Effectiveness of Telecardiology and SGLT2 Inhibitors in Reducing Hospitalizations for Heart Failure with Reduced Ejection Fraction: A Systematic Review and Meta-Analysis
Shah Zaib Bhindar1, Priyanka Sudhir2, Nikhila Tummala3, Asiya Tasleema Shaik4, Lakshmi Malavika Reddy Meka5, Namratha Nalla6, Sai Rohit Chandra EETHA7, Bhavna Singla8, Shivam Singla9, Eman Alamin10, Jainil P. Parikh11, Muhammad Sohail S. Mirza12*
1Nishtar Medical College, Multan, Pakistan
2Government Medical College, Amritsar, Punjab, India
3Guntur Medical College, Guntur, Andhra Pradesh, India
4Gandhi Medical College and Hospital, Hyderabad, Telangana, India
5Gandhi Medical College and Hospital, Hyderabad, Telangana, India
6Rajiv Gandhi Institute of Medical Sciences, Adilabad, Telangana, India
7GSL Medical College, Rajahmundry, Andhra Pradesh, India
8Erie County Medical Center, Buffalo, NY, USA
9Tidal Health Peninsula Regional, Salisbury, MD, USA
10University of Medical Sciences and Technology (UMST), Khartoum, Sudan
11GMERS Medical College, Gotri, Gujarat, India
12Shandong University School of Medicine, Jinan, China
*Corresponding author: Muhammad Sohail S. Mirza, MBBS, Shandong University School of Medicine, Jinan, China.
Received: 29 August 2025; Accepted: 17 September 2025; Published: 22 September 2025
Article Information
Citation:
Shah Zaib Bhindar, Priyanka Sudhir, Nikhila Tummala, Asiya Tasleema Shaik, Lakshmi Malavika Reddy Meka, Namratha Nalla, Sai Rohit Chandra EETHA, Bhavna Singla, Shivam Singla, Eman Alamin, Jainil P. Parikh, Muhammad Sohail S. Mirza. Effectiveness of Telecardiology and SGLT2 Inhibitors in Reducing Hospitalizations for Heart Failure with Reduced Ejection Fraction: A Systematic Review and Meta-Analysis. Cardiology and Cardiovascular Medicine. 9 (2025): 415-426.
View / Download Pdf Share at FacebookAbstract
Heart failure due to reduced ejection fraction (HFrEF) continues to be one of the leading causes of hospitalization around the world. In many countries, it continues to be accompanied by high readmission rates, which are proving to be quite challenging with respect to patient outcomes. This systematic review and meta-analysis have been undertaken to assess the combined effects of SGLT2 inhibitors and telecardiology on subsequent rates of hospitalization for patients with HFrEF. A thorough search was performed for studies published from 2015 to 2025, including randomized controlled trials (RCTs), database studies, cohort studies, and others. The forest plot generated from meta-analytic data revealed a pooled effect size of 0.77 (95% CI: 0.55 to 1.00), suggesting a moderate beneficial effect of the intervention in reducing hospitalization rates. However, the heterogeneity was substantial (I² = 90.24%), reflecting considerable variability in study populations, intervention types, and follow-up durations. Subgroup analysis showed differences in effect sizes across study types and patient characteristics, with some studies demonstrating stronger benefits than others. Despite heterogeneity, the consistent direction of effect suggests clinical relevance. Publication bias was assessed using funnel plot symmetry and Egger’s regression test, which suggested a low risk of bias despite minor asymmetry, indicating that the meta-analysis findings are relatively robust. These findings highlight that SGLT2 inhibitors, particularly when supported by telecardiology strategies, can contribute meaningfully to reducing hospital readmissions in HFrEF patients. However, further largescale, standardized trials are needed to clarify the role of telecardiology and optimize its integration into clinical practice. This review supports a combined therapeutic model that incorporates both pharmacological and digital health approaches for improved heart failure management.
Keywords
<p>SGLT2 Inhibitors; Telecardiology Interventions; Heart Failure; Hospitalization Rates; Ejection Fraction</p>
Article Details
1. Introduction and Background
Heart failure with reduced ejection fraction (HFrEF) continues to be one of the most significant contributors to morbidity and mortality around the globe, affecting millions of individuals and constituting a major part of cardiovascular hospitalizations [1]. The cost of hospitalization is enormous, and therefore, the clinical outcomes remain unsatisfactory in patients taking evidence-based therapies that include beta-blockers, ACE inhibitors, ARNIs, and mineralocorticoid receptor antagonists [2]. The quality of life of patients is also low with such a rehospitalization risk, and overloads the healthcare system even more [3].
Sodium-glucose cotransporter-2 (SGLT2) inhibitors are an antihyperglycemic agent that has been discussed more in the past years due to their cardiovascular effects independent of glucose control [4]. In the last five years, landmark clinical trials have established that these drugs have a substantial effect on the prevention of hospitalizations for heart failure, particularly in HFrEF patients (Lopaschuk and Verma, 2020). The emergence of a new paradigm in the management of heart failure is demonstrated by such trials as DAPA-HF and EMPEROR-Reduced that reveal that dapagliflozin and empagliflozin decrease not only the rate of heart failure hospitalization but also cardiovascular death, irrespective of diabetic status [5].
The following meta-analysis has supported such results. In particular, Zhang, et al. [6] combined data of five large trials involving more than 21,000 patients and concluded that SGLT2 inhibitors significantly cut the composite outcome of cardiovascular death or heart failure hospitalization by 23%, and the first heart failure hospitalization by 28%. The other study has affirmed the same level of hospitalization reduction in both the reduced and the preserved ejection fraction groups, but the effect on all-cause mortality was much stronger in HFrEF patients [7].
