Exercise Echocardiography in Uncomplicated Type 2 Diabetic Patients: which Parameters are Useful?
Iris Schuster1, Claire Maufrais2*, Monika Krastevich1, Gregory Doucende2, Antonia Perez-Martin1, Nathalie Jourdan3, Christophe Demattei4, Stéphane Nottin2
1Phymedexp, UMR_S 1046, Montpellier University, Montpellier, France
2Avignon University, LAPEC EA4278, F-84000, Avignon, France
3Endocrine and Metabolic Disorders Department, Nîmes University Hospital Center France
4Statistical Department (BESPIM), Nîmes University Hospital Center France
*Corresponding author: Claire Maufrais, Avignon University, LAPEC EA4278, F-84000, Avignon, France.
Received: 21 February 2022; Accepted: 01 March 2022; Published: 07 April 2022
Article Information
Citation: Iris Schuster, Claire Maufrais, Monika Krastevich, Gregory Doucende, Antonia Perez-Martin, Nathalie Jourdan, Christophe Demattei, Stéphane Nottin. Exercise Echocardiography in Uncomplicated Type 2 Diabetic Patients: Which Parameters are Useful?. Cardiology and Cardiovascular Medicine 6 (2022): 111-123.
View / Download Pdf Share at FacebookAbstract
Background: The aim of this study was to (1) identify the most sensitive and clinically relevant echocardiographic variables in order to detect early Left Ventricular (LV) dysfunction in uncomplicated Type 2 Diabetic Patients (T2D) in comparison with Sedentary (SC) and Trained Controls (TC), at rest and during exercise, and (2) to understand the mechanisms of exercise intolerance in these patients.
Methods: 20 T2D without micro- or macrovascular complications and without any other uncontrolled risk factor, 20 SC, and 20 TC were studied at rest and during a cardiopulmonary exercise test using conventional, tissue Doppler imaging (TDI) and speckle tracking echocardiography (STE) including twist-untwist mechanics analysis.
Results: LV dysfunction was detectable in T2D by resting echocardiography. During exercise, T2D had the lowest LV diastolic and systolic functional reserve and the most limited vasodilation compared to SC and to TC who showed the highest adaptability. Exercise STE twist-untwist analysis showed lower feasibility and higher variability in T2D compared to SC and TC. T2D patients had lower longitudinal strain and higher untwist/twist ratio compared to SC but not in comparison to TC.
Conclusion: Conventional and TDI data were the most robust echocardiographic parameters, showing early LV dysfunction and evidencing the contribution of diastolic and systolic dysfunction to exercise intolerance with the expected pathophysiological continuum between groups. Exercise STE data, which showed low feasibility and high variability in T2D, did not exhibit the expected distribution between groups, underlining the fact that strain adaptations in different pathophysiological situations need further explorations.
Keywords
<p>Diabetic cardiomyopathy; Exercise echocardiography; Left ventricular twist; Speckle tracking; Type 2 diabetes; Tissue Doppler</p>
Article Details
1. Intruduction
Early detection of preclinical Left Ventricular (LV) dysfunction in Type 2 Diabetic (T2D) patients is crucial because of its associated increased cardiovascular morbidity and mortality [1]. Medical therapy and life-style modifications like exercise training have been shown to prevent or correct myocardial impairment [2,3]. There is increasing evidence for early diabetic cardiomyopathy [4,5] being a distinct entity even in the absence of coronary artery disease and hypertension. Previous echocardiographic studies have focused on LV diastolic dysfunction in T2D with normal ejection fraction and without epicardial coronary lesions[3, 6]. More recently, Tissue Doppler Imaging (TDI) [7-11 ] and Speckle Tracking Echocardiography (STE) [12-21] have documented abnormalities of LV systolic deformation in T2D. However, many studies included patients with an advanced stage of diabetes, presenting micro- and/or macrovascular complications and other cardiovascular risk factors, making it difficult to rule out the effect of comorbidities. An important question is whether diabetes per se is associated with myocardial abnormalities, and how to detect subtle cardiac dysfunction at an early stage of disease. Challenging the heart by exercise echocardiography is a physiological and non-invasive technique for the early diagnosis of myocardial impairment and for a better understanding of exercise intolerance. STE, which has the potential to evaluate myocardial strains, including twist-untwist mechanics, has recently been shown to be feasible during exercise by our group [22, 23] and others [24-26]. The aim of the present study was (1) to identify the most sensitive and the most clinically relevant echocardiographic variables in order to detect early LV dysfunction in uncomplicated T2D without significant comorbidities or diabetes-related complications by exercise echocardiography based on TDI and STE including twist-untwist mechanics, in comparison with age-matched sedentary controls (SC) and with endurance-trained controls (TC) and (2) to understand mechanisms of exercise intolerance in these patients. We hypothesized that TDI and STE analysis during exercise would allow the earliest diagnosis of subtle LV dysfunction compared to conventional parameters and resting echocardiography, exhibiting a pathophysiological continuum between T2D, SC and TC. We furthermore supposed that diastolic and systolic LV dysfunction contribute to exercise intolerance in these patients.
