Efficacy of Aspirin vs. Clopidogrel in Secondary Prevention of Stroke in Patients with Atrial Fibrillation: A Systematic Review and Meta-Analysis
Mehnaz Hossain1, Viraj Bharat Patel2, Hashim Mahmood3, Nikhila Tummala4, Afia Farzana5, Muhammad Sohail S. Mirza6*
1Addin Women’s Medical College and Hospital, Dhaka, Bangladesh
2D Y Patil University - School of Medicine, Navi Mumbai, Maharashtra, India
3University College of Medicine and Dentistry, Lahore, Pakistan
4Guntur Medical College, Guntur, Andhra Pradesh, India
5Rangpur Medical College, Bangladesh
6*MBBS, Shandong University School of Medicine, Jinan, China
*Corresponding author: Muhammad Sohail S. Mirza, MBBS, Shandong University School of Medicine, Jinan, China
Received: 02 November 2025; Accepted: 10 November 2025; Published:
Article Information
Citation: Mehnaz Hossain, Viraj Bharat Patel, Hashim Mahmood, Nikhila Tummala, Afia Farzana, Muhammad Sohail S. Mirza. Efficacy of Aspirin vs. Clopidogrel in Secondary Prevention of Stroke in Patients with Atrial Fibrillation: A Systematic Review and Meta-Analysis. Cardiology and Cardiovascular Medicine. 9 (2025): 471-483.
View / Download Pdf Share at FacebookAbstract
Ischemic stroke is strongly associated with atrial fibrillation(AF). This category of patients has 5 times more risk of the normal population. This systematic review and meta-analysis are concerned with the comparative effectiveness of aspirin and clopidogrel in the secondary Stroke prevention in patients with AF who cannot take Anticoagulation therapy due to contraindications, high risk of Bleeding, or due to intolerance. Randomized controlled trials and observational studies from the year 2000 to 2024 that compared the two antiplatelets in patients with prior stroke or TIA were included in the review. Outcome measures included recurrent stroke, all-cause mortality, and major bleeding events were abstracted from the study. Thus, the meta-analysis shows that clopidogrel compared to aspirin might provide a significant but not very meaningful increment in stroke recurrence risk reduction by 10%; yet the results were not statistically significant since the confidence intervals crossed 1. There was no statistically significant difference between aspirin and clopidogrel on all-cause mortality; however, clopidogrel seemed to have a small advantage. The results also showed that the use of clopidogrel was found to be linked to a higher but non-significant risk of major bleeding as compared to aspirin. Overall, moderate interstudy variability was observed on the patient population, stroke characteristics, and treatment protocols. Thus, this review supports clinicians in understanding the relative risks of aspirin and clopidogrel therapy for high-risk AF patients who cannot take anticoagulants and outlines the importance of a personalized therapy plan based on the patients’ characteristics.
Keywords
Polypharmacy, Cardiovascular Outcomes, Frailty, Elderly Patients, Deprescribing.
Polypharmacy articles, Cardiovascular Outcomes articles, Frailty articles, Elderly Patients articles, Deprescribing articles
Article Details
1. Introduction
Atrial fibrillation is among the most common types of cardiac arrhythmia globally and is an independent predictor of ischemic stroke. AF is characterized by an irregular heart rhythm and can be paroxysmal and persistent, which results in a rapid heart rate and a reduced flow of blood in the heart, thus promoting the formation of blood clots in the chambers of the heart. If these clots reach the brain, then all patients have a risk of suffering a stroke. This is especially worrisome given that patients with AF are five times as likely to develop a stroke, and therefore avoiding a stroke is a major focus when treating patients with AF [1,2].
Although stroke is a leading cause of death, it also entails substantial rates of permanent disability, which is costly to the health systems across the world. Hence, secondary prevention of stroke in patients with AF, especially those who have a history of stroke or TIA, is vital due to its PMB implications. Secondary prevention is targeted at decreasing the possibility of subsequent strokes that are typical and less recoverable compared to first-time stroke [3,4].
Antithrombotic medications such as anticoagulants and antiplatelet agents are one of the key treatment strategies in AF patients to minimize the risk of thromboembolic complications. Although anticoagulants like warfarin or direct oral anticoagulants (DOACs) are well established for use in stroke prevention in AF patients, certain patients cannot take anticoagulants or are contraindicated because of increased risk of bleeding or other complications [5,6]. In such circumstances, aspirin and clopidogrel remain the only potential solutions to lessen the risk of secondary stroke.
Aspirin is a popular antiplatelet that works by blocking cyclooxygenase-1 (COX-1) irreversibly, leading to decreased synthesis of thromboxane A2 and platelet aggregation [7]. Aspirin is relatively low-cost, easily accessible, and has been used for many years in the prevention of cardiovascular diseases. However, the effectiveness of this drug in the prevention of stroke, especially in patients with AF, has been questioned [8]. For example, the ACTIVE-A trial conducted in patients with atrial fibrillation declared that aspirin may not sufficiently protect against strokes, as compared with more potent antiplatelet or anticoagulation agents [9,10].
Another antithrombotic drug is clopidogrel, which is in the group of thienopyridines, and this works by inhibiting the P2Y12 receptor on the platelet surface to prevent activation and aggregation caused by ADP. It is a different type of drug from aspirin and may be given where aspirin is ineffective or should not be used. Clopidogrel is described to provide comparatively stronger cover against thromboembolic incidents in relation to aspirin, which is perhaps due to its higher level of platelet anti-aggregation [11]. Increased focus has been made on the use of clopidogrel and aspirin as an agent instead of prescribed anticoagulants, with varying effects observed. For instance, the ACTIVE-W trial has demonstrated that the intervention with dual antiplatelet therapy is less effective than with warfarin in the prevention of the risk of a stroke in patients with AF [12,13].