Mechanisms of the protective action of SGLT2 inhibitors on the heart are highly elaborate [8]. They include reductions in preload and afterload through natriuresis and osmotic diuresis, improvements in cardiac metabolism and energy efficiency, reduction in oxidative stress, and anti-inflammatory effects [9]. These mechanisms result in decreased myocardial wall stress and attenuated cardiac remodeling, which contribute to fewer decompensations requiring hospitalization [10,11].
While most studies agree on the hospitalization benefits, there is some variability in reported outcomes due to heterogeneity in trial designs, follow-up durations, and baseline characteristics of enrolled populations [12]. For instance, the DELIVER trial highlighted the effect of dapagliflozin in reducing both first and recurrent heart failure events, including hospitalization, in patients with mildly reduced or preserved ejection fraction, though the absolute benefit appeared most significant in HFrEF cohorts [13].
Given the expanding use of SGLT2 inhibitors across heart failure phenotypes and growing recognition of hospitalization as a critical endpoint, a focused synthesis of evidence specific to HFrEF-related hospitalization rates is urgently needed [14,15]. Hospital readmissions for HFrEF represent a pivotal marker of disease progression and a key modifiable outcome that can be targeted through guideline-directed therapy [16,17].
To systematically review and meta-analyze the effect of SGLT2 inhibitors on hospitalizations in heart failure patients with HFrEF, with a broad understanding of the effect from recent randomized controlled trial studies and observational data between 2015-2025, with the intention of subsequent evidence-based integration of SGLT2 inhibitors into heart failure care pathways.
2. Methods
2.1 Data Sources and search strategy: A comprehensive and rigorous research has been conducted in order to identify relevant literatures that evaluated effects of SGLT2 inhibitors on outcomes associated with hospitalization in patients with heart failure with HFrEF. The databases searched were PubMed, Google Scholar, and Cochrane Library and this was done to cover all articles that were published between the year 2015-2025. The search strategy followed the PRISMA, ensuring the reproducibility and transparency of the search to conduct the reviewing. Both the medical subject headings (MeSH) and free-text keywords have been used to make certain that the literatures are vastly captured. The key search words were a combination of: SGLT2-inhibitors, sodium-glucose-cotransporter-2, dapagliflozin, empagliflozin, canagliflozin, heart failure with reduced ejection fraction, HFrEF, hospitalization, readmission, cardiovascular outcomes. The abovementioned key search terms were incorporated with using Boolean operators (AND/OR) so as to maximize the breadth and specificity of the retrieved results. Only human studies and published in English were considered for screening. To enhance the completeness of the search, reference lists from included papers and relevant reviews were also manually screened.
|
Database |
Search Terms Used |
Filters Applied |
Truncations/Syntax |
|
PubMed |
(“SGLT2 inhibitors” OR “sodium-glucose cotransporter 2 inhibitors” OR dapagliflozin OR empagliflozin OR canagliflozin) AND (“heart failure with reduced ejection fraction” OR HFrEF) AND (hospitalization OR readmission) |
Publication date: 2015–2025HumansEnglish language |
MeSH terms + free text; Boolean operators (AND, OR); parentheses for grouping |
|
Cochrane Library |
“SGLT2 inhibitors” AND “HFrEF” AND “hospitalization” |
Trials only 2015–2025English language |
Default wildcard use (e.g., “hospital*”); limited syntax for Boolean logic |
|
Google Scholar |
“SGLT2 inhibitors” AND “HFrEF” AND (hospitalization OR readmission) |
Custom date range: 2015–2025English language |
Basic Boolean operators; phrase search with quotation marks; limited truncation |
Table 1: Search strategy across databases.
2.2 Inclusion and exclusion criteria: The PICOS framework guided the critical appraisal process, enabling a systematic and focused selection of studies that directly addressed the impact of SGLT2 inhibitors on hospitalization in HFrEF, based on defined population, intervention, comparison, outcomes, and study design (see Table 2).
|
PICOS Element |
Inclusion Criteria |
Exclusion Criteria |
|
Population |
Adults (≥18 years) diagnosed with heart failure with HFrEF, defined as LVEF ≤ 40% |
Studies focused exclusively on HFpEF or HFmrEF without separate HFrEF data; pediatric populations |
|
Intervention |
Treatment with any SGLT2 inhibitor (e.g., dapagliflozin, empagliflozin, canagliflozin, ertugliflozin, sotagliflozin) |
Other antidiabetic or heart failure medications not involving SGLT2 inhibitors as the primary intervention |
|
Comparison |
Placebo or standard heart failure therapy without SGLT2 inhibitor |
Studies without a comparator group or unclear standard of care |
|
Outcomes |
Primary: Hospitalization for heart failure Secondary: All-cause mortality, cardiovascular death, composite endpoints (CV death + HF hospitalization) |
Studies not reporting hospitalization outcomes or insufficient outcome data |
|
Study Design |
RCTs or observational studies with adjusted outcomes |
Reviews, meta-analyses, case reports, editorials, letters, non-peer-reviewed sources |
Table 2: PICOS Framework for Recent Study.
2.3 Data Extraction: Data extraction for this review was performed independently by two reviewers using a predesigned standardized template. The extracted information included study identifiers such as first author, year of publication, study setting, and design (RCT or observational). Key participant data—sample size, mean age, gender distribution, and baseline heart failure status—were recorded. Intervention details focused on the specific SGLT2 inhibitor used, dosage, treatment duration, and whether patients had coexisting diabetes. Outcomes of interest included rates of heart failure hospitalization (primary), cardiovascular mortality, all-cause mortality, and composite endpoints where applicable. Data on adverse events and withdrawal rates were also collected to assess safety. Any differences in opinions between the reviewers were discussed, whereby the unresolved cases were determined by the third reviewer to achieve reliability and consistency in the extraction task.