2. Material and Methods
2.1. Study population
We prospectively included 60 male subjects aged 40-70 years, including 20 T2D patients, 20 SC and 20 TC. The following exclusion criteria were applied to all subjects: active smoking, uncontrolled hypertension (>160/100mmHg), clinical evidence or history of cardiovascular disease, screening echocardiography demonstrating a LV ejection fraction <50%, moderate to severe valvular disease or right ventricular dysfunction, no sinus rhythm, or positive stress test. Diabetic patients were recruited according to the following criteria: documented T2D with two fasting plasma glucose readings >7mmol/l according to WHO criteria, treated by diet and/or with oral antidiabetic drugs. Specific exclusion criteria for T2D patients were: HbA1C>10%, insulin treatment, significant microvascular (proliferative retinopathy, nephropathy, autonomic neuropathy) or macrovascular complications (coronary or peripheral vascular disease, stroke), abnormal ECG or positive stress test. In patients with a submaximal exercise test, dobutamine stress-echocardiography or SPECT were performed in order to rule out significant coronary lesions. T2D had undergone blood analysis with glycaemia, lipid profile and HbA1C, plus screening for nephropathy (microalbuminuria, blood creatinine) less than 3 months before the study. They also underwent screening for retinopathy, neuropathy and peripheral vascular disease by echo-Doppler less than one year before. SC were recruited within family members of hospital staff. TC were recruited via local cycling and running clubs and had been exercising for at least 8 hours per week for at least 5 years. This work was co-sponsored by the Nimes University Hospital and by a grant from the Clinical Research Hospital Program from the French Ministry of Health (PHRC-I/2007/IS-05). The protocol was approved by the ethics committee and the institutional committee on human research. Written informed consent was obtained from all subjects.
2.2. Screening echocardiography
Subjects were examined in the supine position with a Vivid 7 echocardiographic system (GE Healthcare, Horten, Norway). M-Mode measurements of the LV were obtained and LV mass was calculated and indexed for height2.7. Left atrial volume index was determined and LV ejection fraction was calculated by the biplane Simpson’s method. Pulsed Doppler LV inflow (E and A waves) and aortic outflow were recorded in apical views. TDI spectral Doppler peak systolic (S’) and diastolic velocities (E’ and A’) at annular level were derived from apical views on the septal and lateral walls of the LV and averaged.
2.3. Exercise protocol
All subjects underwent a maximal incremental test on a dedicated semi-recumbent ergometer (E-Bike EL 240 V, GE Medical Systems, Freiburg, Germany). Baseline echocardiographic images were obtained after 15 minutes of rest on the ergometer. The test included one workload of 6 minutes at a target heart rate (105-115 bpm) characterized by both sufficient exercise intensity and echocardiographic tracking quality in order to assess all submaximal echo parameters. ECG and gas exchanges were recorded continuously by means of a cardiopulmonary exercise system (Ergocard, Medisoft, Sorinnes, Belgium). Systemic Vascular Resistance (SVR) and arterio-venous difference (DAVO2) were calculated with the following formulas: SVR = mean blood pressure / cardiac output, DAVO2 = (oxygen consumption / 1000 x weight) / cardiac output. Subjects were encouraged to exercise to exhaustion with a minimal requirement of respiratory exchange ratio >1.
2.4. Exercise-Echocardiography
Cine-loops of 3-5 cardiac cycles were acquired during the last 4 minutes of submaximal exercise in parasternal short axis and in apical views for blinded STE offline analysis (EchoPac 6.0, GE Healthcare, Horten, Norway). TDI peak Systolic (S’) and diastolic velocities (E’ and A’) at annular level were derived from apical views on the septal and lateral walls of the LV and averaged. LV E wave/E’lateral ratio was used as an index LV filling pressure. Aortic blood flow velocity was recorded in the ascending aorta with a 2.0-MHz transducer (Pedof) placed at the suprasternal notch to assess Stroke Volume (SV) and cardiac output as previously described [27]. STE analysis was conducted as previously described [3]. LV longitudinal peak strain and strain rate were assessed using 4-chamber views. LV rotations were assessed from short-axis views at basal and apical levels according to the recommendations [28]. LV twist was calculated as the instantaneous difference between apical and basal rotations and LV untwisting rate was evaluated [29]. The ratio of untwisting rate/peak twist was calculated as an indicator of systolic-diastolic coupling by twist-untwist variables [30]. At maximal exercise, we only recorded aortic blood flow with the pedof probe to calculate SV and cardiac output.
