Infective Endocarditis After Aortic Valve Replacement with Aorto- Ventricular Discontinuity: A Major Surgical Challenge with Acceptable Midterm Results
Benedikt1*, M. Hohn, V. Kögler1, J. Lacher1, I. Damian1, B. Schachner1, F.Huber1, A. Zierer1
1Johannes Kepler University Linz, Kepler University Hospital, Department for Cardiac-, Vascular- and Thoracic Surgery, Altenberger Strasse 69, 4040 Linz and Krankenhausstrasse 9, 4020 Linz, Austria
*Corresponding author: P. Benedikt, Johannes Kepler University Linz, Kepler University Hospital, Department for Cardiac-, Vascular- and Thoracic Surgery, Altenberger Strasse 69, 4040 Linz and Krankenhausstrasse 9, 4020 Linz, Austria.
Received: 05 March 2024; Accepted: 12 March 2024; Published: 10 April 2024
Article Information
Citation: P. Benedikt, M. Hohn, V. Kögler, J. Lacher, I. Damian, B. Schachner, F.Huber, A. Zierer. Infective Endocarditis After Aortic Valve Replacement with Aorto-Ventricular Discontinuity: A Major Surgical Challenge with Acceptable Midterm Results. Cardiology and Cardiovascular Medicine. 8 (2024): 139-146.
View / Download Pdf Share at FacebookKeywords
Endocarditis; Aortic Valve Replacement; Aorto- Ventricular Discontinuity
Article Details
Introduction
Prosthetic valve endocarditis (PVE) is a serious and potentially life-threatening infection that affects individuals with artificial heart valves[1–4]. Despite advances in surgical techniques and medical therapy, PVE remains a significant problem, with mortality rates ranging from 20% to 80% [5-7]. The incidence of PVE has increased in recent years due to the growing number of individuals receiving prosthetic heart valves, as well as the aging of the population and the rising prevalence of comorbidities such as diabetes and immunosuppression [8-10]. The pathogenesis of PVE is complex and involves a variety of factors such as bacterial virulence, host susceptibility, and prosthetic valve-related factors. The clinical presentation of PVE can vary widely and can include fever, chills, fatigue, malaise, new or changing heart murmurs, embolic phenomena, and sepsis. The diagnosis of PVE can be challenging and requires a high degree of suspicion as well as a combination of clinical, microbiological, and imaging findings [11-13]. The management of PVE involves a multidisciplinary approach with early diagnosis and prompt initiation of appropriate antimicrobial therapy being critical to improving outcomes. Surgical intervention is necessary particularly in cases with persistent infection, valve dysfunction, or complications such as abscess formation, fistulae, or septic emboli. An early cardiothoracic surgical intervention currently represents the gold standard of treatment and is associated with a significant reduction in the mortality rate [14,15].
Surgical management of PVE patients is often challenging, the infection may damage the surrounding tissue and aggressive debridement is mandatory, often leading to severe defects of the outflow tract requiring complex reconstructive procedureshe type of prosthesis, surgical technique used, and type of infection can also affect the success of the operation. Additionally, there is an increased risk of recurrent infections with repeated surgical interventions, which can make the decision to operate challenging in some cases.
In this study, we have investigated the outcomes of our patients with PVE, with a specific focus on the surgical techniques employed for aortic root replacement.
