± 1.8) |
0.009 |
|
- Median (IQR) |
12.6 (± 2.2) |
12.1 (2.4) |
12.7 (2.2) |
12.9 (2.3) |
|
|
Platelets (Thousand/μl) |
|||||
|
- Mean (± SD) |
214.7 (± 82.9) |
206.9 (± 85.1) |
218.7 (± 97.1) |
216.8 (± 68.7) |
0.157 |
|
- Median (IQR) |
202.0 (82.0) |
198.5 (83.0) |
201.0 (75.0) |
208.0 (90.0) |
|
Supplementary Table 2: Echocardiographic measurements before TAVI. Abbreviations: AVA = Aortic Valve Area; IQR = Interquartile Range; LV = Left Ventricular; LVEF = Left Ventricular Ejection Fraction; MPG = Mean Pressure Gradient.
|
All (2010-2019) n = 489 |
Group 1 (2010-2015) n = 132 |
Group 2 (2016-2017) n = 155 |
Group 3 (2018-2019) n = 202 |
p value |
|
|
Echocardiography |
|||||
|
LVEF (%) |
|||||
|
- Mean (± SD) |
52.0 (± 10.1) |
49.7 (± 9.7) |
53.8 (± 9.8) |
52.2 (± 10.2) |
< 0.001 |
|
- Median (IQR) |
55.0 (5.0) |
55.0 (10.0) |
55.0 (0.0) |
55.0 (5.0) |
|
|
LV function |
0.005 |
||||
|
- Normal systolic LV function – n (%) |
382 (78.1 %) |
90 (68.2 %) |
132 (85.2 %) |
160 (79.2 %) |
|
|
- Moderately reduced LV function – n (%) |
30 (6.1 %) |
15 (11.4 %) |
4 (2.6 %) |
11 (5.4 %) |
|
|
- Severely reduced LV function – n (%) |
77 (15.7 %) |
27 (20.5 %) |
19 (12.3 %) |
31 (15.3 %) |
|
|
AVA (cm2) |
|||||
|
- Mean (± SD) |
0.74 (± 0.16) |
0.70 (± 0.14) |
0.72 (± 0.16) |
0.78 (± 0.15) |
0.001 |
|
- Median (IQR) |
0.74 (0,23) |
0.70 (0.20) |
0.70 (0.27) |
0.80 (0.21) |
|
|
MPG (mmHg) |
|||||
|
- Mean (± SD) |
42.2 (± 13.8) |
39.6 (± 15.0) |
43.4 (± 14.0) |
43.0 (± 12.6) |
0.051 |
|
- Median (IQR) |
42.0 (16.0) |
40.0 (21.0) |
43.0 (14.0) |
43.0 (13.5) |
|
|
Aortic annulus diameter (mm) |
|||||
|
- Mean (± SD) |
23.4 (± 3.2) |
23.6 (± 2.1) |
22.7 (± 3.4) |
24.1 (4.5) |
0.039 |
|
- Median (IQR) |
23.0 (4.0) |
23.3 (3.0) |
22.0 (3.6) |
23.5 (5.3) |
|
Supplementary Table 3: Computer Tomography before TAVI. Abbreviations: CT = Computer Tomography; IQR = Interquartile Range; LCA = Left Coronary Artery; RCA = Right Coronary Artery; SD = Standard Deviation.