3. Statistical Analysis
Continuous variables are presented as mean ± SD. Statistical analysis was performed using StatView SE (SAS Institute, Cary, NC). Analysis of variance was used to compare each continuous variable according to groups. Post-hoc tests (i.e. Partial Least-Squares Differences of Fisher) were used for comparisons between two groups when appropriate. A p-value below 0.05 was considered significant. Intra-observer and inter-observer variability for STE analysis were assessed.
4. Results
In T2D, the mean duration of diabetes was 9.3±6.3 years. Mean fasting blood glucose was 150±36 mg/dL, HBA1c 7.10±1.09%, total cholesterol 158±31 mg/dL, triglycerides 174±78mg/dL, HDL-cholesterol 45.2±16.6 mg/dL and LDL-cholesterol 73.1±21.2 mg/dL.
4.1. Study populations
Baseline clinical characteristics, resting echocardiographic data and maximal exercise parameters are shown in table 1. T2D had a significantly greater body mass index than both control groups, and greater waist to hip ratio and higher heart rates than TC. Peak oxygen consumption and maximal power output showed the lowest values in T2D and the highest in TC, while there was no difference in arteriovenous oxygen difference between groups. Screening echocardiographic data at rest showed higher LV mass index in T2D than in SC and lower LV mass index and left atrial volume index than in TC. Analysis of systolic and diastolic function showed greater peak A velocity, lower peak E/A ratio and lower TDI E’ and S’ in T2D patients than in both control groups.
|
Type 2 diabetic patients |
Sedentary controls |
Trained controls |
|||||||
|
Clinical data |
|||||||||
|
Age (years) |
56 |
± 9 |
55 |
± 8 |
56 |
± 8 |
|||
|
Height (cm) |
174 |
± 5 |
176 |
± 6 |
177 |
± 7 |
|||
|
Body weight (kg) |
88 |
± 14**††† |
76 |
± 12 |
74 |
± 8 |
|||
|
Body mass index (kg.m²) |
29 |
± 4***††† |
24 |
± 2.8 |
23 |
± 2 |
|||
|
Waist/hip ratio |
1.0 |
± 0.1† |
0.97 |
± 0.04 |
0.95 |
± 0.06 |
|||
|
Heart rate (bpm) |
70 |
± 11††† |
68 |
± 11 |
56 |
± 8*** |
|||
|
Systolic blood pressure (mmHg) |
133 |
± 11 |
126 |
± 11 |
125 |
± 7 |
|||
|
Diastolic blood pressure (mmHg) |
83 |
± 7 |
85 |
± 6 |
83 |
± 6 |
|||
|
Maximal exercise parameter |
|||||||||
|
Maximal aerobic power (W) |
137 |
± 19***††† |
183 |
± 28 |
269 |
± 38*** |
|||
|
Maximal oxygen uptake (mL.min-1.kg-1) |
25.6 |
± 7.9*††† |
32.6 |
± 7.0 |
42.7 |
± 6.0*** |
|||
|
Arterio-venous difference (mL.100 mL-1) |
14.0 |
± 4.2 |
14.1 |
± 4.0 |
14.6 |
± 2.2 |
|||
|
Resting echo-Doppler data |
|||||||||
|
LV end-diastolic diameter (mm) |
52 |
± 6† |
51 |
± 6 |
57 |
± 4** |
|||
|
LV end-systolic diameter (mm) |
34 |
± 7 |
32 |
± 5 |
35 |
± 6 |
|||
|
LV mass indexed (g.height-2.7) |
52 |
± 11*† |
47 |
± 13 |
62 |
± 13** |
|||
|
Left atrial volume index (ml.m-²) |
29 |
± 8†† |
28 |
± 7 |
36 |
± 8** |
|||
|
Peak E velocity (cm.s-1) |
75 |
± 16 |
71 |
± 13 |
72 |
± 15 |
|||
|
Peak A velocity (cm.s-1) |
80 |
± 18**† |
65 |
± 14 |
67 |
± 14 |
|||
|
E/A ratio |
0.95 |
± 0.16*† |
1.11 |
± 0.23 |
1.1 |
± 0.3 |
|||
|
TDI E’mean (cm.s-1) |
6.4 |
± 1.0**†† |
8.1 |
± 1.7 |
8.2 |
± 1.9 |
|||
|
TDI S’mean (cm.s-1) |
6.1 |
± 1.0** |
7.4 |
± 1.5 |
6.9 |
± 1.0 |
|||
Table 1: Clinical characteristics, maximal exercise parameters and screening echocardiographic data at rest.