Materials and Methods
Study Population
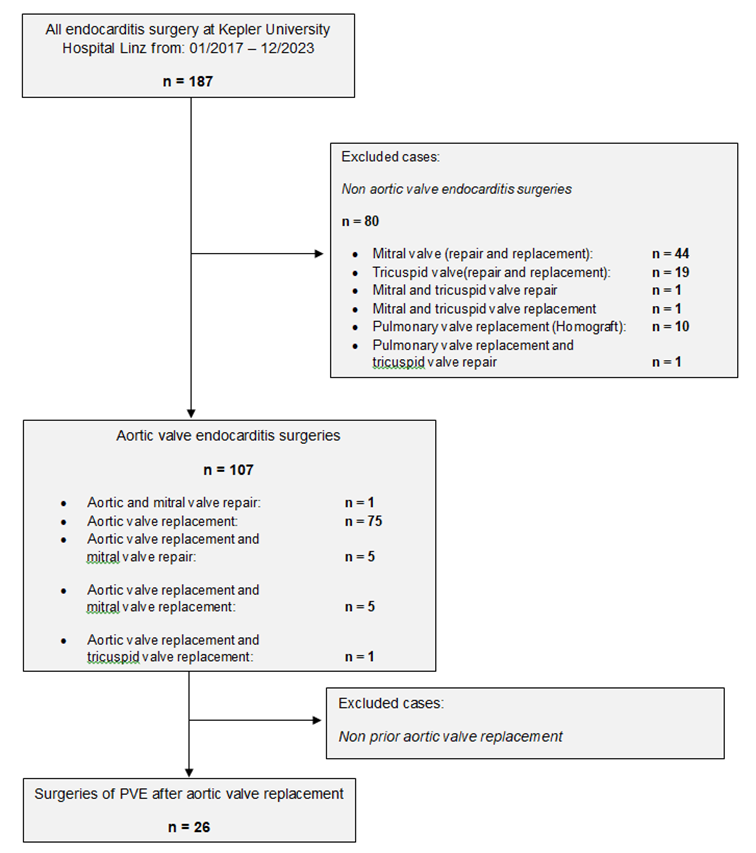
The present study is a single-centre retrospective analysis of patients who underwent surgical treatment for infective endocarditis (IE) following procedures on the aortic valve, from February 2017 to December 2023. The data were extracted from the hospital's internal clinical information system, i.s.h. med (SAP, Walldorf, Germany). During this period, a total of 187 patients were treated with a diagnosis of infective endocarditis (see sample acquisition in Figure 1). Cases with the following characteristics were excluded: native aortic valve endocarditis, additional infective endocarditis of another heart valve requiring surgical intervention, and patients with infections of pacemaker leads. In summary, twenty-six patients were included in the study (see Table 1).
|
Age(y) median [IQR] |
68,5 [11,5] |
|
Female N (%) |
6 (23) |
|
BMI (kg/m2 ) median [IQR] |
26,3 [5] |
|
Hypertension N (%) |
16 (61,5) |
|
Hypercholesterinemia N (%) |
12 (46) |
|
Diabetes mellitus N (%) |
9 (34,6) |
|
Pulmonary hypertension N (%) |
2 (7,7) |
|
PAD N (%) |
3 (11,5) |
|
COPD N (%) |
2 (7,7) |
|
CKD N (%) |
5 (19,2) |
|
Smoking N (%) |
5 (19,2) |
|
EuroScore II median [IQR] |
11,02 [10,5] |
Table 1: Preoperative characteristics of patients undergoing surgery due to PVE (N=26)
Procedures
Previous Operations
All patients underwent a procedure on the aortic valve. The majority (92,3%) underwent open-heart surgery with cardiopulmonary bypass. Two patients (7,7%) were treated with a transcatheter aortic valve implantation (TAVI) through a transapical and a transfemoral approach. Three patients (11,5) had undergone two previous operations on the aortic valve.
Performed Operations
In cases of minimal tissue destruction, the infected aortic valve prostheses were replaced with a biological valve such as the Carpentier-Edwards Magna aortic valve (Edwards Lifesciences, Irvine, CA, USA) or the Perceval sutureless aortic valve (LivaNova, London, United Kingdom).
If replacement or reconstruction of the aortic root was necessary, porcine, bovine or mechanical conduits were used, including the Freestyle stentless porcine aortic root bioprosthesis (Medtronic, Inc, Minneapolis, Minn), the Bio Aortic Conduit NO REACT (Biointegral Surgical Inc, Ontario, Canada), or the St. Jude Mechanical Conduit (Abbott Laboratories, Illinois, USA).
The remaining patients were treated with aortic homografts or self-made biological conduits, which involved sewing a biological aortic valve (Carpentier-Edwards Magna aortic valve; Edwards Lifesciences, Irvine, CA, USA) into a vascular prosthesis such as UniGraft (BBraun, Melsungen, Germany) or Hemashield graft (Getinge, Sweden).