|
All (2010-2019) n = 456 |
Group 1 (2010-2015) n = 100 |
Group 2 (2016-2017) n = 154 |
Group 3 (2018-2019) n = 202 |
p value |
|
|
Computer Tomography (CT) |
|||||
|
CT scan prior to TAVI |
456 (93.3 %) |
100 (75.8 %) |
154 (99.4 %) |
202 (100 %) |
< 0.001 |
|
Aortic annulus diameter (mm) |
|||||
|
- Mean (± SD) |
24.7 (± 2.6) |
24.5 (± 3.8) |
24.4 (± 2.2) |
24.9 (± 2.2) |
0.219 |
|
- Median (IQR) |
24.6 (3.3) |
24.5 (3.0) |
24.3 (3.4) |
24.8 (3.2) |
|
|
Area derived diameter (mm) |
|||||
|
- Mean (± SD) |
24.6 (± 2.2) |
--- |
24.2 (± 2.1) |
24.8 (± 2.2) |
0.051 |
|
- Median (IQR) |
24.4 (3.5) |
--- |
24.1 (3.5) |
24.5 (3.3) |
|
|
Perimeter derived diameter(mm) |
|||||
|
- Mean (± SD) |
24.9 (± 2.2) |
--- |
24.6 (± 2.2) |
25.2 (± 2.2) |
0.039 |
|
- Median (IQR) |
24.9 (3.4) |
--- |
24.5 (3.4) |
25.0 (3.3) |
|
|
Distance RCA to aorticannulus (mm) |
|||||
|
- Mean (± SD) |
1.7 (± 0.4) |
1.5 (± 0.3) |
1.6 (± 0.4) |
1.8 (± 0.4) |
< 0.001 |
|
- Median (IQR) |
1.6 (0.5) |
1.5 (0.3) |
1.6 (0.5) |
1.8 (0.5) |
|
|
Distance LCA to aortic annulus (mm) |
|||||
|
- Mean (± SD) |
1.4 (± 0.3) |
1.4 (± 0.3) |
1.3 (± 0.3) |
1.4 (± 0.3) |
0.021 |
|
- Median (IQR) |
1.4 (0.4) |
1.4 (0.4) |
1.3 (0.4) |
1.4 (0.4) |
|
|
Minimal diameter right femoral artery (mm) |
|||||
|
- Mean (± SD) |
7.3 (± 1.5) |
7.7 (± 1.6) |
7.4 (± 1.2) |
7.0 (± 1.6) |
0.006 |
|
- Median (IQR) |
7.1 (1.7) |
8.0 (2.0) |
7.3 (1.5) |
7.0 (2.0) |
|
|
Minimal diameter right external iliac artery (mm) |
|||||
|
- Mean (± SD) |
7.2 (± 1.7) |
7.6 (± 1.3) |
7.2 (± 2.1) |
7.0 (± 1.5) |
0.002 |
|
- Median (IQR) |
7.0 (1.8) |
8.0 (1.0) |
7.0 (1.9) |
6.9 (1.9) |
|
|
Minimal diameter left external iliac artery (mm) |
|||||
|
- Mean (± SD) |
7.3 (± 1.5) |
7.7 (± 1.3) |
7.2 (± 1.7) |
7.1 (± 1.4) |
0.004 |
|
- Median (IQR) |
7.2 (1.8) |
8.0 (1.0) |
7.0 (2.0) |
7.1 (1.9) |
|
|
Kinking – n (%) |
65 (13.3 %) |
29 (22.0 %) |
20 (12.9 %) |
16 (7.9 %) |
0.001 |
|
Porcelain aorta – n (%) |
12 (2.5 %) |
10 (7.6 %) |
2 (1.3 %) |
0 (0.0 %) |
< 0.001 |
|
Aortic aneurysm - n (%) |
18 (3.7 %) |
6 (4.5 %) |
7 (4.5 %) |
5 (2.5 %) |
0.494 |
Supplementary Table 4: . Abbreviations: AV = Atrioventricular; LAFB = Left Anterior Fascicular Block; LBBB = Left Bundle Branch Block; RBBB = Right Bundle Branch Block.
|
All (2010-2019) n = 489 |
Group 1 (2010-2015) n = 132 |
Group 2 (2016-2017) n = 155 |
Group 3 (2018-2019) n = 202 |
p value |
|
|
Electrocardiography (ECG) |
|||||
|
Atrial fibrillation – n (%) |
146 (29.9 %) |
50 (37.9 %) |
42 (27.3 %) |
54 (26.7 %) |
0.065 |
|
Pacemaker – n (%) |
51 (10.4 %) |
14 (10.6 %) |
11 (7.1 %) |
26 (12.9 %) |
0.208 |
|
AV block 1 - n (%) |
104 (21.3 %) |
32 (24.2 %) |
33 (21.4 %) |
39 (19.3 %) |
0.559 |
|
AV block 2 - n (%) |
1 (0.2 %) |
1 (0.8 %) |
0 (0.0 %) |
0 (0.0 %) |
0.259 |
|
AV block 3 - n (%) |
2 (0.4 %) |
0 (0.0 %) |
2 (1.3 %) |
0 (0.0 %) |
0.113 |
|
LBBB - n (%) |
40 (8.2 %) |
15 (11.4 %) |
8 (5.2 %) |
17 (8.4 %) |
0.164 |
|
RBBB - n (%) |
59 (12.1 %) |
20 (15.2 %) |
16 (10.4 %) |
23 (11.4 %) |
0.432 |
|
LAFB - n (%) |
67 (13.7 %) |
9 (6.8 %) |
27 (17.5 %) |
31 (15.3 %) |
0.022 |
Supplementary Table 5: Procedural characteristics. Abbreviations: F = French; IQR = Interquartile Range; SD = Standard Deviation; THV = Transcatheter Heart Valve.
