Intravascular Ultrasound Imaging for Evaluation of Aortic Elastic Properties: Review of the Literature and a Single-Center Experience
Niya Boykova Milevaa,*, Dobrin Iotkov Vassilevb
aResident Doctor, Cardiology Clinic, “Alexandrovska” University Hospital, Medical University of Sofia, Sofia, Bulgaria
bHead of Cardiology Clinic, “Alexandrovska” University Hospital, Medical University of Sofia, Sofia, Bulgaria
*Corresponding Author: Niya Boykova Mileva, MD, Resident Doctor, Cardiology Clinic, “Alexandrovska” University Hospital, Medical University of Sofia, Sofia, Bulgaria
Received: 18 July 2020; Accepted: 27 July 2020; Published: 06 August 2020
Article Information
Citation: Niya Boykova Milevaa, Dobrin Iotkov Vassilev. Intravascular Ultrasound Imaging for Evaluation of Aortic Elastic Properties: Review of the Literature and a Single-Center Experience. Cardiology and Cardiovascular Medicine 4 (2020): 396-399.
View / Download Pdf Share at FacebookAbstract
Introduction: Aortic stress is a well-known cardio-vascular risk factor. For years, different methods have been studied in the assesment of aortic elastic properties and large arterial stiffness for risk stratification. We asses the role of intravascular ultrasound imaging for the evaluation of aortic elastic properties.
Methods: Intravascular ultrasound imaging of the aorta was performed in 12 patients with transthoracic echocardiography (TTE) and computed tomography (CT) evidence for enlargement of the ascending aorta – diameter ≥ 40.0 mm. Mechanical properties of the aorta were derived from the measured diameters and intra-aortic pressure. Paired samples T-test analysis was performed to determine difference between measurements derived by TTE, CT and IVUS.
Results: Mean values of the calculated elastic properties of the ascending aorta were as follows: compliance 0.021 ± 0.02; strain 205 ± 4.3; aortic stiffness index 4.3 ± 0.75; elastic modulus 0.31 ± 0.05. On paired T-test analysis maximum ascending aortic diameter measured by CT aortography and IVUS did not differ significantly (t = -0.19, p=0.985), but a significant difference between IVUS measurements and TTE derived diameters was found (t = 13.118, p = 0.034). On average, IVUS diameters were 4.1 mm larger than the results acquired by TTE (95% CI [14.21, 17.13]).
Conclusion: IVUS examination of the ascending aorta provided larger diameters than the ones collected by means of TTE. However, IVUS measurements did not differ significantly from diameters derived by CT aortography.
Keywords
<p>Intravascular Ultrasound Imaging</p>
Article Details
Background
Large arteries in the human body, also called elastic, contain high number of collagen and elastin filaments in their medial layer, giving them the ability to stretch in response to the ventricular contractions [1]. They act as an elastic buffering chamber storing nearly fifty percents of the left ventricular (LV) stroke volume during systole. In diastole, the elastic forces of the aortic wall release this residual volume to the peripheral circulation, thus creating a nearly continuous peripheral blood flow. This phenomenon is known as the Windkessel effect [2]. This function of the elastic arteries enables maintaining a relatively constant pressure in the arteries despite intermittent left ventricular ejection and the pulsating nature of blood flow. This interaction impacts not only the peripheral circulation but also the heart, resulting in a reduction of left ventricular afterload and improvement in coronary blood flow and left ventricular relaxation.