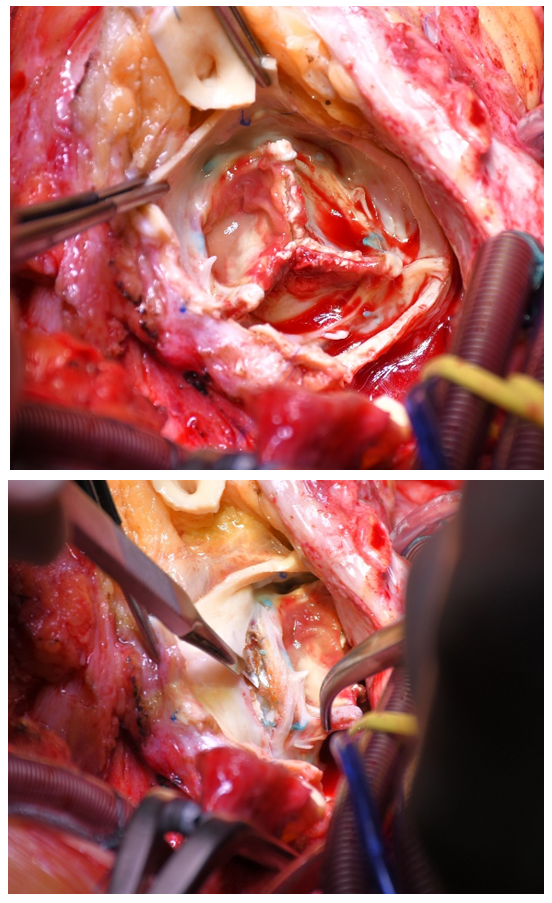
If necessary, econstructive procedures with autologous pericardial patches to reinforce the aortic annulus were done. Intraoperative images of infected valve protheses and replacement of the aortic root with the Biointegral prothesis are shown in Figures 2 to 4.
Specific perioperative approach
For all patients, a focus search is carried out during the postoperative stay, i.e. the dental status is recorded and an ear, nose and throat (ENT) examination is carried out.
Study Approval
The local ethics committee has approved the study (EK Nr: 1161/2022 and 1176/2023).
Statistical Analysis
The statistical analysis is conducted with the pseudonymized relevant data using the software application IBM® SPSS® Statistics (Version 29). Categorical variables are presented as actual counts and relative frequencies. Due to the small sample size, the exact Fisher's test is used to assess independence of variables. A p-value < 0.05 is considered statistically significant at a predetermined significance level of 5%. Given the small sample size and the lack of normal distribution of the metric data, these data are reported as median, first quartile (Q1), and third quartile (Q3) or interquartile range. Graphical representation of the collected data is done using Microsoft® Excel (Version 16.70).
Results
As mentioned before twenty-six patients met the inclusion criteria for PVE ollowing a previous aortic valve surgery. The median time for the development of inflammation at the prosthetic valve was 37 months [(IQR) = 117,5 after the previous procedure. The surgery had to be performed urgently in four patients due to their clinical condition, and one patient required an emergency procedure. A total of ten patients experienced confirmed septic emboli, with the main locations being the spleen (27%) and the brain (19%).
In eighteen (69%) of the patients, due to extensive tissue destruction, replacement and reconstruction of the aortic root with reimplantation of the coronary arteries were necessary. Five patients27,8%) received a replacement with a porcine prosthesis, six (33,3%) with a bovine prosthesis, three patientsach received an aortic homograft or a composite prosthesis consisting of a biological valve and a vascular graft, and one patient was provided with a mechanical conduit. In the remaining eight (31%) patients, the infected valve was replaced with a biological prosthesis. If necessary, the aortic annulus was reinforced with autologous pericardium. Four patients (15,4 %) derwent concomitant CABG.
The 30-day mortality rate was 11,5 %. The patients spent a median of three [2; 5] days at the intensive care unit and 21,5 [14;31] days in the hospital. Acute kidney failure occurred in six (26%) patients. One patientequired re-exploration due to postoperative bleeding. There were no additional neurological events. Four patientseeded a pacemaker due to postoperative sustained AV-block.
The patients were followed up for a median of twenty [1,5;40] months. During this time, there were no cases of recurrent endocarditis. No reoperation on the aortic valve was required. However, two patientsassed away during the follow up, one two years after the surgery due to complications from chronic renal insufficiency, another patient four years after the surgery due to complications arising from chronic heart failure, with no indication of a dysfunction in the aortic valve.