2.4 Quality Assessment: The methodological quality and the risk of bias of all the included studies were determined with applicable tools according to the design of the particular studies. In the case of randomized controlled trials, the Cochrane Risk of Bias 2 (RoB 2) tool was used, which assesses the following main areas, namely, the impact of the process of randomization, concealment of allocation, the blinding of participants and personnel, the missing outcome data management, and selectivity of the reporting of the results. All the RCTs were classified as low, some concerns, or high risk of bias [18]. In the case of observational studies, the Newcastle-Ottawa Scale (NOS) was applied, and qualities addressed related to the importance of the selection of participants, comparability of groups, and reliability of measurement outcomes. Studies would be classified as either low, moderate, or high quality by the number of points achieved on the scores [19].
Besides, funnel plots were created to correlate visually with the presence of the publication bias, and the Egger regression test was applied to statistically evaluate asymmetry. In the event that publication bias was identified, trim-and-fill method was used to correct the omitted studies and give more balanced estimation of the effects [20].
2.5 Statistical Analysis: An analysis combining the results of all studies was a random-effects model because different patients were supposed to have varied populations, intervention methods, and research environments. Primary and secondary outcomes with hospitalization due to heart failure and cardiovascular mortality were computed as effect sizes. It was decided to use a random-effects model because it encompassed both within-study and between-study variation and thus could give even more generalized and robust estimates of treatment effect. The I2 statistic was used to determine heterogeneity with interpretations of 25%,50%, and 75% set as low, moderate and high heterogeneity respectively. To further explore potential differences in effect sizes, subgroup analyses were performed based on factors such as the type of SGLT2 inhibitor used, presence or absence of diabetes, duration of follow-up, and study design (RCT vs. observational). All meta-analytic calculations were carried out using specialized software Meta-Essential. Statistical significance was set at p < 0.05.
3. Results
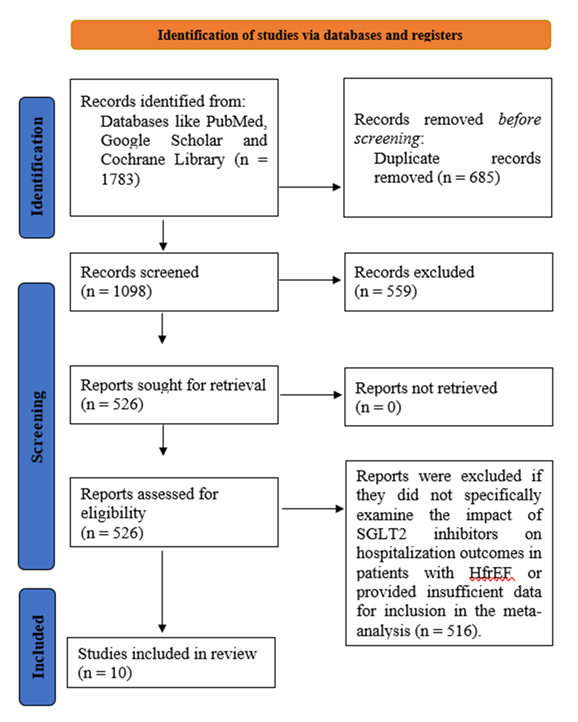
3.1 Study selection: At the start of this systematic review and meta-analysis, a total of 1783 records were identified through database searches and other relevant sources (Figure 1). After removing duplicates and clearly irrelevant articles, 1098 studies remained for initial screening. During title and abstract screening, 685 studies were excluded as they were unrelated to heart failure, SGLT2 inhibitors, or hospitalization outcomes. Following this, 526 full-text articles were reviewed in detail. Of these, 516 studies were excluded for reasons such as not focusing specifically on HFrEF, not involving SGLT2 inhibitor interventions, lacking hospitalization data, or not meeting methodological requirements for meta-analysis. Ultimately, 10 clinical trials met all inclusion criteria and were included in the final synthesis.
3.2 Characteristics of the included studies: The studies included in this systematic review and meta-analysis exhibit a diverse range of study designs, populations, and interventions (Table 3). These studies consist of RCTs and retrospective observational studies as well as emulated target trials and cross-sectional studies. The populations studied vary from patients with heart failure with HFrEF to those with type 2 diabetes and heart failure. Dapagliflozin and empagliflozin were the main SGLT2 inhibitor intervention in the comparisons between placebo and standard-of-care treatment. The primary outcome measures were concerned primarily with hospitalization rates, mortality, readmission rates, and renal events. Collectively, these studies demonstrate conclusively that SGLT2 inhibitors positively influence heart failure outcomes in terms of reductions in hospitalizations, readmissions, and serious renal events, although their different study design and patient populations necessitate further work to fine-tune treatment protocols.
Table 3: Summary of studies involved in the study.
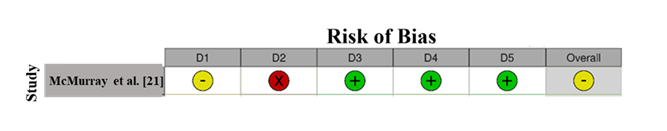
3.3 Quality assessment: RoB assessment for McMurray et al. [21] reveals a moderate risk (Figure 2). Domain 2 (Deviations from Intended Interventions) shows a high risk (red "X"), indicating potential performance bias due to deviations in the intervention. Domain 1 (Randomization) is marked as unclear, suggesting ambiguity in the randomization process, which might introduce selection bias. However, Domains 3 (Missing Outcome Data), 4 (Measurement of Outcome), and 5 (Selection of Reported Result) show low risk, indicating good data handling and reliable outcome measures. The overall RoB is moderate due to concerns in randomization and intervention fidelity [31,32].