With aging mechanical properties of large arteries start to change. As the largest artery in the human body, the aorta is most prone to abnormal stiffening in response to the cumulative exposure to hemodynamic loading, lifestyle and cardiovascular (CV) risk factors. The most distensible segment of the vessel is the ascending aorta, hence it exhibits the earliest changes with aging [3]. The medial layer of the aortic wall (tunica media) is the main determinant of this proccess. With aging, exposure to risk factors and the development of variety of chronic diseases, the vascular wall is being progressively injured [4]. This injury is provoking inflammatory proccess with elastin degradation and collagen deposition in the vessel wall. Furthermore, inflammation promotes endothelial disfunction with production of bone morphogenetic protein-2 and vascular calcification. Genetics seems to play an important role in the development of large artery stiffness (LAS). It has been shown that mutations in the matrix metalloprotein-9 gene are independent predictors of increased aortic stiffness [5]. Also, patients with connective tissue disorders - Marfan and Ehlers – Danlos syndrome, experience increased aortic wall stiffness early in childhood which progresses with aging [6]. There is plenty of evidence demonstrating the relationship between diabetes and aortic stress. Hyperglycemia and insulin-resistance cause endothelial disfunction and arterial stiffening [7,8]. In patients with chronic kidney disease (CKD) there is dysregulation of bone and mineral metabolism. Serum concentrations of osteoprotegerin and fibroblast growth factor-23 increase in impaired kidney function. Furthermore, there is elevated production of inflammatory biomarkers—such as C-reactive protein, tumor necrosis factor-alpha, and interleukin-6 [9]. All these factors are strongly associated with increased LAS. Patients with CKD are known to be more susceptible to electrolyte fluctuations, increases in reninangiotensin-aldosterone system, and sympathetic hyperactivity, all of which also induce arterial stress [10]. On the other hand, in patients with chronic aortic regurgitation there is increased arterial compliance and distensibility. Probably this is a consequence of a compensatory mechanism to lessen the impact of the large systolic volume ejected into the conduit arteries [11]. Lack of such increased compliance is associated with faster hemodynamic deterioration and disease progression [12].
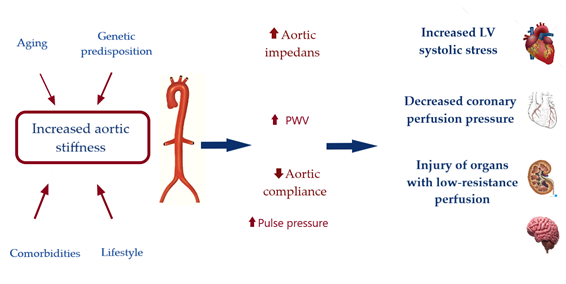
Hemodynamic Consequences of Increased Aortic Stiffness
As stated early a compliant aorta in a young healthy individual has the quality to effectively buffer excess pulsatility caused by the intermittent left ventricular ejection and exhibits a slow pulse wave velocity [12]. In such conditions, reflected waves arrive to the heart during diastole, increasing diastolic coronary perfusion pressure but not systolic ventricular load. However, with decrease of aortic distensibility and rise in aortic root impedance, there is an increase in forward wave amplitude and pulse-wave velocity (PWV). The higher PWV induce earlier arrival of the reflected waves – in mid to late systole. This in turn, leads to systolic pressure augmentation with increased LV oxygen demand and decrease of coronary perfusion pressure on the other hand [13]. Therefore, aortic stiffening plays central role in a viscous cycle of hemodynamic consequences, causing systolic hypertension, promoting LV remodelling and heart failure [14]. Importantly, aortic stiffening may be harmful farer than the heart. Transmission of pulsatile energy with excessive shear forces into the microvasculature is injuring organs in low resistance beds such as the brain and the kidney [15, 16], See Figure 1.
Aortic stress and cardio-vascular outcomes
Aortic stiffening may have major impact on cardiovascular health. A large body of evidence has demonstrated the prognostic value of artery stress for the prediction of cardiovascular events [17, 18]. LAS has been proved as an independent predictor of mortality as well as predictor of ischemic heart disease and stroke in the general population [19]. Furthermore, decreased arterial distensibility has been associated with increased morbidity and both all-cause and CV mortality in hypertensive patients [20,21]. Meta-analysis of prospective studies including 17 635 participants found that increased arterial stiffness is a strong predictor for coronary heart disease, stroke, and overall cardiovascular disease, respectively [22].
Definitions of aortic mechanical properties (23,24):
For the calculation of wall properties, it is assumed that the cross-section of an artery is circular.
Diameter D = √(A/π)
Arterial compliance (C) is the absolute change in area (or change in diameter D) for a given pressure step (P) at a fixed vessel length. The terms “compliance” and “elasticity” are often used interchangeably.
C= ΔD/ΔP.
Stress is defined as the force applied to a particular object or area. It can be applied in radial, circumferential, and longitudinal direction. Circumferential wall stress, defined by Laplace’s law, is directly proportional to the vessel pressure and radius and inversely proportional to its thickness.
Stress= F/A.
Strain is the resulting deformation (percentage change in length) of an object/material subjected to a stress force.
Strain = (DD–SD)/DD.
Stiffness is defined as the resistance offered by an elastic body to deformation. Aortic stiffness index β = ln (SBP/DBP) / strain, where ‘ln’ means natural logarithm.