Pathogen spectrum
A pathogen detection is present in 96.1% of the underlying patient collective. In 34,6% of the cases the preoperative blood culture was negative. The intraoperatively obtained tissue was sent for both conventional microbiological analysis and, since December 2021, broad range 16S rDNA PCR. The microbiological spectrum of infective endocarditis (PVE) is predominantly characterized by staphylococci (39,2%), with Staphylococcus aureus (28,5%) being the most common pathogen, surpassing coagulase-negative staphylococci (10,7). Enterococci and Streptococci follow with 25% and 17,8 respectively. Polymicrobial endocarditis is detected in two cases (7,6%).
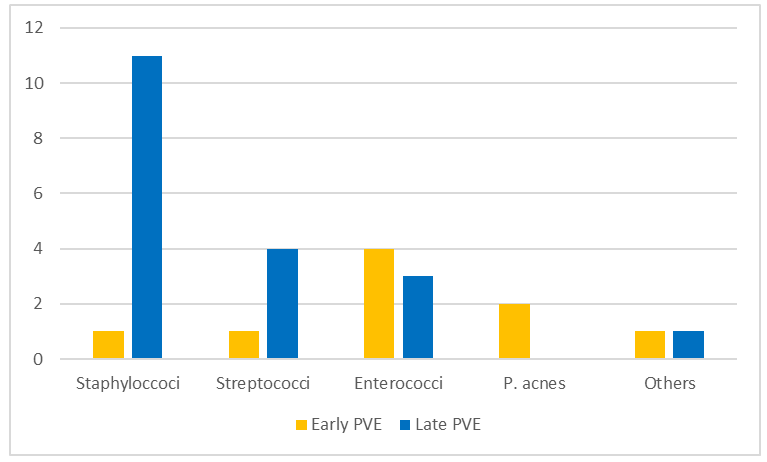
A differentiation of the pathogen spectrum regarding the temporal manifestation of PVE is to be carried out. About one-third of the patient collective (30.7 %)have early PVE (< 12 months), while more than two-thirds (69,3%) have late PVE (> 12 months). According to the exact Fisher test, the early and late PVE differ significantly in their pathogen spectrum (p = 0.024). Figure 5 presents the pathogen spectrum of both early and late PVE.
The pathogen spectrum of early PVE is primarily characterized by Enterococcus faecalis (37,5%). The second most common pathogen is the Gram-positive anaerobic bacterium Propionibacterium acnes (25%), which is part of the skin flora.
In contrast, Staphylococci (56%) clearly predominate in the pathogen spectrum of late PVE, with Staphylococcus aureus (44%) being predominantly detected. About one-quarter of the identified pathogen spectrum of late PVE is determined by streptococci, with the Streptococcus mitis group (16,7 %) being the dominant subtype.
Table 2 provides a concise overview of the underlying pathogen spectrum of PVE and the results of the conducted investigations.
|
Variables |
n = 26 |
Early PVE 9 (30,7 %) |
Late PVE 17 (69,2 %) |
p-value |
|
Blood cultures positive |
17 (65,4 %) |
4 (15,3 %) |
13 (38,5 %) |
|
|
Growth on prothesis |
21 (80,7 %) |
9 (21,4 ) |
12(46,2 %) |
|
|
No growth |
1 (3,8 %) |
1 (3,8 %) |
||
|
Multiple microorganismsl |
2 (7,7 %) |
1 (3,8 %) |
1 (3,8 %) |
|
|
Pathogens |
n = 28 |
0,024* |
||
|
Staphylococci |
11 (39,2 %) |
1 (3,6 %) |
10 (35,7 %) |
|
|
Staphylococcus aureus |
8 (28,5 %) |
8 (28,5 %) |
||
|
CoNS |
3 (10,7 %) |
1 (3,6 %) |
2 (7,1 %) |
|
|
Staphylococcus epidermidis |
2 (7,1 %) |
1 (3,6 %) |
1 (3,6 %) |
|
|
Staphylococcus hominis |
1 (3,6 %) |
1 (3,6 %) |
||
|
Streptococci |
5 (17,8 %) |
1 (3,6 %) |
4 (14,2 %) |
|
|
Streptococcus mitis |
2 (7,1 %) |
2 (7,1 %) |
||
|
Streptococcus oralis |
2 (7,1 %) |
1 (3,6 %) |
1 (3,6 %) |
|
|
Streptococcus dysgalactiae |
1 (3,6 %) |
1 (3,6 %) |
||
|
Enterococci |
7 (25 %) |
4 (10,7 %) |
3 (14,3 %) |
|
|
Enterococcus faecalis |
6 (21,4 %) |
4(10,7 %) |
2 (7,1 %) |
|
|
Enterococcus faecium |
1 (3,6 %) |
1 (3,6 %) |
||
|
Propionibacterium acnes |
2 (7,1 %) |
2 (7,1 %) |
||
|
Acinetobacter lwoffii |
1 (3,6 %) |
1 (3,6 %) |
||
|
Pseudomonas aeruginosa |
1 (3,6 %) |
1 (3,6%) |
Table 2: Spectrum of pathogens causing PVE and the conducted analysis
Worth of mentioning is that all patients who had an infection with streptococci required a replacement of the entire aortic root, while patients with an enterococcal infection more frequently underwent only aortic valve replacement (p=0.027). In the group with a staphylococcal infection, procedures involving complete replacement of the aortic root were also more common (p=0,054). However, no significance was demonstrated.