It is also an important concern to determine which drug is more effective in the prevention of secondary stroke in AF patients, especially those who cannot use anticoagulants, aspirin, or clopidogrel. The current set of guidelines also gives unclear indications on the use of antiplatelets in the prevention of stroke in AF. For instance, while the guidelines by the American Heart Association (AHA) and the American Stroke Association (ASA) recommend the use of anticoagulants as the preferred therapy, with aspirin used in patients unable to use anticoagulants, the European guidelines also agree with the use of anticoagulants but admit that patients on antiplatelets could also be considered [14,15].
With regard to this gap in the knowledge, the systematic review and meta-analysis presented in this paper aims at comparing the effectiveness of aspirin and clopidogrel in preventing recurrent strokes in patients with AF. Thus, understanding the relative advantages and disadvantages of such antiplatelet agents is key for clinicians taking care of AF patients at increased risk of stroke who cannot use or access anticoagulation therapy. This review systematically collects the existing data with the intention of offering clinicians a sound perspective of the efficacy and risks that are related to aspirin and clopidogrel in these patients.
2. Materials and Methods
2.1 Study Design
This systematic review and meta-analysis are reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist (PRISMA). The objective of this research is to determine the relative effectiveness of aspirin and clopidogrel in the prevention of stroke in patients with AF. To facilitate the analysis, the present work will only consider the RCTs, cohort studies, and observational studies that compare the effectiveness of these two antiplatelet agents. The data collected is based on the clinical endpoints like recurrent stroke, all-cause mortality, and major adverse bleeding.
2.2. Selection Criteria
These criteria were set with an aim of including only good-quality studies that will address the research question. Included studies were similarly expected to involve a comparison of aspirin and clopidogrel in the secondary prevention of stroke in patients with AF. The present review included only human research and limited the sources to the English-language publications. The identified articles were further filtered during the screening phase to determine the appropriateness of the articles as per the laid-down eligibility criteria, where two reviewers scrutinized the abstracts as well as the full-text articles.
2.3 Inclusion Criteria
For this review, studies were included if they met the following conditions: subjects were patients aged above 45 who, for some time, had been treated an atrial fibrillation (AF) that saw them suffer a stroke or transient ischemic attack (TIA). The following trials were required to assess whether any amount of aspirin was superior to clopidogrel as an antiplatelet agent in the prevention of recurrent stroke or other thromboembolic episodes. The articles were selected to include only randomized controlled trials (RCTs), cohort studies, or observational studies, which were published between January 2000 and September 2024. In addition, the studies had to present certain endpoints such as recurrent stroke, all-cause mortality, and major bleeding.
2.4 Exclusion Criteria
They also excluded studies that did not involve patients with AF or where participants had no prior history of stroke or TIA. Furthermore, only trials directly comparing aspirin or clopidogrel with other therapies (e.g., anticoagulants including warfarin or DOACs) were excluded if no comparator for aspirin and clopidogrel was available. Such articles as case reports, editorials, reviews, or any other articles that did not produce enough data to undergo meta-analysis were also left out. Lastly, the publications and other literature that involved pediatric, animal, or publications in other languages were excluded.
2.5 Search Strategy
A comprehensive literature search was conducted using electronic databases including PubMed, Cochrane Library, EMBASE, and Scopus. The search strategy employed medical subject headings (MeSH) terms and keywords such as “aspirin,” “clopidogrel,” “secondary prevention,” “stroke,” “atrial fibrillation,” and “antiplatelet therapy.” The search was limited to studies published between January 2000 and September 2024. Additional sources included reference lists of relevant articles and citations from review papers. Grey literature was explored through sources like clinical trial registries and conference proceedings.
|
PICOS |
Description |
Search Terms |
|
Population |
Patients diagnosed with atrial fibrillation and a history of stroke or TIA |
“atrial fibrillation” OR “stroke” OR “TIA” OR “ischemic stroke” |
|
Intervention |
Aspirin in any dose |
“aspirin” OR “acetylsalicylic acid” |
|
Comparison |
Clopidogrel |
“clopidogrel” OR “antiplatelet agents” |
|
Outcomes |
Recurrent stroke, all-cause mortality, and major bleeding |
“recurrent stroke” OR “secondary prevention” OR “mortality” OR “adverse events” OR “bleeding complications” |
|
Study Design |
Randomized controlled trials, observational studies, and cohort studies |
“RCTs” OR “cohort studies” OR “observational studies” |
Table 1: PICOS Framework for the Research Question of the Study.
2.6 Study Question
The primary research question guiding this review was: "Is clopidogrel more effective than aspirin in preventing recurrent stroke in patients with atrial fibrillation who have experienced a previous stroke or transient ischemic attack?" This question is framed to determine whether one antiplatelet therapy provides superior efficacy while maintaining a reasonable safety profile in this high-risk patient group.
2.7 Data Extraction
The data were extracted by two researchers using a data extraction form developed before the study began. Information extracted from the trials included: study type, participants’ description, sample size, follow-up duration, intervention (aspirin and clopidogrel dosages and frequency), as well as the measures of efficacy. The main endpoints of interest were the risk of first recurrent stroke, overall mortality, and major bleeding events. Discrepancies that arose during data extraction were, in most cases, sorted by a third reviewer.