|
All (2010-2019) n = 489 |
Group 1 (2010-2015) n = 132 |
Group 2 (2016-2017) n = 155 |
Group 3 (2018-2019) n = 202 |
p value |
|
|
General procedural characteristics |
|||||
|
Procedure duration (min) |
|||||
|
- Mean (± SD) |
81.2 (± 49.1) |
112.7 (± 46.9) |
81.5 (± 48.2) |
60.4 (± 39.3) |
< 0.001 |
|
- Median (IQR) |
71.0 (43.0) |
97.0 (52.0) |
71.5 (36.0) |
54.5 (31.0) |
|
|
Contrast medium (ml) |
|||||
|
- Mean (± SD) |
139.5 (± 72.1) |
136.0 (± 66.7) |
167.6 (± 84.6) |
119.6 (± 56.7) |
< 0.001 |
|
- Median (IQR) |
124.0 (95.0) |
123.5 (100.0) |
147.0 (105.0) |
110.0 (70.0) |
|
|
Fluoroscopy time (min) |
|||||
|
- Mean (± SD) |
10.6 (± 6.0) |
9.6 (± 6.8) |
10.8 (± 5.8) |
11.0 (± 5.4) |
0.003 |
|
- Median (IQR) |
9.7 (8.2) |
7.1 (10.9) |
9.7 (7.0) |
10.0 (7.2) |
|
|
Radiation dose (cGycm2) |
|||||
|
- Mean (± SD) |
2900.1(± 1949.7) |
2728.7 (± 1595.0) |
2715.2(± 1906.3) |
3172.4(± 2178.7) |
0.056 |
|
- Median (IQR) |
2463.5 (2013) |
2470 (1768) |
2235.5 (1530) |
2799 (2414) |
|
|
Access route and valve types |
|||||
|
Transapical |
144 (29.4 %) |
80 (60.6 %) |
41 (26.5 %) |
23 (11.4 %) |
< 0.001 |
|
Valve types |
< 0.001 |
||||
|
Balloon-expandable |
239 (48.9 %) |
132 (100 %) |
60 (38.7 %) |
47 (23.3 %) |
|
|
Self-expandable |
250 (51.1 %) |
0 (0.0 %) |
95 (61.3 %) |
155 (76.7 %) |
|
|
Valve models: Balloon-expandable THV |
|||||
|
- Edwards SAPIEN |
12 (2.5 %) |
12 (9.1 %) |
0 (0.0 %) |
0 (0.0 %) |
|
|
- Edwards SAPIEN XT |
79 (16.2 %) |
79 (59.8 %) |
0 (0.0 %) |
0 (0.0 %) |
|
|
- Edwards SAPIEN 3 |
148 (30.3 %) |
41 (31.1 %) |
60 (38.7 %) |
47 (23.2 %) |
|
|
Valve models: Self-expandable THV |
|||||
|
- Symetis ACURATE Neo |
67 (13.7 %) |
0 (0.0 %) |
37 (23.9 %) |
30 (14.9 %) |
|
|
- Symetis ACURATE TA |
15 (3.1 %) |
0 (0.0 %) |
15 (9.7 %) |
0 (0.0 %) |
|
|
- CoreValve Evolut R |
168 (34.4 %) |
0 (0.0 %) |
43 (27.7 %) |
125 (61.9 %) |
|
|
Valve sizes (mm) |
< 0.001 |
||||
|
- 20 mm |
1 (0.2 %) |
1 (0.8 %) |
0 (0.0 %) |
0 (0.0 %) |
|
|
- 23 mm |
97 (19.8 %) |
46 (34.8 %) |
34 (21.9 %) |
17 (8.4 %) |
|
|
- 25 mm |
30 (6.1 %) |
0 (0.0 %) |
18 (11.6 %) |
12 (5.9 %) |
|
|
- 26 mm |
129 (26.4 %) |
64 (48.5 %) |
29 (18.7 %) |
36 (17.8 %) |
|
|
- 27 mm |
33 (6.7 %) |
0 (0.0 %) |
18 (11.6 %) |
15 (7.4 %) |
|
|
- 29 mm |
140 (28.6 %) |
21 (15.9 %) |
45 (29.0 %) |
74 (36.6 %) |
|
|
- 34 mm |
59 (12.1 %) |
0 (0.0 %) |
11 (7.1 %) |
48 (23.8 %) |
|
|
Sheath sizes (F) |
< 0.001 |
||||
|
- 14 F |
232 (67.2 %) |
19 (36.5 %) |
91 (79.8 %) |