Regarding the type of valve prosthesis, no difference in the pathogen spectrum is confirmed (p = 0.143). Therefore, based on this data, it is not assumed that specific pathogens preferentially infect a biological or mechanical prosthesis.
Discussions
PVE is a severe complication following valve replacement surgery, carrying a significant risk of mortality and morbidity [5]. In the recent ESC guidelines for the management of endocarditis the recommendation for early surgical intervention in PVE has been further emphasized. It has also been highlighted that the treatment principles for PVE do not differ from those of NVE, but PVE exhibits a higher incidence of tissue destruction, thereby increasing the complexity of the procedure [1]. The occurrence of an aorto-ventricular discontinuity involving the intervalvular fibrous body (IVFB) due to abscesses or pseudoaneurysms in particular necessitates complex surgical procedures, such as the "UFO" procedure [16]. In our study, we focused on surgical treatment options for PVE, particularly in cases with aortic root reconstructions [17,18]. In line with previous studies, our findings revealed a high surgical mortality rate of 20 to 30% for PVE [10,19,20]. These results underscore the complexity and inherent risks associated with treating this condition. The choice of surgical intervention depends on the extent of tissue destruction, which can vary widely among patients. Abscess formation, fistulae, or septic emboli and aorto-ventricular discontinuity further complicate the decision-making process, as it requires innovative techniques to restore valve function and maintain structural integrity [21].
Full root replacement emerged as a frequently employed surgical approach in our study, with 70% of patients undergoing this procedure. This finding aligns with previous research that has shown the necessity and the benefits of full root replacement in managing PVE. The utilization of different types of prosthetic grafts, such as porcine full root prostheses, bovine bioconduits, aortic homografts, biological composite conduits, and mechanical valves, demonstrates the need for individualized treatment strategies based on the specific characteristics of each case. Even in the absence of clear evidence, but out of our experience, we think especially in young patients surgical procedures should employ methods that use minimal foreign material and result in low aortic valve gradients [22,23].
The observation that all patients with a streptococcal infection required complete reconstruction of the aortic root due to the extent of tissue destruction was surprising. In the case of Staphylococcus infection, we anticipated the high number, as it aligns with the scientific opinion that infections caused by Staphylococcus often progress fulminantly with pronounced tissue destruction. This is not the case with streptococcal infections [24,25].
The PVE cases associated with Enterococcus infection exhibited a milder course in our cohort and could generally be surgically managed with a simple replacement of the aortic valve prosthesis [26].
Notably, the occurrence of early PVE within 12 months after the initial valve replacement was observed in 35% of our patients. This finding is consistent with previous studies that have reported a higher incidence of early PVE [5,27,28]. The identification of risk factors associated with early PVE and the development of preventive measures are crucial areas for further investigation. Early detection and prompt intervention are vital to improve outcomes and reduce the morbidity and mortality associated with this complication.
Regarding postoperative outcomes, our study revealed a 30-day mortality rate of 11,5%. Although this rate is in the lower range reported in the literature, efforts should be directed toward reducing perioperative mortality further. The absence of perioperative strokes in our patient cohort is encouraging, as stroke is a significant concern in surgical interventions for PVE. These findings support the notion that careful patient selection, comprehensive preoperative evaluation, and meticulous surgical techniques can contribute to improved outcomes.
Complications rates of bleeding, temporary atrioventricular (AV) block, and the need for pacemaker implantation were observed in our study and are consistent with previous reports that have emphasized the potential for conduction abnormalities and the necessity of close follow-up in PVE patients [1,3,5,10,29-31].