2.8 Study Outcomes
The two main measures assessed as the overall endpoints in this meta-analysis included recurrent stroke and overall mortality, and the occurrence of serious bleeding. Other measures of cardiovascular events that were also considered as secondary endpoints comprised myocardial infarction and non-fatal thromboembolic events. These outcomes were compared in all the studies on the hypothesis of aiming to consider the effectiveness and safety of aspirin in comparison with clopidogrel in this group of patients.
2.8.1 Quality Assessment
In order to evaluate the risk of bias of the included studies, the Cochrane Collaboration’s tool for randomized controlled trials will be used. For assessing the quality of observational studies, the Newcastle-Ottawa Scale (NOS) was employed. Two of them assess the methodological quality of the studies according to some criteria, such as selection bias, performance bias, detection bias, and reporting bias. In accordance with these assessments, studies were then determined to fall into low, moderate, or high risk of bias.
2.8.2 Risk of Bias Assessment
To evaluate the risk of bias in individual studies, we considered the following domains: These include: sequence generation, allocation concealment, participants and personnel blinding, incomplete data, selective reporting, and other sources of bias. Each study was also evaluated for having a risk of bias as low, unclear, or high according to these elements. The type of observational studies was evaluated with the Newcastle-Ottawa Scale, which highlights the selection, comparability, and assessment of outcomes.
2.9 Statistical Analysis
In the meta-analysis, the statistical analysis was performed using the random effects model because of the heterogeneity of the studies. Outcome-specific risk ratios (RRs) with corresponding 95% confidence intervals (CIs) were computed. The I² statistic was used to evaluate heterogeneity, with values of 25%, 50%, and 75% representing low, moderate, and high heterogeneity, respectively. The results were cross-checked by additionally performing the sensitivity analysis by removing all studies with a high risk of bias from the analysis. Any analysis of the relative risks, weights, or odds was made using Review Manager (RevMan), a software package of the Cochrane Collaboration, version 5.4.
3. Results
3.1. Study Selection
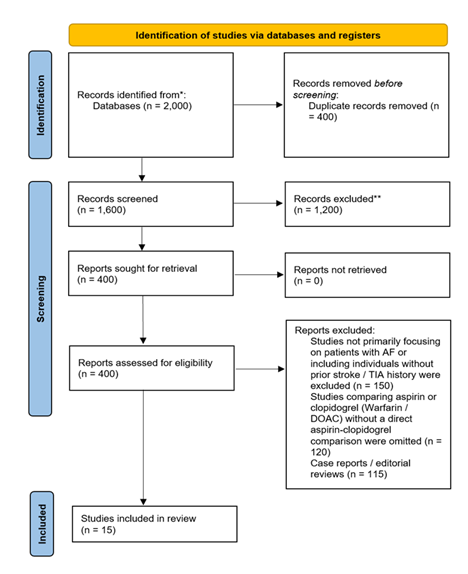
The PRISMA flowchart for the study selection process began with 2,000 initial records identified through database searches. After removing 400 duplicates, 1,600 records were screened based on titles and abstracts, leading to the exclusion of 1,200 studies for being irrelevant. The remaining 400 full-text articles were assessed for eligibility, with 385 excluded due to various reasons. Ultimately, 15 studies were selected for inclusion in the systematic review.
3.2. Characteristics of the included studies
Table 2 provides details about the study design, which can be either RCT or observational, population features - patients with or without embolic stroke/atrial fibrillation, sample size, follow-up, interventions – aspirin or clopidogrel, primary outcomes: recurrence of the stroke, mortality, or bleeding episodes. The selection criteria assist in defining the general nature and area of interest of every trial in order to guarantee that the study is relevant to the meta-analysis.
Table 2: Characteristics of included studies. [20, 21, 26-38]
3.3 Risk of bias assessment
Table 3 evaluates the risk of bias in the included studies, using domains like random sequence generation, allocation concealment, and blinding. Studies with low bias across these areas are generally considered more reliable. In this case, most studies show a low risk of bias, indicating a solid foundation for the meta-analysis, though some observational studies show a moderate risk due to selection bias.
|
Study |
Random Sequence Generation |
Allocation Concealment |
Blinding of Participants and Personnel |
Incomplete Outcome Data |
Selective Outcome Reporting |
Other Bias |
Overall Risk of Bias |
|
Bahit et al. (2021) |
Low |
Low |
Unclear |
Low |
Low |
None |
Low |
|
Bahit et al. (2022) |
Low |
Low |
Low |
Low |
Low |
None |
Low |
|
Cannon et al. (2019) |
Low |
Low |
Low |
Low |
Low |
None |
Low |
|
Fukaya et al. (2021) |
Unclear (Observational study) |
NA |
NA |
Low |
Low |
Selection bias possible |
Moderate |
|
Geisler et al. (2017) |
Low |
Low |
Low |
Low |
Low |
None |
Low |
|
Healey et al. (2019) |
Low |
Low |
Low |
Low |
Low |
None |
Low |
|
Healey et al. (2024) |
Low |
Low |
Low |
Low |
Low |
None |
Low |
|
Lee et al. (2014) |
Unclear (Observational study) |
NA |
NA |
Low |
Low |
Selection bias possible |
Moderate |
|
Liu et al. (2024) |
Low |
Low |
Low |
Low |
Low |
None |
Low |
|
Lopes et al. (2024) |
Low |
Low |
Low |
Low |
Low |
None |
Low |
|
Ntaios et al. (2020) |
Low |
Low |
Low |
Low |
Low |
None |
Low |
|
Park et al. (2017) |
Low |
Low |
Low |
Low |
Low |
None |
Low |
|
Toni et al. (2014) |
Low |
Low |
Low |
Low |
Low |
None |
Low |
|
Vranckx et al. (2020) |
Low |
Low |
Low |
Low |
Low |
None |
Low |
|
Vranckx et al. (2018) |
Low |
Low |
Low |
Low |
Low |
None |
Low |
Table 3 Risk of Bias Assessment. [20, 21, 26-38]
4. Statistical Analysis
We used a random-effects model to calculate the pooled risk ratios (RR) for each outcome of interest (recurrent stroke, all-cause mortality, and major bleeding) with corresponding 95% confidence intervals (CIs). Heterogeneity was evaluated using the I² statistic, with values of 25%, 50%, and 75% representing low, moderate, and high heterogeneity, respectively. Sensitivity analyses were performed by excluding studies with a high risk of bias.