The elastic modulus (E), is the stress/strain ratio. Elastic modulus E(p) = (SBP−DBP) / strain
Distensibility is defined as the change in diameter/area/pressure step increase. It is the inverse of the elastic modulus (E). Aortic distensibility = (2 x strain) / (SBP−DBP)
Methods for evaluation arterial elastic properties
Although, current diagnostic methods do not provide direct in vivo measurement of arterial mechanical properties, parameters can be derived by evaluation of the relation of changes in arterial pressure and changes in arterial volume, cross-sectional area and diameter or by assessment of the speed at which the pressure waves propagate along the arterial tree - arterial PWV.
Measurement of PWV is the most validated method for noninvasive evaluation of arterial stress. Given its simplicity and accuracy for prediction of adverse CV outcomes [25, 26] it is recommended by the European Society of Cardiology guidelines for the management of arterial hypertension as the first-line method for assessing of arterial stiffness [27]. PWV can be determined by measuring the pulse transit time from the pressure waveforms at two sites along a vascular segment. Carotid-femoral pulse wave velocity (CFPWV) is the current reference method for aortic stiffness and is considered to be a global estimate of arterial PWV through the entire aorta [28]. CFPWV may be measured by applanation tonometry, oscillometric method or by doppler. Main advantage of the method is the superficial location of the common carotid and femoral arteries. However, the acquisition of carotid and femoral pulse waveforms can be technically difficult in heavy weight patients. Another method for evaluation of large artery stiffness is the acquiring of phonocardiographic data, along with brachial and ankle cuff waveforms and the estimation of the cardiac-ankle vascular index (CAVI) [29]. The heart-to-ankle travel time is derived as the time between the pulse onset at the heart and the upstroke of the ankle waveform. A potential disadvantage is the inclusion of a long muscular arterial segment (femoral to ankle), which may discord LAS measurements.
Transthoracic echocardiography is easily accessible imaging modality that is commonly used in everyday clinical practise. Vessels mechanical properties may be derived from measurements of systolic and diastolic diameters or cross-sectional area. M-mode measurements of aortic diameters should be obtained at 3 cm above the aortic valve on parasternal long-axis view [30]. On transesophageal echocardiography, recommended measurements are done at the level of pulmonary artery bifurcation and in the descending thoracic aorta just distal to the branching site of the left subclavian artery [31]. Magnetic resonance imaging enables measuring of PWV and the detection of more subtle changes in regional wall kinetics with 3-dimensional visualization of the vessel is possible. What is more, velocity data can be acquired simultaneously within 1 acquisition plane in 2 aortic locations, and distance between the two aortic locations can be measured precisely [32].
Intravascular Ultrasound Imaging of the Aorta
Weintraub in 1990 is the first to use intravascular ultrasound (IVUS) for the assessment of a patient with aortic dissection [33]. Since then, there are several reports and studies described in the literature about the application of IVUS for imaging of the diseased aorta [34-36].
In 1985 Hughes et al. described an in vivo experiment of evaluation of aortic distention in dogs by intravascular ultrasonic catheter with simultaneous measurement of intra-aortic pressure [37]. Consequently, the canine aorta were excised and pressure/radius ratio measurement were performed once again. The authors concluded that measurement of arterial dimensions with this ultrasonic system could provide useful evaluation of the elastic properties of the aorta. Nearly, 10 years later Hansen et al, performed a study with evaluation of aortic mechanical properties of 6 patients via intravascular ultrasound imaging [38]. Minimum and maximal aortic diameters were measured, with simultaneous recording of intraaortic pressures. The authors concluded that IVUS provides an accurate in vivo evaluation of human arterial compliance, the practical value of which needs to be established.