LV: left ventricle; TDI: tissue Doppler imaging. Significantly different from sedentary controls: *p<0.05, **p<0.01, ***p<0.001, different from trained controls: †p<0.05, ††p<0.01, †††p<0.001
4.2. Exercise variables
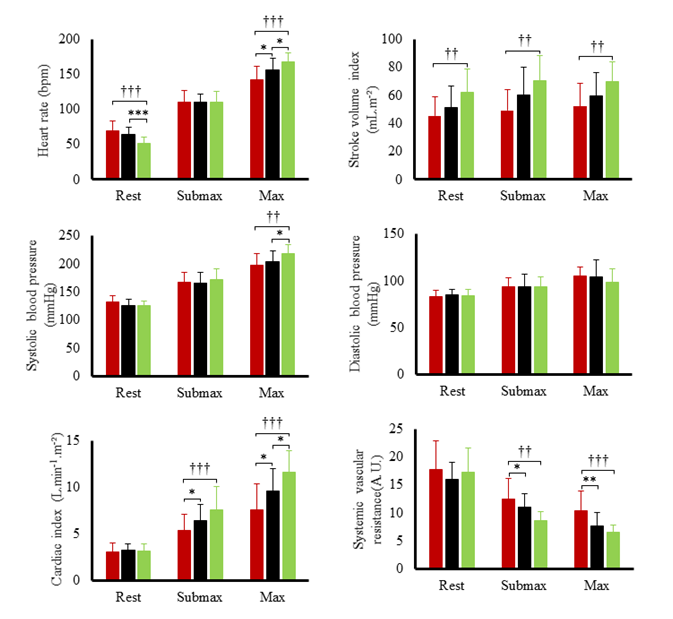
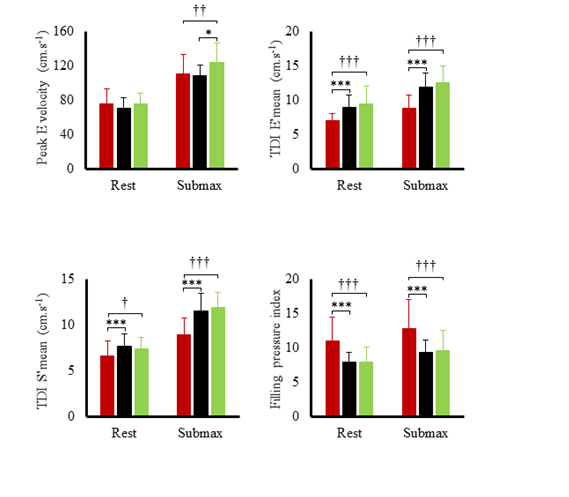
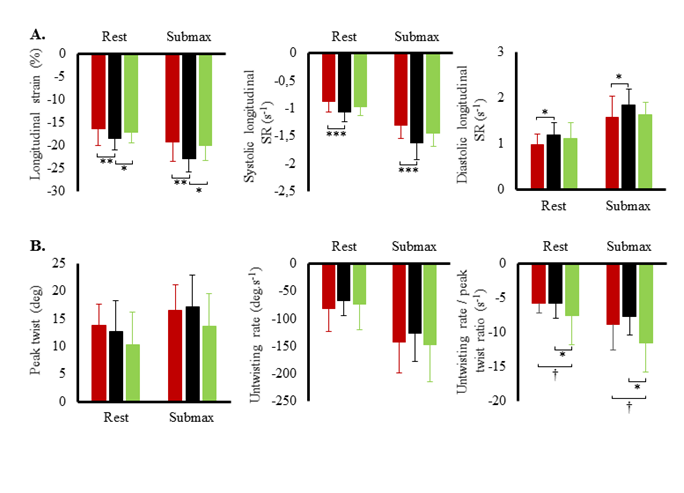
Hemodynamic variables at rest and during submaximal and maximal effort are exposed in figure 1. Systolic blood pressure, SV index and cardiac output index were the highest in TC and lowest in T2D. Systemic vascular resistance was lowest in TC and highest in T2D. Ejection fraction was the lowest in T2D both at rest (60 ± 6 %) and during effort (66 ± 9 %) compared to SC (rest: 71 ± 9 %; exercise: 78 %, p<0.001) and TC (rest: 70 ± 7%; T2D: 79 ± 7%, p<0.001). Heart rate reserve and cardiac index reserve were lowest in T2D patients (73 ± 19 bpm, 4.4 L.min-1.m-2) compared to SC (92 ± 17 bpm, 6.3 ± 1.9 L.min-1.m-2, p<0.001) and TC (116 ± 14 bpm, 8.5 ± 1.9 L.min-1.m-2,p<0.001). We observed no differences in SV index reserve between groups (T2D: 7.4 ± 9.7 mL.m-2; SC: 8.3 ± 9.8 mL.m-2, TC: 8.2 ± 10.4 mL.m-2). Both resting LV end-diastolic diameter (r²=0.32; p<0.001) and heart rate reserve (r²=0.50, p<0.001) were positively correlated with maximal oxygen uptake. LV peak E and TDI data at rest and during submaximal exercise are presented in (Figure 2). During effort, diastolic TDI E’ and systolic TDI S’ were lower in T2D than in both control groups, while E/E’, used as an index of LV filling pressure, was greater in T2D. STE parameters at rest and during submaximal exercise are shown in figure 3. T2D showed lower longitudinal strain and lower longitudinal systolic and diastolic strain rates than SC only but no difference compared with TC. Peak twist and untwisting rate showed important standard deviations with no statistically significant differences between groups. Only untwisting rate/peak twist ratio showed significant differences, with T2D and TC showing higher values compared to SC.