As mentioned in the method section, focusarch is conducted for all patients. In our experience the ENT examination rarely reveals a source of infection, but infected teeth are regularly discovered. These are usually drawn during the primary hospital stay under the existing antibiotic therapy. In accordance with the current guidelines, the antibiotic therapy is given intravenously for six weeks in consultation with the microbiologist and following the antibiogram.
When considering mid-term survival and freedom from reintervention, our study demonstrated satisfactory outcomes, with 91% of patients experiencing favorable results. While this finding is promising, the long-term durability of the surgical interventions performed in PVE cases remains an area of interest. Long-term follow-up studies and registry data are essential to assess the durability and late complications associated with various surgical treatment options for PVE.
Study Limitations
It is important to acknowledge certain limitations of our study, including its retrospective nature and the relatively small sample size. Larger, multicenter studies are needed to validate our findings and provide more comprehensive insights into the surgical management of PVE. Additionally, the inclusion of a control group and comparative analyses with alternative treatment strategies, such as medical management and transcatheter interventions, would further enhance the understanding of optimal therapeutic approaches.
Conclusions
Despite the initial expected, yet acceptable, increase of early postoperative mortality and morbidity, the extensive surgical resection and reconstruction of all infected tissue in PVE provides satisfactory midterm outcome in the majority of patients.
Overall, surgical management of PVE patients requires a high level of expertise and experience, as well as careful consideration of benefits and risks. The decision to operate should be made in the context of the individual patient's circumstances and should be made by an experienced team of cardiologists, infectious disease specialists, and cardiac surgeons. Despite advances in diagnostic and therapeutic approaches, PVE remains a challenging clinical entity, with a high morbidity and mortality rate. Further research is needed to better understand the pathogenesis of PVE, improve diagnostic accuracy, optimize treatment strategies, and identify new therapeutic targets.
However early cardiothoracic surgical intervention is intended to constitute the gold standard for the therapy of PVE, as it is associated with a significant reduction in mortality rates.
References
- Delgado V, Ajmone Marsan N, de Waha S, et al. 2023 ESC Guidelines for the management of endocarditis. Eur Heart J 44 (2023): 3948-4042.
- Satriano UM, Nenna A, Spadaccio C, et al. Guidelines on prosthetic heart valve management in infective endocarditis: a narrative review comparing American Heart Association/American College of Cardiology and European Society of Cardiology guidelines. Ann Transl Med 8 (2020): 1625-1625.
- Mahesh B, Angelini G, Caputo M, et al. Prosthetic Valve Endocarditis. Ann Thorac Surg 80 (2005): 1151-1158.
- Cahill TJ, Prendergast BD. Infective endocarditis. The Lancet 387 (2016): 882-893.
- Khalil H, Soufi S. Prosthetic Valve Endocarditis (2023).
- Le Bot A, Lecomte R, Gazeau P, et al. Is Rifampin Use Associated With Better Outcome in Staphylococcal Prosthetic Valve Endocarditis? A Multicenter Retrospective Study. Clinical Infectious Diseases 72 (2021): e249-255.
- Galar A, Weil AA, Dudzinski DM, et al. Methicillin-Resistant Staphylococcus aureus Prosthetic Valve Endocarditis: Pathophysiology, Epidemiology, Clinical Presentation, Diagnosis, and Management. Clin Microbiol Rev 32 (2019): e00041-18.
- Brouwer J, van den Brink FS, Nijenhuis VJ, et al. Incidence and outcome of prosthetic valve endocarditis after transcatheter aortic valve replacement in the Netherlands. Netherlands Heart Journal 28 (2020): 520-525.
- Goldsweig AM, Baron SJ. Prosthetic valve endocarditis: Literally a growing concern following transcatheter aortic valve replacement. Catheterization and Cardiovascular Interventions 99 (2022): 904-905.
- Luehr M, Bauernschmitt N, Peterss S, et al. Incidence and Surgical Outcomes of Patients With Native and Prosthetic Aortic Valve Endocarditis. Ann Thorac Surg 110 (2020): 93-101.
- Lo Presti S, Elajami TK, Zmaili M, et al. Multimodality imaging in the diagnosis and management of prosthetic valve endocarditis: A contemporary narrative review. World J Cardiol 13 (2021): 254-270.