122 (68.2 %) |
|
|
- 16 F |
83 (24.1 %) |
3 (5.8 %) |
23 (20.2 %) |
57 (31.8 %) |
|
|
- 18 F |
11 (3.2 %) |
11 (21.2 %) |
0 (0.0 %) |
0 (0.0 %) |
|
|
- 19 F |
19 (5.5 %) |
19 (36.5 %) |
0 (0.0 %) |
0 (0.0 %) |
|
|
Specific procedural characteristics |
|||||
|
Predilatation - n (%) |
304 (62.2 %) |
131 (99.2 %) |
109 (70.3 %) |
64 (31.7 %) |
< 0.001 |
|
Rapid Pacing - n (%) |
351 (71.8 %) |
132 (100 %) |
114 (73.5 %) |
105 (52.0 %) |
< 0.001 |
|
Postdilatation - n (%) |
70 (14.3 %) |
13 (9.8 %) |
17 (11.0 %) |
40 (19.8 %) |
0.014 |
|
Conversion to surgery - n (%) |
5 (1.0 %) |
2 (1.5 %) |
2 (1.3 %) |
1 (0.5 %) |
0.612 |
|
Conversion to transapical access - n (%) |
6 (1.2 %) |
4 (3.0 %) |
2 (1.3 %) |
0 (0.0 %) |
0.048 |
|
Cardiopulmonary bypass - n (%) |
9 (1.8 %) |
4 (3.0 %) |
4 (2.6 %) |
1 (0.5 %) |
0.171 |
|
Valve-in-Valve – n (%) |
6 (1.2 %) |
2 (1.5 %) |
1 (0.6 %) |
3 (1.5 %) |
0.728 |
Supplementary Table 6: Univariate logistic regression analysis. Abbreviations as previously mentioned.
|
Univariate logistic regression analysis: In-hospital mortality |
|||
|
Variables |
p value |
Odds Ratio (OR) |
95 % Confidence interval (CI) |
|
Group (years) |
0.01 |
0.518 |
0.313 – 0.855 |
|
Demography |
|||
|
Age (years) |
0.036 |
1.095 |
1.006 – 1.192 |
|
Gender |
0.189 |
0.581 |
0.258 – 1.307 |
|
Body mass index (kg/m2) |
0.009 |
0.868 |
0.781 – 0.966 |
|
Body surface area (m2) |
0.146 |
0.21 |
0.026 – 1.724 |
|
Logistic EuroSCORE (%) |
< 0.001 |
1.051 |
1.029 – 1.073 |
|
EuroSCORE II (%) |
0.057 |
1.057 |
0.998 – 1.120 |
|
Risk factors |
|||
|
Hypertension |
0.436 |
0.608 |
0.174 – 2.127 |
|
Diabetes mellitus |
0.379 |
0.672 |
0.277 – 1.631 |
|
Hyperlipidemia |
0.607 |
0.812 |
0.367 – 1.794 |
|
Renal insufficiency |
0.061 |
2.333 |
0.962 – 5.656 |
|
Obesity |
0.087 |
0.345 |
0.102 – 1.169 |
|
Family history of cardiovascular disease |
0.942 |
1.036 |
0.405 – 2.646 |
|
Clinical features |
|||
|
Cardiac decompensation |
0.023 |
2.534 |
1.137 – 5.645 |
|
NYHA class |
0.978 |
1.382 |
0.648 – 2.945 |
|
CCS class |
0.627 |
0.916 |
0.642 – 1.306 |
|
Comorbidities |
|||
|
Myocardial infarction |
0.055 |
2.428 |
0.980 – 6.017 |
|
- STEMI |
0.298 |
1.952 |
0.553 – 6.885 |
|
- NSTEMI |
0.094 |
2.624 |
0.850 – 8.106 |
|
Coronary heart disease |
0.998 |
1.001 |
0.450 – 2.227 |
|
- 1-vessel disease |
0.733 |
1.178 |
0.460 – 3.015 |
|
- 2-vessels disease |
0.679 |
0.771 |
0.225 – 2.639 |
|
- 3-vessels disease |
0.983 |
1.01 |
0.396 – 2.581 |
|
- left main artery stenosis |