Figure 2: Left ventricular peak E velocity and tissue Doppler data during submaximal exercise in type 2 diabetic patients (red bars), sedentary controls (black bars) and trained controls (green bars). Significantly different from controls: *p<0.05, ***p<0.001; different from athletes: †p<0.05, ††p<0.01, †††p<0.001.
Figure 3: A. Left ventricular strains and strain rates (SR) at rest and during submaximal exercise in type 2 diabetic patients (red circles), sedentary controls (black circles) and trained controls (green circles). B. Left ventricular peak twist, untwisting rate and untwisting rate/peak twist ratio at rest and during submaximal exercise. Significantly different from controls: *p<0.05, **p<0.01, ***p<0.001; different from athletes: †p<0.05.
4.3. Feasibility and variability of STE analysis
At baseline, image quality was good in all groups as the echographer had enough time to save images with high grey scale quality on a still subject. As a result, tracking quality and feasibility at rest was 100% in the three groups for longitudinal strain and twist-untwist analysis. The intra-observer variability was 6% for longitudinal strain and 13% for twist-untwist analysis without significant inter-group differences. During exercise, images had to be saved in short time intervals on a moving subject. Image quality was still good for longitudinal strain analysis in all groups with 100% feasibility and 7.5% variability. For twist-untwist analysis measurements feasibility was only 60% in T2D patients vs 95% in control groups with measurement variabilities of 13% vs 19% respectively. This was due to the fact that high quality images from short axis apical and from the short axis basal level of the left ventricle are necessary for these calculations at the same level of exercise.
5. Discussion
To our knowledge this is the first study in T2D with a comprehensive TDI and STE analysis including twist-untwist mechanics during a cardiopulmonary exercise test. The main results are that: (1) early signs of LV dysfunction can be evidenced in T2D patients without significant comorbidities or diabetes-related complications even by resting echocardiography; (2) exercise intolerance in T2D can be at least partly explained by a lower adaptability of systolic and diastolic function (3) conventional and TDI parameters are the most robust criteria with excellent feasibility and exhibiting a pathophysiologic continuum between T2D, SC and TC, while STE data did not show the same distribution and exhibited lower feasibility and higher measurement variability.
5.1. Early LV dysfunction
In this strictly selected T2D sample without micro or macrovascular complications or other uncontrolled risk factors, the resting echocardiographic examination demonstrated preclinical LV impairment, which can thus be attributed to diabetes per se. Cardio-pulmonary exercise testing revealed exercise intolerance in T2D paralleled by LV systolic and diastolic impairment, which persisted or worsened during exercise. These findings support the concept of specific diabetic cardiomyopathy [4], in the absence of comorbidities or complications. While some authors underlined the need for exercise or dobutamine stress echocardiography for the detection of early cardiac dysfunction in T2D [10, 11, 31], other recent studies are in line with our results, showing that cardiac dysfunction assessed by conventional, TDI or STE parameters is already present at the time of diagnosis in asymptomatic and normotensive T2D [7,17,32] and even before the onset of diabetes (Fontes-Carvalho et al., 2015) in patients with insulin resistance.
5.2. Mechanisms of exercise intolerance in T2D
As expected, maximal power output and aerobic capacity were lowest in T2D and highest in TC. At maximal effort, heart rate, systolic blood pressure and standard echocardiographic variables like SV index and cardiac output index showed the same pathophysiologic continuum between T2D, SC and TC, indicating reduced cardiac adaptability in T2D, while systemic vascular resistances were highest and arteriovenous oxygen difference was unchanged. Moreover, as reported in the study of Roberts et al. [33], cardiac index reserve was decreased in T2D patients due to a diminished heart rate reserve while SV index reserve was preserved in this population. This impaired heart rate reserve which can be linked to reduced physical activity and autonomic dysregulation [34,35 ] also has an impact on aerobic capacity as evidenced by its robust positive correlation with maximal aerobic consumption uptake (r²=0.50, p<0.001). Results from detailed echocardiographic analysis during submaximal exercise in our study suggested a diastolic dysfunction in T2D as evidenced by lower TDI E’ and diastolic STE strain rate as well as increased filling pressures estimated by E/E’ ratio. It is well-known that diabetic cardiomyopathy is associated with an impaired LV compliance and relaxation [34] which were also highlighted in the present study. Furthermore, we observed systolic LV dysfunction as evidenced by ejection fraction, TDI S’, STE longitudinal strain and systolic strain rate that can be explained by an impaired contractility in T2D [36]. All these alterations were already present at rest and were not exacerbated during effort. Taken together, our results demonstrate a limited cardiac adaptability to exercise of the uncomplicated diabetic heart, due to diastolic and systolic LV dysfunction already present in resting conditions.