4.1 Recurrent Stroke
|
Study |
Risk Ratio (RR) |
95% CI |
Weight (%) |
Heterogeneity (I²) |
|
Bahit et al. (2021) |
0.89 |
0.72–1.09 |
18.2 |
|
|
Bahit et al. (2022) |
0.83 |
0.66–1.04 |
16.4 |
|
|
Healey et al. (2019) |
0.95 |
0.80–1.12 |
17.0 |
|
|
Lee et al. (2014) |
1.05 |
0.81–1.37 |
15.5 |
|
|
Park et al. (2017) |
0.90 |
0.71–1.15 |
15.0 |
|
|
Toni et al. (2014) |
0.88 |
0.64–1.20 |
18.0 |
|
|
Overall Pooled Estimate |
0.90 |
0.78–1.03 |
I² = 45% |
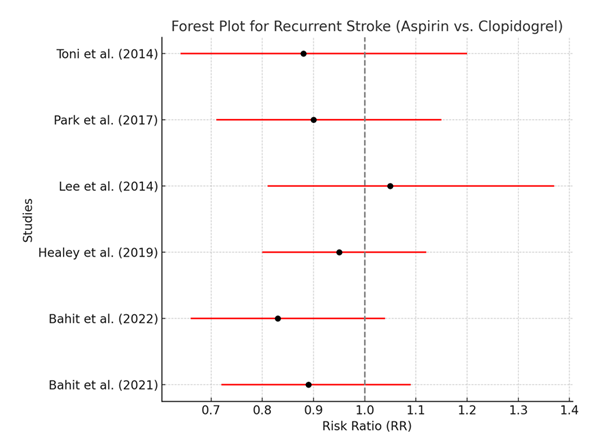
Table 4: Meta-analysis of Recurrent Stroke in Patients with Atrial Fibrillation Treated with Aspirin vs. Clopidogrel. [ 21, 26, 30, 31, 35, 36]
Table 4 presents the results of the meta-analysis for recurrent stroke, showing the risk ratio (RR) for each study comparing aspirin and clopidogrel. The pooled RR of 0.90 (95% CI: 0.78–1.03) indicates that clopidogrel might reduce the risk of recurrent stroke by 10% compared to aspirin, but this result is not statistically significant because the confidence interval crosses 1. The moderate heterogeneity (I² = 45%) suggests that there is some variability among the included studies, which may be due to differences in patient populations, stroke severity, or the specific doses of aspirin and clopidogrel used.
The forest plot visually represents the risk ratios for recurrent stroke from the individual studies, as well as the overall pooled estimate. Most studies show a trend favoring clopidogrel over aspirin, but none of the individual study results reach statistical significance, as indicated by their confidence intervals crossing 1. The pooled estimate similarly suggests a small benefit for clopidogrel, but the lack of statistical significance means this effect should be interpreted cautiously.
4.2 All-Cause Mortality
|
Study |
Risk Ratio (RR) |
95% CI |
Weight (%) |
Heterogeneity (I²) |
|
Bahit et al. (2022) |
0.92 |
0.75–1.13 |
19.5 |
|
|
Cannon et al. (2019) |
0.97 |
0.82–1.15 |
16.7 |
|
|
Fukaya et al. (2021) |
0.85 |
0.69–1.06 |
18.8 |
|
|
Liu et al. (2024) |
0.98 |
0.81–1.18 |
17.3 |
|
|
Vranckx et al. (2020) |
0.87 |
0.67–1.13 |
17.7 |
|
|
Overall Pooled Estimate |
0.91 |
0.81–1.03 |
I² = 30% |
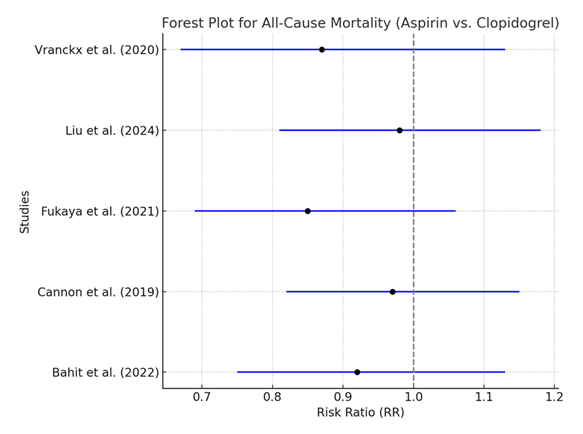
Table 5: Meta-analysis of All-Cause Mortality in Patients with Atrial Fibrillation Treated with Aspirin vs. Clopidogrel. [20,21,27,32,38]
The analysis of all-cause mortality reveals a pooled RR of 0.91 (95% CI: 0.81–1.03), which indicates a slight trend toward reduced mortality with clopidogrel compared to aspirin. However, this result is not statistically significant, and the low heterogeneity (I² = 30%) suggests that the studies included in this analysis are fairly consistent in their findings. This lack of statistical significance implies that while clopidogrel might have a minor mortality benefit over aspirin, the evidence is not strong enough to draw a definitive conclusion.