Methods
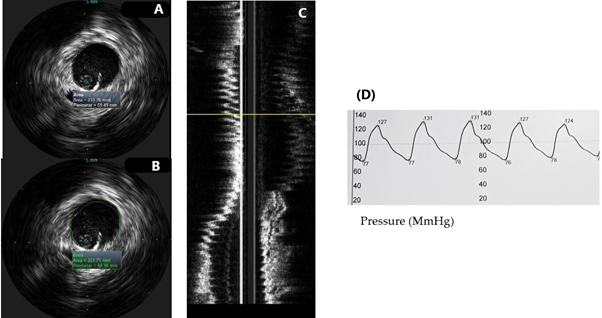
We performed intravascular ultrasound imaging examination of aorta in 12 patients with transthoracic echocardiography (TTE) and computed tomography (CT) evidence for enlargement of the ascending aorta – diameter ≥ 40.0 mm. We used a 20 MHz, Visions PV probe (Volcano Philips Corp, USA) through a 8.5 F femoral sheath. By virtue of manual pullback of the probe, the vessel was visualized through its whole length from the aortic root to the aorto-iliac bifurcation. Measurements of maximal systolic and diastolic cross-sectional area and diameters of the vessel were obtained, as well as, simultaneous recording of intra-aortic systolic (Ps) and diastolic pressures (Pd). (See Figure 2). Then elastic properties of the aorta were calculated using the following formulas: aortic compliance - C= ΔD/ΔP; strain - (Ds + Dd)/Dd x 100; elastic modulus E = Ps-Pd/strain, aortic stiffness index β = ln(Ps/Pd)/strain. Continuous variables were presented as mean ± SD. Categorical data were presented as numbers in percentages. Paired samples T-test analysis was performed to determine difference between measurements derived by TTE, CT and IVUS. If distribution was not normal (verified with the Kolmogorov-Smirnov test), Wilcoxon signedrank test was used. P values of <0.05 were considered statistically significant. Statistical analysis was performed using IBM SPSS Statistics 23.
Results
IVUS was perfomed in 12 patients with transthoracic echocardiography evidence for ascending aortic enlargement. Of them 91% were males with mean age 67 ± 9.5 years, all of them had arterial hypertension, 28.6% were with ischemic heart disease, 42.9% - with lower extremity artery disease, 42.9% were diabetics, 28.6% - smokers. Mean IVUS ascending aortic diameter was 49.6 mm. (See Table 1). Mean values of the calculated elastic properties of the ascending aorta were as follows: compliance 0.021 ± 0.02; strain 205 ± 4.3; aortic stiffness index 4.3 ± 0.75; elastic modulus 0.31 ± 0.05. On paired T-test analysis maximum ascending aortic diameter measured by CT aortography and IVUS did not differ significantly (t = -0.19, p=0.985). However, there was a significant difference between IVUS measurements and TTE derived diameters (t = 13.118, p = 0.034). On average, IVUS diameters were 4.1 mm larger than the results acquired by TTE (95% CI [14.21, 17.13]). IVUS examination of the ascending aorta provided larger diameters than the ones collected by means of TTE. However, IVUS measurements did not differ significantly from diameters derived by CT aortography.
Discussion
The heterogenecity in the arterial stiffness is an important fact that should be taken into consideration when evaluating vascular elastic properties. Every arterial segment has different viscoelastic properties, and it is inaccurate to extrapolate segmental arterial properties to the whole arterial tree. For instance, in hypertensive patients and those with other CV risk factors, cases, the aorta is stiffened earlier than the others elastic arteries, such as carotid and femoral arteries. Thus, aortic and carotid stiffness cannot be used as interchangeable predictors in high-risk patients. Furthermore, with the commonly used methods, measurements are conducted between two peripheral sites, whereas the most distensible segment of the aorta – the ascending part, which exhibits the earliest changes with aging, is actually excluded from the analysis. The distance between measurement sites needs to be derived from surface measurements and thus represents only an approximation. What also deserves to be mentioned is the amplification phenomenon. It is well-known that there is a progressive increase in the amplitude of the pressure wave due to reflections in the distal and more muscular arteries owing to their lesser elasticity [39]. Furthermore, the stiffness of medium-sized arteries is under vasomotor regulation by the endothelial function, the sympathetic nervous system [40, 41] and the renin–angiotensin system [42]. Hence, blood pressure varies along the arterial tree and a method evaluating aortic wall properties locally and measuring arterial pressure simultaneously may be of specific value.
We suggest, that IVUS may provide accurate in-vivo measurements of aortic diameters during the cardiac cycle. What is more, during the examination simultaneous recording of intra-aortic pressures is attainable. Although invasive, IVUS imaging modality may be of specific value for the assessment of the aortic elastic properties. Thus, further study evaluating a larger cohort of patients comparing IVUS with the current referent method – PWV, is needed.
|
Maximal TTE diameter (mm) |
Maximal CT diameter (mm) |
Maximal IVUS diameter (mm) |
|
|
Patient 1 |
47 |
46 |
50 |
|
Patient 2 |
46 |
44 |
49 |
|
Patient 3 |
48 |
49 |
51 |
|
Patient 4 |
49 |
48 |
50 |
|
Patient 5 |
49 |
50 |
50 |
|
Patient 6 |
50 |
49 |
47 |
|
Patient 7 |
48 |
46 |
46 |
|
Patient 8 |
57 |
59 |
58 |
|
Patient 9 |
39 |
41 |
50 |
|
Patient 10 |
50 |
53 |
52 |
|
Patient 11 |
52 |
60 |
60 |
|
Patient 12 |
43 |
45 |
46 |
Table 1: Maximal measurements of ascending aorta derived by transthoracic echocardiography (TTE), computed tomography (CT) aortography and intravascular ultrasound (IVUS).