0.076 |
3.225 |
0.886 – 11.739 |
|
Previous bypass operation |
0.114 |
0.197 |
0.026 – 1.479 |
|
Previous balloon valvuloplasty |
0.98 |
1.019 |
0.231 – 4.490 |
|
Previous valve intervention |
0.149 |
3.132 |
0.663 – 14.789 |
|
Previous PCI |
0.561 |
1.265 |
0.572 – 2.796 |
|
Peripheral vascular disease |
0.009 |
2.887 |
1.296 – 6.431 |
|
Cerebrovascular disease |
0.031 |
2.733 |
1.099 – 6.798 |
|
COPD |
0.07 |
2.424 |
0.930 – 6.314 |
|
Previous stroke |
0.14 |
2.158 |
0.777 – 5.996 |
|
History of cancer |
0.113 |
0.373 |
0.110 – 1.264 |
|
Anemia |
0.482 |
1.329 |
0.602 – 2.935 |
|
Blood examination |
|||
|
Creatinine before TAVI (mg/dl) |
0.002 |
1.498 |
1.161 – 1.933 |
|
Hemoglobin before TAVI (g/dl) |
0.506 |
0.929 |
0.749 – 1.153 |
|
NT-pro-BNP before TAVI (pg/ml) |
0.062 |
1 |
1.000 – 1.000 |
|
Troponin-T before TAVI (pg/ml) |
0.944 |
1 |
0.997 – 1.003 |
|
Platelets before TAVI (Tsd. /μl) |
0.026 |
0.992 |
0.985 – 0.999 |
|
Echocardiography |
|||
|
LVEF (%) |
0.512 |
0.988 |
0.952 – 1.025 |
|
Normal systolic LV function ( ≥ 50 %) |
0.9 |
0.942 |
0.368 – 2.407 |
|
Moderately reduced LV function ( 41-49 %) |
0.248 |
2.106 |
0.595 – 7.458 |
|
Severely reduced LV function ( ≤ 40 %) |
0.53 |
0.675 |
0.198 – 2.305 |
|
AVA (cm2) |
< 0.001 |
0.007 |
0.000 – 0.110 |
|
MPG (mmHg) |
0.177 |
0.979 |
0.949 – 1.010 |
|
Diameter aortic annulus (Echocardiography) (mm) |
0.788 |
1.022 |
0.871 – 1.199 |
|
Computer tomography (CT) |
|||
|
CT prior to TAVI |
0.002 |
0.206 |
0.077 – 0.556 |
|
Diameter aortic annulus (mm) |
0.412 |
1.094 |
0.883 – 1.355 |
|
Area-derived diameter (mm) |
0.333 |
1.139 |
0.875 – 1.484 |
|
Perimeter-derived diameter (mm) |
0.271 |
1.159 |
0.891 – 1.506 |
|
Distance right coronary artery to aortic annulus (mm) |
0.34 |
0.518 |
0.134 – 2.000 |
|
Distance left coronary artery to aortic annulus (mm) |
0.528 |
0.613 |
0.134 – 2.803 |
|
Minimal diameter right femoral artery (mm) |
0.664 |
0.928 |
0.664 – 1.299 |
|
Minimal diameter right external iliac artery (mm) |
0.573 |
0.914 |
0.668 – 1.250 |
|
Minimal diameter left external iliac artery (mm) |
0.399 |
0.867 |
0.623 – 1.207 |
|
Kinking |
0.747 |
1.198 |
0.399 – 3.596 |
|
Electrocardiography (ECG) |
|||
|
Atrial fibrillation before TAVI |
0.037 |
2.419 |
1.056 – 5.540 |
|
Permanent pacemaker before TAVI |
0.283 |
0.33 |
0.044 – 2.491 |
|
AV block degree 1 before TAVI |
0.821 |
1.114 |
0.436 – 2.850 |
|
LBBB before TAVI |
0.923 |
0.93 |
0.212 – 4.085 |
|
RBBB before TAVI |