5.3. Feasibility and usefulness of STE exercise analysis
Feasibility of twist-untwist analysis was low in T2D with higher measurement variability compared to control groups. Using the same analysis package, our group and others have shown feasibility of STE analysis during submaximal exercise in athletes, healthy subjects and patients with lower body mass index [16,20,21,23,37], pointing out the usefulness of STE for the understanding of cardiac exercise adaptations. In accordance with these studies, TC, who were lean and used to exercise, showed high image quality and feasibility even during effort. T2D and SC, who were overweight and/or unaccustomed to physical exercise, showed important body and respiratory movements. This results in out-of-plane movements of the LV and in lower image quality with subsequent loss of tracking. Interestingly, STE exercise strain data did not exhibit the expected pathophysiologic continuum between T2D, SC and TC as observed with standard and TDI parameters. T2D had impaired longitudinal strain and ratio of untwisting rate/peak twist only in comparison with SC but showed similar values to TC. This seems intriguing for groups with different exercise tolerance and is likely to reflect different underlying mechanisms of exercise adaptation. In T2D reduced longitudinal strain has been attributed to dysfunction of subendocardial longitudinal fibres, which are the most vulnerable to ischemia and fibrosis [12-14,18-21]. Their higher untwist/twist ratio at submaximal exercise might reflect a compensatory mechanism for longitudinal dysfunction and reduced filling. At higher intensities, this mechanism is likely to fail in these patients and might contribute to their lower maximal exercise tolerance. In athletes, the question about whether trained athletes have supra-normal, normal or reduced measures of cardiac strain at rest is still under debate (for review: La Gerche et al. [38,39]. However, we can hypothesize that TC would develop a greater deformational reserve at maximal effort, while T2D would show a reduced contractile exercise reserve, as documented in studies based on TDI measurements at maximal effort [31, 9-11]. We have previously shown that the ratio of untwisting rate/peak twist is greater in endurance athletes than in controls [3], reflecting the fact that LV untwist is more important for a given amount of elastic energy stored during systolic twist. During early diastole, LV untwist creates an intraventricular pressure gradient, which is a fundamental mechanism to support filling, particularly during exercise when diastolic time shortens [30]. This mechanism probably ensures optimal systolic-diastolic coupling via twist-untwist mechanics in athletes until maximal effort and helps them achieve important SV during exercise.
5.4. Study limitations
The study population was small. However, it was large enough to support the main conclusions of our paper. Because of its temporal resolution, STE analysis is limited to submaximal exercise with heart rates <110-120 bpm. Furthermore, because of very strict selection criteria, our T2D population does not reflect the general diabetic population in whom exercise intolerance results from the interaction of different mechanistic factors like sedentary lifestyle, overweight, hypertension and dyslipidemia. However, these criteria were applied in order to determine the most sensitive technique for the detection of the earliest myocardial changes in uncomplicated T2D.
5.5. Clinical implications
The identification of sensitive and reproducible echocardiographic parameters for the detection of early cardiac impairment in T2D and for the understanding of exercise intolerance is essential. Conventional and TDI echocardiographic parameters are the most robust criteria in this context with high feasibility and reproducibility during exercise, exhibiting a pathophysiologic continuum between T2D, SC and TC and underlining the contribution of systolic and diastolic dysfunction to exercise tolerance. STE strain analysis, however, showed lower feasibility and higher measurement variability in T2D, especially for twist-untwist mechanics during exercise. The distribution among groups at submaximal exercise was intriguing, underlining the fact that strain adaptations during exercise are still incompletely understood and that there is a need for more “pathophysiological” studies comparing healthy, trained and pathologic groups. The newest techniques might not always be the easiest to analyze in every clinical situation, holding its own restrictions like the need for high image quality and the limitation to submaximal exercise for STE.
Acknowledgements
This work was co-sponsored by the Nimes University Hospital and by a grant from the Clinical Research Hospital Program from the French Ministry of Health (PHRC-I/2007/IS-05). We express our sincere thanks to the volunteers who participated in the study. We thank Nathalie Bedos from the clinical research department of Nîmes hospital for helpful assistance.