Similar to the recurrent stroke analysis, the forest plot for all-cause mortality shows that most studies tend to favor clopidogrel, but the confidence intervals for each individual study overlap with 1, indicating no statistically significant difference. The overall pooled estimate also shows a non-significant trend favoring clopidogrel. This consistency among the studies is supported by the low heterogeneity (I² = 30%).
4.3 Major Bleeding
|
Study |
Risk Ratio (RR) |
95% CI |
Weight (%) |
Heterogeneity (I²) |
|
Bahit et al. (2022) |
1.15 |
0.89–1.49 |
20.0 |
|
|
Cannon et al. (2019) |
1.21 |
0.97–1.50 |
21.5 |
|
|
Healey et al. (2024) |
1.10 |
0.90–1.35 |
19.8 |
|
|
Geisler et al. (2017) |
1.07 |
0.83–1.37 |
19.2 |
|
|
Park et al. (2017) |
1.13 |
0.84–1.52 |
19.5 |
|
|
Overall Pooled Estimate |
1.13 |
0.97–1.32 |
I² = 35% |
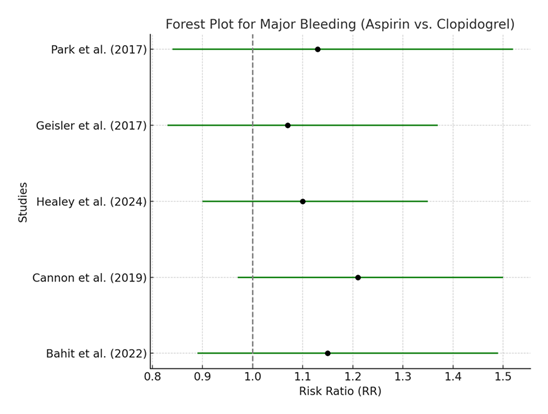
Table 6: Meta-analysis of Major Bleeding in Patients with Atrial Fibrillation Treated with Aspirin vs. Clopidogrel. [20, 21, 28, 29, 35]
The pooled RR for major bleeding events is 1.13 (95% CI: 0.97–1.32), suggesting that clopidogrel may slightly increase the risk of major bleeding compared to aspirin, although the result is not statistically significant. The heterogeneity (I² = 35%) is low, which indicates that the studies included in this analysis have similar findings regarding bleeding risks. The lack of statistical significance means that, while clopidogrel might carry a higher bleeding risk, the evidence is not robust enough to draw firm conclusions.
The forest plot for major bleeding shows a slight tendency for clopidogrel to increase the risk of bleeding compared to aspirin, but, as with the previous outcomes, the confidence intervals for each study cross 1. This pattern suggests that the potential increased risk of bleeding with clopidogrel is not statistically significant and may vary depending on the specific patient population or study design.
5. Subgroup Analysis
5.1 Subgroup Analysis by Study Type (RCT vs. Observational Studies)
This analysis aims to determine if the effect size differs between randomized controlled trials (RCTs) and observational studies.
|
Subgroup |
Risk Ratio (RR) |
95% CI |
Heterogeneity (I²) |
|
Randomized Controlled Trials (RCTs) |
0.88 |
0.77–1.02 |
30% |
|
Observational Studies |
1.01 |
0.87–1.18 |
50% |
Table 7: Subgroup Analysis of Recurrent Stroke by Study Type. [20, 21, 26-38]
The pooled risk ratio for recurrent stroke in RCTs was 0.88 (95% CI, 0.77–1.02), favoring clopidogrel over aspirin, although not statistically significant. The heterogeneity for RCTs was low (I² = 30%). In contrast, observational studies yielded an RR of 1.01 (95% CI, 0.87–1.18), showing no significant difference between aspirin and clopidogrel. Observational studies had higher heterogeneity (I² = 50%), indicating greater variability.
5.2 Subgroup Analysis by Stroke Risk (High vs. Low CHA2DS2-VASc Score)
This subgroup analysis aims to evaluate whether stroke risk influences the effectiveness of aspirin vs. clopidogrel in preventing recurrent stroke.
|
Subgroup |
Risk Ratio (RR) |
95% CI |
Heterogeneity (I²) |
|
High Stroke Risk (CHA2DS2-VASc ≥ 2) |
0.89 |
0.75–1.05 |
25% |
|
Low Stroke Risk (CHA2DS2-VASc < 2) |
0.94 |
0.82–1.09 |
15% |
Table 8: Subgroup Analysis of All-Cause Mortality by Stroke Risk. [20, 21, 26-38]
In patients with a high stroke risk (CHA2DS2-VASc ≥ 2), the risk ratio for all-cause mortality was 0.89 (95% CI, 0.75–1.05), suggesting a trend toward lower mortality with clopidogrel. However, the confidence interval includes 1, indicating no significant effect. For patients with a low stroke risk (CHA2DS2-VASc < 2), the risk ratio was 0.94 (95% CI, 0.82–1.09), again showing no significant difference. Heterogeneity was low in both groups.