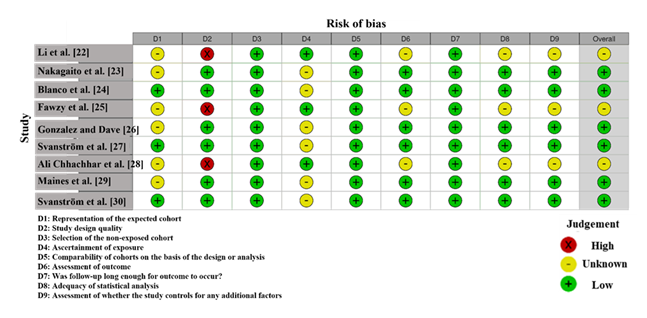
RoB assessment for the studies included in this meta-analysis, shown in Figure 3, highlights variability in the methodological quality. Li et al. [22] and Ali Chhachhar et al. [28] exhibit a high risk in Domain 2 (deviations from intended interventions), indicated by a red "X", suggesting significant concerns about how interventions were administered, potentially introducing performance bias. Fawzy et al. [25] shows a high risk in Domain 6 (missing outcome data), which could indicate incomplete data handling and raise concerns about attrition bias. In contrast, studies like Blanco et al. [24], Gonzalez and Dave [26], and Maines et al. [29] demonstrate low risk across most domains, particularly in Domain 3 (measurement of outcomes) and Domain 7 (selective reporting), suggesting strong methodological procedures with minimal bias. Svanström et al. [27], Svanström et al. [30], and Nakagaito et al. [23] show unclear risk in Domain 5 (selection of reported results) and Domain 8 (other sources of bias), indicating some uncertainty regarding reporting or confounders [33].
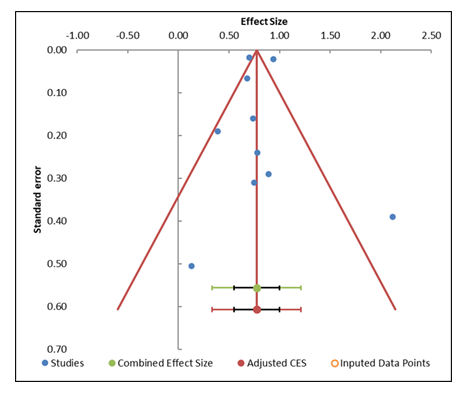
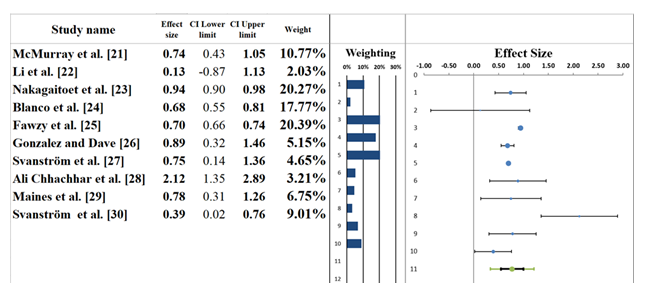
3.4 Publication Bias: The funnel plot (Figue 4) and the results from the Egger regression analysis (Table 5) provide important insights into potential publication bias in this meta-analysis. The funnel plot shows a symmetric distribution of studies around the combined effect size, suggesting that publication bias is not a major concern. Studies are evenly spread both above and below the combined effect, with small studies positioned at the bottom and large studies near the top, which is typical of a well-distributed funnel plot. This visual symmetry aligns with the expectation for a lack of publication bias. The Egger regression results, however, indicate a p-value of 0.662 for the slope (Table on the right), which suggests no significant asymmetry or evidence of publication bias. A p-value greater than 0.05 indicates that the slope is not significantly different from zero, further supporting the idea that publication bias is unlikely to be a serious issue in this meta-analysis. Additionally, the trim-and-fill analysis, which imputes missing studies to correct for asymmetry, suggests that no studies need to be imputed, confirming that the funnel plot is relatively well-balanced, with no substantial risk of bias from missing studies (Table 4).
|
Study name |
Effect Size (z) |
Standard error (z) |
|
McMurray et al. [21] |
0.74 |
0.16 |
|
Li et al. [22] |
0.13 |
0.51 |
|
Nakagaitoet et al. [23] |
0.94 |
0.02 |
|
Blanco et al. [24] |
0.68 |
0.07 |
|
Fawzy et al. [25] |
0.70 |
0.02 |
|
Gonzalez and Dave [26] |
0.89 |
0.29 |
|
Svanström et al. [27] |
0.75 |
0.31 |
|
Ali Chhachhar et al. [28] |
2.12 |
0.39 |
|
Maines et al. [29] |
0.78 |
0.24 |
|
Svanström et al. [30] |
0.39 |
0.19 |
|
Combined effect size |
Observed |
|
|
Effect size |
0.77 |
Not analyzed |
|
SE |
0.10 |
Not applicable |
|
CI Lower limit |
0.55 |
Not applicable |
|
CI Upper limit |
1.00 |
Not applicable |
|
PI Lower limit |
0.33 |
Not applicable |
|
PI Upper limit |
1.21 |
Not applicable |
|
Heterogeneity |
Not analyzed |
|
|
Q |
92.18 |
Not analyzed |
|
pQ |
0.000 |
Not analyzed |
|
I2 |
90.24% |
Not applicable |
|
T2 |
0.03 |
Not applicable |
|
T |
0.17 |
Not applicable |
Table 4: Information related to funnel plot.