0.085 |
2.315 |
0.890 – 6.023 |
|
LAFB before TAVI |
0.367 |
0.509 |
0.117 – 2.205 |
|
General procedural characteristics |
|||
|
Hybrid operation room |
0.028 |
0.408 |
0.184 – 0.907 |
|
Procedure duration (min) |
< 0.001 |
1.014 |
1.008 – 1.020 |
|
Laboratory time (min) |
< 0.001 |
1.011 |
1.005 – 1.016 |
|
Contrast medium consumption (ml) |
0.015 |
1.006 |
1.001 – 1.010 |
|
Fluoroscopy time (min) |
0.047 |
1.058 |
1.001 – 1.120 |
|
Radiation dose (cGycm2) |
0.263 |
1 |
1.000 – 1.000 |
|
Route access |
|||
|
Transfemoral |
0.303 |
0.652 |
0.288 – 1.473 |
|
Transapical |
0.303 |
1.535 |
0.679 – 3.467 |
|
Valve types |
|||
|
Edwards valve |
0.038 |
2.464 |
1.050 – 5.779 |
|
Medtronic valve |
0.219 |
0.557 |
0.219 – 1.416 |
|
Symetis valve |
0.218 |
0.399 |
0.092 – 1.722 |
|
Sheath size |
0.753 |
1.109 |
0.583 – 2.111 |
|
Valve size |
0.239 |
0.87 |
0.690 – 1.097 |
|
Specific procedural characteristics |
|||
|
Predilatation |
0.118 |
2.101 |
0.828 – 5.332 |
|
Rapid pacing |
0.3 |
1.693 |
0.625 – 4.582 |
|
Postdilatation |
0.151 |
0.228 |
0.030 – 1.713 |
|
Postprocedural Outcomes |
|||
|
Stroke |
0.447 |
2.275 |
0.274 – 18.906 |
|
Major bleeding |
0.641 |
1.644 |
0.204 – 13.240 |
|
Minor bleeding |
0.832 |
0.802 |
0.104 – 6.192 |
|
Blood transfusion |
< 0.001 |
9.606 |
3.929 – 23.487 |
|
Acute kidney injury (all stages) |
0.001 |
4.064 |
1.729 – 9.548 |
|
AKIN I |
0.638 |
0.808 |
0.332 – 1.965 |
|
AKIN III |
< 0.001 |
19.239 |
7.694 – 48.106 |
|
Major vascular complication |
< 0.001 |
7.871 |
2.802 – 22.110 |
|
Minor vascular complication |
0.42 |
0.435 |
0.057 – 3.296 |
|
New AV block 1 |
0.709 |
0.755 |
0.173 – 3.300 |
|
New AV block 3 |
0.913 |
1.086 |
0.246 – 4.795 |
|
New LBBB |
0.187 |
0.439 |
0.129 – 1.492 |
|
New RBBB |
0.244 |
3.664 |
0.412 – 32.557 |
|
New LAFB |
0.879 |
1.122 |
0.254 – 4.963 |
|
Hemodynamic relevant arrhythmias |
< 0.001 |
14.605 |
5.857 – 36.423 |
|
New permanent pacemaker implantation |
0.529 |
1.383 |
0.504 – 3.792 |
|
Paravalvular insufficiency |
0.669 |
0.749 |
0.200 – 2.810 |
|
Hospitalization after TAVI (days) |
0.044 |
1.013 |
1.000 – 1.025 |
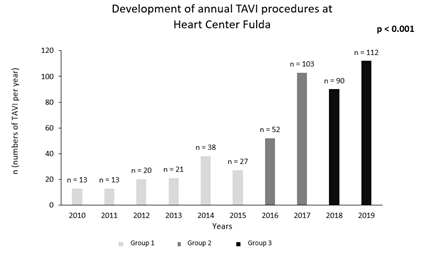
Supplementary Figure 1: Development of annual TAVI procedures at Heart Center Fulda. Description: Group 1 (years 2010-2015), Group 2 (years 2016-2017) and Group 3 (years 2018-2019).


 Impact Factor: * 5.6
Impact Factor: * 5.6 Acceptance Rate: 74.36%
Acceptance Rate: 74.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 