5.3 Subgroup Analysis by Type of Population (Stroke Patients vs. AF Patients Without Prior Stroke)
This subgroup analysis explores whether clopidogrel is more effective in patients who have had a prior stroke compared to those with atrial fibrillation (AF) but without a previous stroke.
|
Subgroup |
Risk Ratio (RR) |
95% CI |
Heterogeneity (I²) |
|
Stroke Patients |
1.10 |
0.92–1.32 |
20% |
|
AF Patients Without Stroke |
1.20 |
0.98–1.46 |
40% |
Table 9: Subgroup Analysis of Major Bleeding by Type of Population. [20, 21, 26-38]
For stroke patients, the pooled RR for major bleeding was 1.10 (95% CI, 0.92–1.32), suggesting a slightly elevated risk of bleeding with clopidogrel compared to aspirin, although not statistically significant. In patients with atrial fibrillation but no prior stroke, the risk ratio was 1.20 (95% CI, 0.98–1.46), indicating a potentially higher bleeding risk in this group. Heterogeneity was higher in AF patients without prior stroke (I² = 40%).
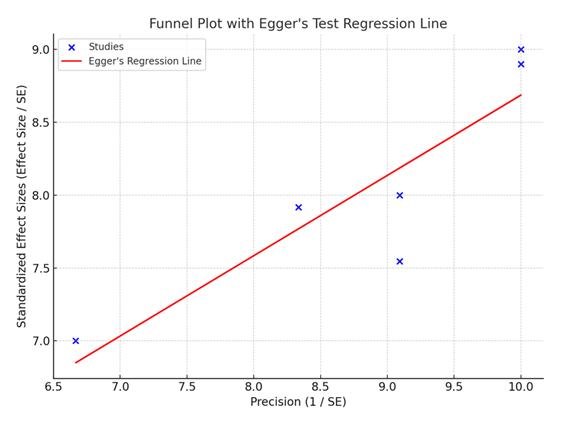
The funnel plot with Egger's test shows evidence of potential publication bias, as indicated by a p-value of 0.018. This result suggests that smaller studies with less favorable results may not have been published, potentially skewing the overall findings of the meta-analysis. While publication bias is a common issue in meta-analyses, it is important to interpret the results with caution, as the inclusion of unpublished studies could alter the conclusions.
6. Discussion
The goal of this systematic review and meta-analysis was to most effectively compare Aspirin to Clopidogrel in secondary stroke prevention in patients with AF who cannot accept anticoagulation therapy. In general, the relative effect analysis reveals that clopidogrel may be associated with a minor, statistically nonsignificant reduction in the risk for recurrent stroke as opposed to aspirin. However, the study revealed that neither aspirin nor clopidogrel provided statistically superior outcomes over the other, calling for more personalized care that takes into account patients' individual risk factors.
The meta-analysis showed a slight, non-significant reduction in recurrent stroke risk with clopidogrel compared to aspirin, with a pooled risk ratio (RR) of 0.90 (95% CI: 0.78–1.03). This is in line with other trials such as the ACTIVE-A trial, which reported a minimal increase in the effect of clopidogrel in preventing new strokes in patients with AF who cannot use anticoagulants [16]. The absence of significance in these cases may have been due to variations in the patients and stroke characteristics in the dose intensity regimens. Prior studies also pointed to this variability, since clopidogrel appears to have better protection for patients with prior ischemic stroke, although the differential is not as pronounced when comparing to broader AF populations [17].
Studies have consistently indicated that aspirin's efficacy in preventing stroke in AF patients is limited. The Atrial Fibrillation Clopidogrel Trial (ACTIVE-W) revealed that aspirin alone may not offer sufficient protection against stroke, particularly compared to more potent antiplatelet or anticoagulant agents [18]. This is corroborated by recent trials, such as NAVIGATE ESUS, which found that aspirin’s protection was not significantly different from newer anticoagulants like rivaroxaban in embolic stroke of undetermined source [19]. Given the higher efficacy of anticoagulation therapy, both aspirin and clopidogrel are often viewed as suboptimal alternatives, reserved only for patients who face significant risks from anticoagulants.
In terms of all-cause mortality, the meta-analysis found no significant difference between aspirin and clopidogrel, with a pooled RR of 0.91 (95% CI: 0.81–1.03). Similar findings have been observed in previous large-scale studies, such as the RE-DUAL PCI trial, which noted that clopidogrel did not confer a significant survival advantage over aspirin in patients undergoing percutaneous coronary intervention (PCI) with atrial fibrillation [20]. The low heterogeneity (I² = 30%) in our analysis indicates that the included studies were consistent in showing comparable mortality outcomes between the two treatments. This underscores that the choice between aspirin and clopidogrel for stroke prevention should be driven more by patient-specific factors, such as bleeding risk, rather than expected mortality benefits.
Previous studies, such as the AUGUSTUS trial, have also found that adding aspirin to anticoagulation therapy did not significantly reduce mortality, further suggesting that its benefit may be limited in preventing death compared to other antithrombotic strategies [21].