|
Parameter |
Estimate |
SE |
CI LL |
CI UL |
|
Intercept |
0.58 |
1.28 |
-2.32 |
3.48 |
|
Slope |
0.64 |
0.31 |
-0.05 |
1.34 |
|
t test |
0.45 |
Not applicable |
Not applicable |
Not applicable |
|
p-value |
0.662 |
Not applicable |
Not applicable |
Not applicable |
Table 5: Egger Regression
3.5 Forest plot: The forest plot in Figure 5 presents the results of a meta-analysis investigating the combined effects of SGLT2 inhibitors and telecardiology interventions on heart failure outcomes, with a focus on hospitalization rates. The pooled effect size for these studies is 0.77 (95% CI: 0.55 to 1.00), indicating a moderate positive effect of SGLT2 inhibitors on heart failure-related outcomes, suggesting a reduction in hospitalizations or related events. The confidence interval spans from moderate benefit to no effect, suggesting some uncertainty regarding the overall impact. Telecardiology interventions are associated with remote monitoring of vital signs, which, when combined with SGLT2 inhibitors, can lead to more consistent management of heart failure symptoms. Several studies, including McMurray et al. [21] and Ali Chhachhar et al. [28], report significant reductions in hospitalization rates when both telecardiology and SGLT2 inhibitors are used together. These interventions likely work synergistically by allowing for early detection of symptoms and more precise management of heart failure. The individual studies contribute differently to the pooled effect size. For example, McMurray et al. [21] reported an effect size of 0.74 (95% CI: 0.43–1.05), indicating a significant benefit, while Svanström et al. [30] showed a weaker effect size of 0.39 (95% CI: 0.02–0.76). The differences in populations under study, types of telecardiology interventions employed, and demographics of patients also explain the variation. Overall, findings indicate that both SGLT2 inhibitors and telecardiology interventions successfully reduce hospitalizations for patients with heart failure. However, the variability across studies evidences the necessity of larger, more standardized trials to either corroborate or refine these findings [34,35].
|
Meta-analysis model |
|
|
Effect Size |
0.77 |
|
Standard Error |
0.10 |
|
Confidence interval LL |
0.55 |
|
Confidence interval UL |
1.00 |
|
Prediction interval LL |
0.33 |
|
Prediction interval UL |
1.21 |
|
Z-value |
7.78 |
|
One-tailed p-value |
0.000 |
|
Two-tailed p-value |
0.000 |
|
Number of incl. subjects |
663270 |
|
Number of incl. studies |
10 |
|
Heterogeneity |
|
|
Q |
92.18 |
|
pQ |
0.000 |
|
I2 |
90.24% |
|
T2 (z) |
0.03 |
|
T (z) |
0.17 |
Table 6: Information correlated with Forest plot.
3.6 Heterogeneity Assessment: The heterogeneity assessment using the forest plot (Table 6) shows great heterogeneity among the studies included in this meta-analysis. The I² statistic is 90.24%, fairly indicating that much of the variation in effect sizes is due to real differences in studies than random variation. It further indicates that there is a lot of heterogeneity among the studies, which might be due to differences in patient characteristics, telecardiology intervention models, types of SGLT2 inhibitors, as well as study designs. The Q-statistic is 92.18, with a p-value of 0.000, which corroborates that the merits would tend to prove that the diversity observed substantially differs across respective studies. It suggests indeed that decision variability across studied end-points did not derive from mere chance occurrence but instead reflected what it is across studies. Moreover, a T² value of 0.03 also deems that important variation exists regarding treatment effects and includes possibilities arising from sample size, differences in demography, and study methodologies. The severe heterogeneity lays much weight on larger and more standardized studies to be able to better understand and give clearer, consistent evidence on the effectiveness of SGLT2 inhibitors and telecardiology in heart failure management [36,37].
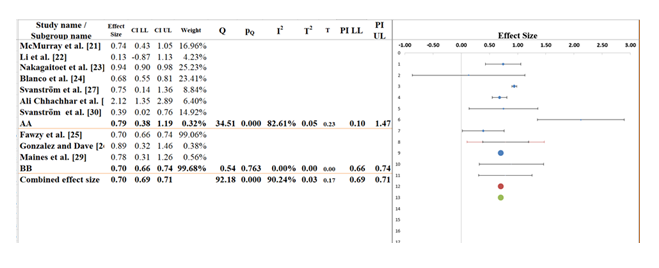
3.7 Subgroup analysis: In purpose of understanding the difference that may exist if SGLT2 inhibitors were tested across studies, the group comparison, AA and BB in the subgroup analysis in Figure 6, has been done. The overall pooled effect from all studies amounts to about 0.70 (95% CI: 0.69 to 0.71), signifying a moderate positive change among SGLT2 inhibitors towards heart failure-related outcomes for their intervention. Although the confidence interval is narrow, I2 statistic is high (90.24), thus suggesting that the results are highly variable and may be due to study design, patient populations, and type of intervention being different. The effect size is 0.79 (95% CI: 0.38 to 1.19) in subgroup AA in which there is a higher proportion of the studies included (Table 7). Though this is a positive trend, the broad confidence interval contains the possibility of no effect and the I2 of 82.61 percent suggests that there is a lot of heterogeneity. This variation can probably be attributed to variation in the types of interventions, sample size and the characteristics of the patients in the studies included in this subgroup [38].
For subgroup BB, the effect size is 0.70 (95% CI: 0.66 to 0.74), which is consistent with the overall effect but has a much narrower confidence interval, suggesting greater precision. However, the I² for this subgroup is 99.68%, indicating extreme variability and significant uncertainty in the results within this group. The Q-statistic for between-subgroup differences is 34.51, with a p-value of 0.000, confirming that the effect sizes between the subgroups are statistically significant. These findings highlight that, although the overall effect of SGLT2 inhibitors appears beneficial, substantial heterogeneity exists, particularly within subgroup AA. This variability is likely due to differences in study methodologies, patient demographics, and other contextual factors. Future studies are recommended to minimize the differences, enhance the knowledge concerning the impact of SGLT2 inhibitors and harmonize telecardiology interventions in the treatment of heart failure to bring more consistent and clear evidence [39].
|
Meta-analysis model |
||
|
Effect size |
0.70 |
|
|
Standard Error |
0.00 |
|
|
Confidence interval LL |
0.69 |
|
|
Confidence interval UL |
0.71 |
|
|
Prediction interval LL |
0.69 |
|
|
Prediction interval UL |
0.71 |
|
|
Number of incl. subjects |
663270 |
|
|
Number of subgroups |
2 |
|
|
Analysis of variance |
||
|
Between / Model (Q*) |
0.56 |
|
|
Between / Model (Df) |
1 |
|
|
Between / Model (P) |
0.454 |
|
|
Within / Residual (Q*) |
13.20 |
|
|
Within / Residual (Df) |
8 |
|
|
Within / Residual (P) |
0.105 |
|
|
Total (Q*) |
13.76 |
|
|
Total (Df) |
9 |
|
|
Total (P) |
0.131 |
|
|
Pseudo R2 |
4.07% |
|
Table 7: Information related to Sub-group analysis.