- Bayer AS, Chambers HF. Prosthetic Valve Endocarditis Diagnosis and Management— New Paradigm Shift Narratives. Clinical Infectious Diseases 72 (2021): 1687-1692.
- Durack DT, Lukes AS, Bright DK, Duke Endocarditis Service. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Am J Med 96 (3): 200-209.
- Chirouze C, Alla F, Fowler VG, et al. Impact of Early Valve Surgery on Outcome of Staphylococcus aureus Prosthetic Valve Infective Endocarditis: Analysis in the International Collaboration of Endocarditis–Prospective Cohort Study. Clinical Infectious Diseases 60 (2015): 741-749.
- Weber C, Petrov G, Luehr M, et al. Surgical results for prosthetic versus native valve endocarditis: A multicenter analysis. J Thorac Cardiovasc Surg 161 (2021): 609-619.e10.
- Misfeld M, Davierwala PM, Borger MA, et al. The “UFO” procedure. Ann Cardiothorac Surg 8 (2019): 691-698.
- Szczechowicz MP, Weymann A, Mkalaluh S, et al. Aortic Root Replacement for Destructive Endocarditis – Clinic and Microbiology. Braz J Cardiovasc Surg 36 (2021): 614-622.
- Levine D, Patel P, Zhao Y, et al. Reoperative aortic root replacement for prosthetic aortic valve endocarditis: impact of aortic graft. European Journal of Cardio-Thoracic Surgery 64 (2023): ezad268.
- Grubitzsch H, Christ T, Melzer C, et al. Surgery for prosthetic valve endocarditis: associations between morbidity, mortality and costs. Interact Cardiovasc Thorac Surg 22 (2016): 784-791.
- Lalani T. In-Hospital and 1-Year Mortality in Patients Undergoing Early Surgery for Prosthetic Valve Endocarditis. JAMA Intern Med 173 (2013): 1495.
- Galeone A, Trojan D, Gardellini J, et al. Cryopreserved aortic homografts for complex aortic valve or root endocarditis: a 28-year experience. European Journal of Cardio-Thoracic Surgery 62 (2022): ezac193.
- Kirklin JK. Challenging homografts as the holy grail for aortic valve endocarditis. J Thorac Cardiovasc Surg 151 (2016): 1230-1231.
- Abdelsattar ZM, Elsisy MF, Schaff H, et al. Comparative Effectiveness of Mechanical Valves and Homografts in Complex Aortic Endocarditis. Ann Thorac Surg 111 (2021): 793-799.
- MURRAY RJ. Staphylococcus aureus infective endocarditis: diagnosis and management guidelines. Intern Med J 35 (2005): S25-44.
- Cahill TJ, Prendergast BD. Infective endocarditis. The Lancet 387 (2016): 882-893.
- Danneels P, Hamel JF, Picard L, et al. Impact of Enterococcus faecalis Endocarditis Treatment on Risk of Relapse. Clinical Infectious Diseases 76 (2023): 281-290.
- Galar A, Weil AA, Dudzinski DM, et al. Methicillin-Resistant Staphylococcus aureus Prosthetic Valve Endocarditis: Pathophysiology, Epidemiology, Clinical Presentation, Diagnosis, and Management. Clin Microbiol Rev 32 (2019): e00041-18.
- Siciliano RF, Randi BA, Gualandro DM, et al. Early-onset prosthetic valve endocarditis definition revisited: Prospective study and literature review. International Journal of Infectious Diseases 67 (2018): 3-6.
- Salem M, Friedrich C, Saad M, et al. Active Infective Native and Prosthetic Valve Endocarditis: Short- and Long-Term Outcomes of Patients after Surgical Treatment. J Clin Med 10 (2021): 1868.
- Pyo WK, Kim HJ, Kim JB, et al. Comparative Surgical Outcomes of Prosthetic and Native Valve Endocarditis. Korean Circ J 51 (2021): 504.
- Ivanovic B, Trifunovic D, Matic S, Petrovic J, Sacic D, Tadic M. Prosthetic valve endocarditis – A trouble or a challenge? J Cardiol 73 (2019): 126–133.



 Impact Factor: * 5.6
Impact Factor: * 5.6 Acceptance Rate: 74.36%
Acceptance Rate: 74.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 