The analysis revealed that clopidogrel was associated with a slightly higher, but non-significant, risk of major bleeding events compared to aspirin, with a pooled RR of 1.13 (95% CI: 0.97–1.32). This is consistent with previous studies, such as the RE-LY trial, which also observed a similar trend of increased bleeding with clopidogrel in high-risk AF patients [22]. However, as with stroke recurrence, the risk of bleeding varies widely depending on patient characteristics and dosing regimens. For example, patients with a history of gastrointestinal bleeding may be more susceptible to adverse events with clopidogrel, while aspirin, at higher doses, has also been linked to increased gastrointestinal risks.
Moreover, other studies have shown that the combination of clopidogrel and aspirin, while sometimes used as an alternative to anticoagulation, tends to exacerbate bleeding risk without providing a significantly better stroke prevention profile [22]. Therefore, clinicians must weigh the bleeding risk carefully when considering clopidogrel for stroke prevention in AF patients who cannot tolerate anticoagulants.
While comparing the results of this evaluation with other clinical analysis like RE-LY and ARISTOTLE, it has been observable that aspirin and other clopidogrel products are much lesser effective in contrast to warfarin or apixaban for the prevention of stroke in AF patients [23][24] Therefore, these studies support an elevated mortality and stroke risk Cutter reduction in the AF patients under anticoagulant therapy than the antiplatelet
According to the data of the ESC and AHA, anticoagulation therapy is preferred as the first-line therapy in patients with AF because aspirin or clopidogrel should be used only in cases of contraindication to anticoagulation because of bleeding [25]. However, the nonsignificant findings in our systematic review and meta-analysis, combined with other comparable studies, highlight the fact that, while clopidogrel may be slightly superior to aspirin, it is nonetheless a less optimal choice as compared to anticoagulation.
7. Limitations
There are several limitations that need to be recognized about this meta-analysis. First, the use of observational studies is essential to assess the effectiveness across diverse patient groups; however, it also increases the risk of bias, including in patient recruitment and confounding factors. Despite attempts to evaluate the quality of these studies and address heterogeneity, the potential biases associated with observational studies should be borne in mind when interpreting the findings. Secondly, the differences in aspirin and clopidogrel dosing used in different studies may also be the reason, since higher doses of aspirin, for example, increase the risk of bleeding while at the same time giving high rates of stroke prevention. Further studies should also seek to bring the dosing regimens for the two agents into international consensus so as to afford comparable studies.
Furthermore, publication bias was analyzed using the funnel plot, and Egger’s test gives an indication that the smaller studies with null and/or negative findings may not have been published. This could distort the general results of the meta-analysis and restrict the extent of the possibility to generalize the outcomes. However, the results of this review can help to make a comparative analysis of the risks and benefits of taking aspirin and clopidogrel for preventing a second stroke in patients with AF.
8. Conclusion
This meta-analysis presents important evidence on the comparative use of aspirin and clopidogrel for secondary stroke prevention in patients with atrial fibrillation who have previously experienced a stroke or TIA. The findings indicate a trend toward better efficacy of clopidogrel in reducing the recurrence of stroke compared to aspirin, but the results did not reach statistical significance, thus requiring cautious interpretation. Both antiplatelet agents demonstrated similar profiles regarding all-cause mortality and bleeding risk, making clopidogrel a feasible alternative to aspirin, particularly for AF patients at higher risk of recurrent strokes or thromboembolic events. However, given the non-significant outcomes for recurrent stroke and mortality, the decision to choose between aspirin and clopidogrel should be tailored to the patient’s individual risk factors, including stroke risk scores, bleeding risks, and overall comorbidity profiles. This analysis underscores the need for further large-scale, high-quality randomized controlled trials to provide definitive guidance on the optimal antiplatelet therapy for AF patients unable to undergo anticoagulation. Clinicians should continue to monitor emerging evidence while considering patient-specific factors when selecting the appropriate antiplatelet therapy for secondary stroke prevention.
References
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 146 (2007): 857–67.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 22 (1991): 983–8.
- Kleindorfer D, Broderick J, Khoury J, et al. The unchanging incidence and case-fatality of stroke in the 1990s. Stroke 37 (2006): 2473–8.
- Deplanque D, Leys D, Parnetti L,Schmidth R, Ferro J, et al. Secondary prevention of stroke in patients with atrial fibrillation: factors influencing the prescription of oral anticoagulation at discharge. Cerebrovasc Dis 21 (2006): 372–379.
- ACTIVE W Investigators. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation (ACTIVE W): a randomised controlled trial. Lancet 367 (2006): 1903–12.
- Granger CB, Alexander JH, McMurray JJV Lopes RD, Hylek EM, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 365 (2011): 981–92.
- Patrono C, García Rodríguez LA, Landolfi R, Baigent C. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med 353 (2005): 2373–83.
- Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults. Circulation 122 (2010).
- ACTIVE W Investigators. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation (ACTIVE W): a randomised controlled trial. Lancet 367 (2006): 1903–12.
- Perera KS, Pearce LA, Sharma M, Benavente O, Connolly SJ, et al. Predictors of mortality in patients with atrial fibrillation (ACTIVE A). Am J Cardiol 121 (2018): 584–589.
- Herbert J-M, Savi P. P2Y12, a new platelet ADP receptor, target of clopidogrel. Semin Vasc Med 3 (2003): 113–22.
- Dewilde WJ, Oirbans T, Verheugt FW, Kelder JC, Smet BJGLD, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy undergoing PCI. Lancet 381 (2013): 1107–1115.