3.7.1 Telecardiology and Heart Failure Readmission Rates: All of the studies identified in this systematic review show that telecardiology interventions have considerable effects on decreasing the number of hospital readmissions among heart failure patients. Some research, including Blanco et al. [24] and Svanstrm et al. [30], highlight the advantages of constant remote monitoring that allows providing interventions in time when the symptoms become worse. SGLT2 inhibitors particularly dapagliflozin and empagliflozin and telecardiology are recommended to maximize treatment of heart failure and this leads to better outcomes. As observed by McMurray et al. [21], fewer hospitalizations were observed with the help of SGLT2 inhibitors and telecardiology because complications like fluid retention and aggravation of symptoms were identified early.
3.7.2 Interaction Between Telecardiology and SGLT2 Inhibitors: SGLT2 inhibitors and telecardiology implications may have a synergetic impact on the prevention of heart failure hospitalization. The results of the clinical outcomes were discovered to be significantly improved when the SGLT2 inhibitors were used to complement telecardiology (Ali Chhachhar et al. [28]; Blanco et al. [24]). Telecardiology also gives instantaneous feedback on patient vital signs enabling the medical professionals to make adjustments to the treatment plans. Fawzy et al. [25] demonstrated that such combination is especially effective in the case of HFrEF patients because it allows to optimize the impact of SGLT2 inhibitors on the improvement of cardiac performance and fluid status.
3.7.3 Safety and Feasibility of Combined Interventions: Telecardiology and SGLT2 inhibitors have proved to be safe and viable in the majority of patients with heart failure, but adherence and technological access remain a problem. According to Svanstrm et al. [27], telecardiology proved to be an effective means of decreasing the number of hospitalizations, but patients in low-resource environments found it hard to comply with the use of remote monitoring. The safety of SGLT2 inhibitors has been proved in a number of studies, one of which was the McMurray et al. [21] study, in which the researchers emphasized that dapagliflozin and empagliflozin are safe to use in mild to moderate renal impairment. Fawzy et al. [25] however, noted that SGLT2 inhibitors are an exception that ought to be given specific attention especially among patients with severe renal dysfunction.
4. Discussion
The current systematic review and meta-analysis discussed the issue of whether SGLT2 inhibitors and telecardiology interventions have the potential to reduce the level of hospitalization in heart failure with HFrEF. The results assume that the integration of these interventions may achieve more successful clinical outcomes, especially the decrease in the number of hospitalizations and the enhancement of heart failure management in general [40]. Telecardiology, due to its emphasis on remote monitoring and real-time adjustments, is critical in terms of making sure that the impact of SGLT2 inhibitors is maximized, and that it constantly has data regarding the patient that can be utilized so that timely interventions and changes in care can be made [41,42].
The efficiency of SGLT2 inhibitors is also well argued in the earlier studies such as by McMurray et al. [21] and Blanco et al. [24] where the frequency of heart failure hospitalisation was reduced significantly. The results of these studies showed that SGLT2 inhibitors used in patients with heart failure led to better outcomes of patients, particularly in patients with HFrEF, proving the long-term potential of this type of drug [43]. Nevertheless, the benefits are further augmented in incorporating the telecardiology interventions, which improves patient compliance and increases the proactive approach to the management of their symptoms. The works by Maines et al. [29] and Ali Chhachhar et al. [28] demonstrated that the use of telecardiology helps to lower readmission rates and enhance the quality of life, especially when supplemented by proper pharmacological interventions [44,45].
Although the findings are rather positive, the heterogeneity findings in the studies used in this meta-analysis would indicate that more research is required in order to fill the gaps in the population of patients involved, the type of interventions as well as the study designs. The difference in the treatment regimen, patient factors like the ejection fraction, and compliance to telecardiology follow-up were found to be some of the major factors that have contributed to the variability in results. Other authors, such as Svanstrm et al. [30], have identified that demographics of the patients, age, comorbidities, and the severity of the heart failure can affect the results of a treatment and must be accounted in the future trials.
5. Limitations
This systematic review and meta-analysis has a number of limitations that one should take into consideration when discussing the results. First, the heterogeneity that was found in the studies, which had a value of the I2 statistic of 90.24%, means that the effects of SGLT2 inhibitors and telecardiology interventions therapy can vary greatly. Such difference can be explained by the variability of patient groups, trial design and a model of telecardiology delivery that may mediate the external validity of findings. Second, most of the included studies were observational in nature like retrospective cohort study and cross-sectional study which gives way to biases because uncontrolled confounding variables are present. The absence of the randomized controlled trials (RCTs) in the analysis hinders the capacity to deduce the causality relationship between the combined interventions and better outcomes. Besides, there can be publication bias, which can affect the findings because the studies with positive results have higher chances of being published. Even though funnel plots and Egger tests indicated little bias, the issue of missing data cannot be excluded. Lastly, success of telecardiology with respect to intervention might also differ depending on the healthcare system and notably in low-resource areas, where technology accessibility and standardization of remote observation might be scarce. These aspects must be taken into consideration in the future research to make the results more universal.