Conflict Of Interest
The authors declare no conflict of interest, and no funding was obtained for this study
References
- Kannel WB. Framingham Study Insights on Diabetes and Cardiovascular Disease. Clinical Chemistry 57 (2011): 338-339.
- HollekimStrand SM, Høydahl SF, Follestad T, et al. Exercise Training Normalizes Timing of Left Ventricular Untwist Rate, but Not Peak Untwist Rate, in Individuals with Type 2 Diabetes and Diastolic Dysfunction: A Pilot Study. J Am Soc Echocardiogr 29 (2016): 421-430.
- Maufrais C, Schuster I, Doucende G, et al. Endurance Training Minimizes Age-Related Changes of Left Ventricular Twist-Untwist Mechanics. Journal of the American Society of Echocardiography 27 (2014): 1208-1215.
- Rubler S, Dlugash J, Yuceoglu YZ, et al. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol 30 (1972): 595-602.
- Seferovic PM, Paulus WJ.. Clinical diabetic cardiomyopathy: a two-faced disease with restrictive and dilated phenotypes. Eur Heart J 36 (2015): 1718-1727.
- Zabalgoitia M., Ismaeil MF, Anderson L, et al. Prevalence of diastolic dysfunction in normotensive, asymptomatic patients with well-controlled type 2 diabetes mellitus. Am J Cardiol 87 (2001): 320-323.
- Chaudhary AK, Aneja GK, Shukla S, et al. Study on Diastolic Dysfunction in Newly Diagnosed Type 2 Diabetes Mellitus and its Correlation with Glycosylated Haemoglobin (HbA1C). J Clin Diagn Res 9 (2015): 20-22.
- Fontes-Carvalho R, Ladeiras-Lopes R, Bettencourt P, et al. Diastolic dysfunction in the diabetic continuum: association with insulin resistance, metabolic syndrome and type 2 diabetes. Cardiovasc Diabetol 14 (2015): 4.
- Galderisi M, de Simone G, Innelli P, et al. Impaired inotropic response in type 2 diabetes mellitus: a strain rate imaging study. Am J Hypertens 20 (2007): 548-555.
- Ha JW, Lee HC, Kang ES, et al. Abnormal left ventricular longitudinal functional reserve in patients with diabetes mellitus: implication for detecting subclinical myocardial dysfunction using exercise tissue Doppler echocardiography. Heart 93 (2007): 1571-1576.
- Jellis CL, Stanton T, Leano R, et al. Usefulness of at rest and exercise hemodynamics to detect subclinical myocardial disease in type 2 diabetes mellitus. Am J Cardiol 107 (2011): 615-621.
- Blomstrand P, Engvall M, Festin K, et al. Left ventricular diastolic function, assessed by echocardiography and tissue Doppler imaging, is a strong predictor of cardiovascular events, superior to global left ventricular longitudinal strain, in patients with type 2 diabetes. Eur Heart J Cardiovasc Imaging 16 (2015): 1000-1007.
- Ernande L, Bergerot C, Girerd N, et al. Longitudinal myocardial strain alteration is associated with left ventricular remodeling in asymptomatic patients with type 2 diabetes mellitus. J Am Soc Echocardiogr 27 (2014): 479-488.
- Nakai H, Takeuchi M, Nishikage T, et al.. Subclinical left ventricular dysfunction in asymptomatic diabetic patients assessed by two-dimensional speckle tracking echocardiography: correlation with diabetic duration. Eur J Echocardiogr 10 (2009): 926-932.
- Ng ACT, Bertini M, Ewe SH, et al. 2019. Defining Subclinical Myocardial Dysfunction and Implications for Patients With Diabetes Mellitus and Preserved Ejection Fraction. The American Journal of Cardiology.
- Ng ACT, Delgado V, Bertini M, et al. Myocardial steatosis and biventricular strain and strain rate imaging in patients with type 2 diabetes mellitus. Circulation 122 (2010): 2538-2544.
- Tadic M, Cuspidi C, Vukomanovic V, et al. Layer-specific deformation of the left ventricle in uncomplicated patients with type 2 diabetes and arterial hypertension. Arch Cardiovasc Dis 111 (2018): 17-24.
- Tadic M, Ilic S, Cuspidi C, et al. Left Ventricular Mechanics in Untreated Normotensive Patients with Type 2 Diabetes Mellitus: A Two- and Three-dimensional Speckle Tracking Study. Echocardiography 32 (2015): 947-955.
- Zhang X, Wei X, Liang Y, et al. Differential changes of left ventricular myocardial deformation in diabetic patients with controlled and uncontrolled blood glucose: a three-dimensional speckle-tracking echocardiography-based study. J Am Soc Echocardiogr 26 (2013): 499-506.