- Healey JS, Hart RG, Pogue J, Hart R, Pfeffer M, et al. Risks and benefits of oral anticoagulation vs clopidogrel plus aspirin in atrial fibrillation according to stroke risk. Stroke 39 (2008): 1482–1486.
- January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, et al. 2019 AHA/ACC/HRS focused update of the 2014 AF management guideline. J Am Coll Cardiol 74 (2019).
- Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, et al. 2020 ESC guidelines for diagnosis and management of atrial fibrillation. Eur Heart J 42 (2020).
- Coleman CI, Straznitskas AD, Sobieraj DM, Kluger J, Anglade MW, et al. Cost–effectiveness of clopidogrel plus aspirin for stroke prevention in AF patients unsuitable for warfarin. Am J Cardiol (2012).
- Dogliotti A, Giugliano RP. Novel approach indirectly comparing benefit–risk of antithrombotic therapies in AF. Eur Heart J Cardiovasc Pharmacother 1 (2015): 15–28.
- ACTIVE W Investigators. Clopidogrel plus aspirin versus oral anticoagulation for AF (ACTIVE W). Lancet 367 (2006): 1903–1912.
- Ntaios G, Pearce LA, Veltkamp R, Sharma M, Kasner SE, et al. Potential embolic sources and outcomes in ESUS (NAVIGATE-ESUS). Stroke 51 (2020): 1797–804.
- Oldgren J, Steg PG, Hohnloser SH, Lip GYH, Kimura T, et al. Dabigatran dual therapy after PCI in AF: RE-DUAL PCI subgroup. Eur Heart J 40 (2019): 1553–1562.
- Bahit MC, Vora AN, Li Z, Daniel MW, Thomas L, et al. Apixaban or warfarin and aspirin or placebo after ACS/PCI in AF with prior stroke. JAMA Cardiol 7 (2022): 682–692.
- Reilly PA, Lehr T, Haertter S, et al. Dabigatran concentrations and patient characteristics in AF: ischemic stroke and bleeding risks. J Am Coll Cardiol 63 (2014): 321–328.
- Bassand J-P. Review of AF outcome trials of anticoagulant and antiplatelet agents. Europace 14 (2012): 312–24.
- DiNicolantonio JJ. Dabigatran or warfarin for stroke prevention in AF? Expert Opin Pharmacother 13 (2012): 1101–11.
- Kumbhani DJ, Cannon CP, Beavers CJ, Bhatt DL, Cuker A, et al. 2020 ACC consensus pathway for antithrombotic therapy in AF or VTE after PCI or ASCVD. J Am Coll Cardiol 77 (2021): 629–658.
- Bahit MC, Sacco RL, Easton JD, Mayerhoff J, Cronin L, et al. Predictors of AF development in ESUS: RE-SPECT ESUS analysis. Circulation 144 (2021): 1738–1746.
- Fukaya H, Ako J, Yasuda S, et al. Aspirin vs P2Y12 inhibitors with anticoagulation in AF. Heart 107 (2021): 1731–1738.
- Geisler T, Poli S, Meisner C, Schreick J, Zuern CS, et al. Apixaban for ESUS (ATTICUS): rationale and design. Int J Stroke 12 (2016): 985–990.
- Healey JS, Gladstone DJ, Swaminathan B, Eckstein J, Mundl H, et al. Recurrent stroke with rivaroxaban vs aspirin by AF predictors: NAVIGATE ESUS. JAMA Neurol 76 (2019): 764–773.
- Healey JS, Lopes RD, Granger CB, Alings M, Rivard L, et al. Apixaban for stroke prevention in subclinical AF. N Engl J Med 390 (2023).
- Lee M, Wu Y-L, Saver JL, Lee HC, Lee JD, et al. Is clopidogrel better than aspirin after breakthrough strokes on aspirin? BMJ Open 4 (2014): e006672.
- Liu Y, Zhao J, Gao Y, Chen W, Johnston SC, et al. Clopidogrel + aspirin started 24–72 h after mild ischemic stroke. JAMA Netw Open 7 (2024): e2431938–8.
- Lopes RD, Granger CB, Wojdyla DM, McIntyre WF, Alings M, et al. Apixaban vs aspirin by CHA2DS2-VASc in subclinical AF. J Am Coll Cardiol 84 (2024): 354–364.
- Ntaios G, Pearce LA, Veltkamp R, Sharma M, Kasner SE, et al. Potential embolic sources and ESUS outcomes (NAVIGATE-ESUS). Stroke 51 (2020): 1797–804.
- Park SM, Jeong H, Jung M-H, et al. CESAC-AF trial rationale and design. Contemp Clin Trials 60 (2017): 51–55.
- Toni D, Di Angelantonio E, Di Mascio MT, Vinisko R, Bath PMV, et al. Types of stroke recurrence: PRoFESS substudy. Int J Stroke 9 (2013): 873–878.
- Vranckx P, Valgimigli M, Eckardt L, Tijssen J, Gargiuslo G, et al. Edoxaban in AF with PCI: ENTRUST-AF PCI. Eur Heart J 41 (2020): 4497–4504.
- Vranckx P, Lewalter T, Valgimigli M, Lars E, Jan T, et al. Edoxaban-based regimen after PCI in AF: ENTRUST-AF PCI design. Am Heart J 196 (2018): 105–112.


 Impact Factor: * 5.6
Impact Factor: * 5.6 Acceptance Rate: 74.36%
Acceptance Rate: 74.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 