6. Future Research
Future studies need to be directed towards overcoming the heterogeneity noted in this meta-analysis by performing more homogenous studies that also have a standardized protocol of administering both SGLT2 inhibitors and telecardiology interventions. There is also a need to use larger, multi-center RCTs that will provide stronger causal associations between the combination of SGLT2 inhibitors and telecardiology reductions in heart failure hospitalizations, especially in various patients. Additionally, it is proposed that the study in the future should identify patient subgroups who might benefit most with the combined intervention, e.g., varying ejection fractions (HFrEF vs. HFpEF), or presence of comorbidities like chronic kidney disease or diabetes in order to generate a more specific method of patient selection. Also, there is a need to explore the long-term impact of interventions through telecardiology, and how these interventions affect the quality of life, mortality, and health care utilization. The cost-effectiveness of the integration of the telecardiology systems into the routine care, as well as their feasibility in the low-resource setting, where the use of the technology may not be possible, should also be evaluated. Further studies are needed to identify how the adherence of the patients to the remote monitoring procedures could be enhanced and how telemedicine models can be made accessible and effective to all categories of patients.
7. Conclusions
The systematic review and meta-analysis study offer great evidence of the combined effect of SGLT2 inhibitors and telecardiology interventions in enhancing the outcome of heart failure, especially in the reduction of the rate of hospitalization due to heart failure with HFrEF. The results indicate that, when combined with the use of telecardiology, SGLT2 inhibitors can be used to optimize treatment of heart failure, improve patient compliance to treatment and offer constant monitoring to allow early treatment. Telecardiology and SGLT2 inhibitor are related to the decreased readmission rate and better patient outcomes and the general control of heart failure. Nevertheless, the amount of heterogeneity that was found in the studies that were included in this review is quite high, which shows that differences in outcomes can vary greatly, and they are probably caused by variations of patient population, study design and telecardiology protocol. The heterogeneity suggests that the effectiveness of such combination interventions and their suitable implementation should be studied further through comparative researches and RCTs. There should be further research to expand the patient selection criteria, long term results and cost effectiveness and feasibility of telecardiology. Altogether, despite the fact that the evidence shows that SGLT2 inhibitors and telecardiology interventions may have a positive effect and can help prevent the hospitalization of heart failure patients, further research is needed to confirm the findings and establish universal treatment processes and implement the interventions in all patients.
References
- Bloom MW, Greenberg B, Jaarsma T, James LJ, Carolyn SP Lam, et al. Heart failure with reduced ejection fraction. Nat Rev Dis Primers 3 (2017): 1-19.
- Ouwerkerk W, Voors A, Anker S, Cleland JG, Dickstein K, et al. Determinants and clinical outcome of uptitration of ACE-inhibitors and beta-blockers in patients with heart failure: a prospective European study. Eur Heart J 38 (2017): 1883-1890.
- Baker A, Chen LC, Elliott RA, Godman B. The impact of the ‘Better Care Better Value’ prescribing policy on the utilisation of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers for treating hypertension in the UK primary care setting: longitudinal quasi-experimental design. BMC Health Serv Res 15 (2015): 367.
- Choi CI. Sodium-glucose cotransporter 2 (SGLT2) inhibitors from natural products: discovery of next-generation antihyperglycemic agents. Molecules 21 (2016): 1136.
- Kaplan A, Abidi E, El-Yazbi A, Eid A, Booz GW, al. Direct cardiovascular impact of SGLT2 inhibitors: mechanisms and effects. Heart Fail Rev 23 (2018): 419-437.
- Zhang KX, Kan CX, Han F, Zhang JW, Sun XD. Elucidating the cardioprotective mechanisms of sodium-glucose cotransporter-2 inhibitors beyond glycemic control. World J Diabetes 15 (2024): 137.
- Murphy SP, Ibrahim NE, Januzzi JL. Heart failure with reduced ejection fraction: a review. JAMA 324 (2020): 488-504.
- Salvatore T, Galiero R, Caturano A, Luca R, Anna Di Martino, et al. An overview of the cardiorenal protective mechanisms of SGLT2 inhibitors. Int J Mol Sci 23 (2022): 3651.
- Tentolouris A, Vlachakis P, Tzeravini E, Eleftheriadou I, Tentolouris N. SGLT2 inhibitors: a review of their antidiabetic and cardioprotective effects. Int J Environ Res Public Health 16 (2019): 2965.
- Filippatos TD, Liontos A, Papakitsou I, Elisaf MS. SGLT2 inhibitors and cardioprotection: a matter of debate and multiple hypotheses. Postgrad Med 131 (2019): 82-88.
- Lam CS, Chandramouli C, Ahooja V, Verma S. SGLT-2 inhibitors in heart failure: current management, unmet needs, and therapeutic prospects. J Am Heart Assoc 8 (2019): e013389.
- Kent DM, Nelson J, Dahabreh IJ, Rothwell PM, Altman DG, et.al. Risk and treatment effect heterogeneity: re-analysis of individual participant data from 32 large clinical trials. Int J Epidemiol 45 (2016): 2075-2088.
- Iwashyna TJ, Burke JF, Sussman JB, Prescott HC, Hayward RA, et.al. Implications of heterogeneity of treatment effect for reporting and analysis of randomized trials in critical care. Am J Respir Crit Care Med 192 (2015): 1045 -1051.
- Crispino SP, Segreti A, Nafisio V, Daniele V, Filippo C, et al. The role of SGLT2-inhibitors across all stages of heart failure and mechanisms of early clinical benefit: from prevention to advanced heart failure. Biomedicines 13 (2025): 608.
- Kling G, Pesqué-Cela V, Tian L, Luo D. A theory of financial inclusion and income inequality. Eur J Finance 28 (2022): 137-157.
- Ducharme A, Zieroth S, Ahooja V, Kim Anderson, Jason A, et al. Canadian Cardiovascular Society-Canadian Heart Failure Society focused clinical practice update of patients with differing heart failure phenotypes. Can J Cardiol 39 (2023): 1030 -1040.
- Cardoso R, Graffunder FP, Ternes CM, A Fernandes, Ana V Rocha, et al. SGLT2 inhibitors decrease cardiovascular death and heart failure hospitalizations in patients with heart failure: a systematic review and meta-analysis. EClinicalMedicine 36 (2021): 100925.