- Zhen Z, Chen Y, Shih K, et al.. Altered myocardial response in patients with diabetic retinopathy: an exercise echocardiography study. Cardiovasc Diabetol 14 (2015): 123.
- Zoroufian A, Razmi T, Taghavi-Shavazi M, et al. Evaluation of subclinical left ventricular dysfunction in diabetic patients: longitudinal strain velocities and left ventricular dyssynchrony by two-dimensional speckle tracking echocardiography study. Echocardiography 31 (2014): 456-463.
- Doucende G, Schuster I, Rupp T, et al. Kinetics of left ventricular strains and torsion during incremental exercise in healthy subjects: the key role of torsional mechanics for systolic-diastolic coupling. Circ Cardiovasc Imaging 3 (2010): 586-594.
- Soullier C, Obert P, Doucende G. Exercise response in hypertrophic cardiomyopathy: blunted left ventricular deformational and twisting reserve with altered systolic-diastolic coupling. Circ Cardiovasc Imaging 5 (2012): 324-332.
- Burns AT, La Gerche A, MacIsaac AI, et al. Augmentation of left ventricular torsion with exercise is attenuated with age. J Am Soc Echocardiogr 21 (2008): 315-320.
- Stöhr EJ, González-Alonso J, Shave R. Left ventricular mechanical limitations to stroke volume in healthy humans during incremental exercise. Am J Physiol Heart Circ Physiol 301 (2011): 478-487.
- Wang J, Fang F, Wai-Kwok Yip G, et al. Changes of ventricular and peripheral performance in patients with heart failure and normal ejection fraction: insights from ergometry stress echocardiography. Eur J Heart Fail 16 (2014): 888-897.
- Vinet A, Nottin S, Lecoq AM, et al. Reproducibility of cardiac output measurements by Doppler echocardiography in prepubertal children and adults. Int J Sports Med 22 (2001): 437-441.
- vanDalen BM, Vletter WB, Soliman OII, et al. Importance of Transducer Position in the Assessment of Apical Rotation by Speckle Tracking Echocardiography. Journal of the American Society of Echocardiography 21 (2008): 895-898.
- Dong SJ, Hees PS, Siu CO, et al. MRI assessment of LV relaxation by untwisting rate: a new isovolumic phase measure of tau. Am J Physiol Heart Circ Physiol 281 (2001): 2002-2009.
- Notomi Y, Martin-Miklovic MG, Oryszak SJ, et al. Enhanced ventricular untwisting during exercise: a mechanistic manifestation of elastic recoil described by Doppler tissue imaging. Circulation 113 (2006): 2524-2533
- Cadeddu C, Nocco S, Piano D, et al. Early impairment of contractility reserve in patients with insulin resistance in comparison with healthy subjects. Cardiovasc Diabetol 12 (2013): 66.
- Holland DJ, Marwick TH, Haluska BA, et al, Subclinical LV dysfunction and 10-year outcomes in type 2 diabetes mellitus. Heart 101 (2015): 1061-1066.
- Roberts TJ, Burns AT, MacIsaac RJ, et al. Exercise capacity in diabetes mellitus is predicted by activity status and cardiac size rather than cardiac function: a case control study. Cardiovascular Diabetology 17 (2018): 44.
- Baldi JC, Wilson GA, Wilson LC, et al. The Type 2 Diabetic Heart: Its Role in Exercise Intolerance and the Challenge to Find Effective Exercise Interventions. Sports Med 46 (2016): 1605-1617.
- La Gerche A, Burns AT, Taylor AJ, et al. Maximal oxygen consumption is best predicted by measures of cardiac size rather than function in healthy adults. Eur. J. Appl. Physiol. 112 (2012): 2139-2147.
- Montaigne D, Marechal X, Coisne A, et al. Myocardial contractile dysfunction is associated with impaired mitochondrial function and dynamics in type 2 diabetic but not in obese patients. Circulation 130 (2014): 554-564.
- Simsek Z, Hakan Tas M, Degirmenci H, et al. Speckle Tracking Echocardiographic Analysis of Left Ventricular Systolic and Diastolic Functions of Young Elite Athletes with Eccentric and Concentric Type of Cardiac Remodeling. Echocardiography 30 (2013): 1202-1208.
- LaGerche A, Taylor AJ, Prior DL. Athlete’s Heart: The Potential for Multimodality Imaging to Address the Critical Remaining Questions. JACC: Cardiovascular Imaging 2 (2009): 350-363.
- Boyer JK, Thanigaraj S, Schechtman KB, et al. Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am J Cardiol 93 (2004): 870-875.


 Impact Factor: * 5.6
Impact Factor: * 5.6 Acceptance Rate: 74.36%
Acceptance Rate: 74.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 