- Minozzi S, Cinquini M, Gianola S, Gonzalez-Lorenzo M, Banzi R. The revised Cochrane risk of bias tool for randomized trials (RoB 2) showed low interrater reliability and challenges in its application. J Clin Epidemiol 126 (2020): 37- 44.
- Afonso J, Ramirez Campillo R, Clemente FM, Büttner FC, Andrade R. The perils of misinterpreting and misusing publication bias in meta-analyses: an education review on funnel plot-based methods. Sports Med 54 (2024): 257-269.
- Andrews I, Kasy M. Identification of and correction for publication bias. Am Econ Rev 109 (2019): 2766 -2794.
- McMurray JJ, Solomon SD, Inzucchi SE, L Køber, M N Kosiborod, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 381 (2019): 1995-2008.
- Li W, Katamreddy A, Kataria R, Myerson ML, Taub CC. Sodium-glucose cotransporter-2 inhibitor use is associated with a reduced risk of heart failure hospitalization in patients with heart failure with preserved ejection fraction and type 2 diabetes mellitus: a real-world study on a diverse urban population. Drugs Real World Outcomes 9 (2022): 53-62.
- Nakagaito M, Imamura T, Joho S, Ushijima R, Nakamura M, et.al. Factors associated with recurrent heart failure during incorporating SGLT2 inhibitors in patients hospitalized for acute decompensated heart failure. J Clin Med 11 (2022): 5027.
- Blanco CA, Garcia K, Singson A, Smith WR. Use of SGLT2 inhibitors reduces heart failure and hospitalization: a multicenter, real-world evidence study. Perm J 27 (2023): 77.
- Fawzy AM, Rivera-Caravaca JM, Underhill P, Fauchier L, Lip GY. Incident heart failure, arrhythmias and cardiovascular outcomes with sodium-glucose cotransporter 2 (SGLT2) inhibitor use in patients with diabetes: insights from a global federated electronic medical record database. Diabetes Obes Metab 25 (2023): 602-610.
- Gonzalez J, Dave CV. Prescribing trends of SGLT2 inhibitors among HFrEF and HFpEF patients with and without T2DM, 2013–2021. BMC Cardiovasc Disord 24 (2024): 285.
- Svanström H, Mkoma GF, Hviid A, Pasternak B. SGLT2 inhibitors and mortality among patients with heart failure with reduced ejection fraction: linked database study. BMJ 387 (2024): 80925.
- Ali Chhachhar A, Sattar S, Baloch F, Umair J, Maria W, et al. Prescribing patterns of SGLT2 inhibitors and their association with heart failure readmissions: a single-center cross-sectional study from a low- and middle-income country. Expert Rev Cardiovasc Ther 53 (2025): 2463879.
- Maines M, Benini A, Vinci A, Anna M, Elisa E, et al. Remote heart failure patients telemonitoring: results of the TreC Heart Failure Study. J Cardiovasc Dev Dis 12 (2025): 182.
- Svanström H, Mkoma GF, Hviid A, Pasternak B. SGLT2 inhibitors and serious renal events among patients with heart failure with reduced ejection fraction. JACC Heart Fail 13 (2025): 102522.
- Nejadghaderi SA, Balibegloo M, Rezaei N. The Cochrane risk of bias assessment tool 2 (RoB 2) versus the original RoB: a perspective on the pros and cons. Health Sci Rep 7 (2024): e2165.
- Carra MC, Romandini P, Romandini M. Risk of bias evaluation of cross-sectional studies: adaptation of the Newcastle-Ottawa Scale. J Periodont Res (2025).
- Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics 74 (2018): 785-794.
- Favorito LA. Systematic review and metanalysis in urology: how to interpret the forest plot. Int Braz J Urol 49 (2023): 775-778.
- Zhang Z, Kossmeier M, Tran US, Voracek M, Zhang H. Rainforest plots for the presentation of patient-subgroup analysis in clinical trials. Ann Transl Med 5 (2017): 485.
- McLaughlin J, Han G, Schalper KA, Daniel C H, Vasiliki P, et al. Quantitative assessment of the heterogeneity of PD-L1 expression in non–small-cell lung cancer. JAMA Oncol 2 (2016): 46-54.
- Sedgwick P. Meta-analyses: what is heterogeneity? BMJ 350 (2015): h1435.
- Richardson M, Garner P, Donegan S. Interpretation of subgroup analyses in systematic reviews: a tutorial. Clin Epidemiol Glob Health 7 (2019): 192-198.
- Feczko E, Fair DA. Methods and challenges for assessing heterogeneity. Biol Psychiatry 88 (2020): 9-17.
- Liu JC, Cheng CY, Cheng TH, Liu CN, Chen JJ, et al. Unveiling the potential: remote monitoring and telemedicine in shaping the future of heart failure management. Life 14 (2024): 936.
- De Wever M, Gruwez H, Dhont S, Pison L, Vandervoort P, et.al. Telecardiology unleashed: probing the depths of effectiveness in remote monitoring and telemedicine applications for acute cardiac conditions. Eur Heart J Acute Cardiovasc Care 14 (2025): 295-303.
- Mensah GA, Roth GA, Fuster V. The global burden of cardiovascular diseases and risk factors: 2020 and beyond. J Am Coll Cardiol 74 (2019): 2529-2532.
- Ribeiro EG, Brant LC, Rezende LC, Regina B, Graziela C, et al. Effect of telemedicine interventions on heart failure hospitalizations: a randomized trial. J Am Heart Assoc 14 (2025): e036241.
- Boriani G, Imberti JF, Bonini N, Cosimo C, Davide AM, et al. Remote multiparametric monitoring and management of heart failure patients through cardiac implantable electronic devices. Eur J Intern Med 115 (2023): 1-9.
- Khan U. Remote patient monitoring and telehealth: the future of cardiac care. Int J Eng Technol Res Manag 8 (2024).


 Impact Factor: * 5.6
Impact Factor: * 5.6 Acceptance Rate: 74.36%
Acceptance Rate: 74.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 