Conclusion
Aortic stiffness is now recognized as an important determinant of cardio-vascular morbidity and mortality. For years, different methods have been studied in the assesment of aortic mechanics and stress. However, none of the described methods allows simultaneous measuerements of local vascular changes – pressure/diameter. We suggest that IVUS may be the answer to this unmet clinical need.
List of Abbreviations
LV – left-ventricular
CV – cardio-vascular
LAS – large artery stiffness
CHD – chronic kidney disease
PWV – pulse wave velocity
C – compliance
D – diameter
P – pressure
SP systolic pressure
DP – diastolic pressure
SD – systolic diameter
DD – diastolic diameter
CFPWV – carotid femoral pulse wave velocity
IVUS – intravascular ultrasound imaging
TTE – transthoracic echocardiography
CT – computed tomography
References:
- Kassab G. Biomechanics of the cardiovascular system: the aorta as an illustratory example. Journal of the Royal Society Interface 3 (2006): 719-740. i
- Belz G. Elastic properties and Windkessel function of the human Cardiovascular Drugs and Therapy 9 (1995): 73-83.
- Redheuil A, Yu WC, Wu CO, et al. Reduced ascending aortic strain and distensibility: earliest manifestations of vascular aging in humans. Hypertension 55 (2010): 319-326.n
- Cavalcante JL, Lima JA, Redheuil A, et al. Aortic stiffness: current understanding and future directions. Journal of the American College of Cardiology 57 (2011): 1511-1522.
- Yasmin, McEniery CM, O’Shaughnessy KM, et al. Variation in the human matrix metalloproteinase-9 gene is associated with arterial stiffness in healthy individuals. Arteriosclerosis, Thrombosis, and Vascular Biology 26 (2006): 1799-1805.am
- Harada K, Yasuoka K, Shimada Y. Usefulness of tissue doppler imaging for assessing aortic wall stiffness in children with the Marfan syndrome. The American Journal of Cardiology 93 (2004): 1072-1075.
- Lee JM, Shirodaria C, Jackson CE, et al. Multi-modal magnetic resonance imaging quantifies atherosclerosis and vascular dysfunction in patients with type 2 diabetes mellitus. Diabetes and Vascular Disease Research 4 (2007): 44-48.
- Stacey RB, Bertoni AG, Eng J, et al. Modification of the effect of glycemic status on aortic distensibility by age in the multi-ethnic study of atherosclerosis. Hypertension 55 (2010): 26-32.
- Safar ME, London GM, Plante GE. Arterial stiffness and kidney function. Hypertension 43 (2004): 163-168.
- Doyle A, Mark PB, Johnston N, et al. Aortic stiffness and diastolic flow abnormalities in end-stage renal disease assessed by magnetic resonance imaging. Nephron Clinical Practice 109 (2008): c1-c8.
- Kopel L, Tarasoutchi F, Medeiros C, et al. Arterial distensibility as a possible compensatory mechanism in chronic aortic regurgitation. Arquivos Brasileiros de Cardiologia 77 (2001): 262-265.
- Wilson RA, McDonald RW, Bristow JD, et al. Correlates of aortic distensibility in chronic aortic regurgitation and relation to progression to surgery. Journal of the American College of Cardiology 19 (1992): 733-738.
- Gillebert TC, Lew WY. Influence of systolic pressure profile on rate of left ventricular pressure fall. American Journal of Physiology-Heart and Circulatory Physiology 261 (1991): H805-H813.
- Chirinos JA, Segers P, Hughes T, et al. Large-artery stiffness in health and disease: JACC state-of-the-art review. Journal of the American College of Cardiology 74 (2019): 1237-1263.
- O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 46 (2005): 200-204.
- Hashimoto J, Ito S. Central pulse pressure and aortic stiffness determine renal hemodynamics: pathophysiological implication for microalbuminuria in hypertension. Hypertension 58 (2011): 839-846.
- Ben-Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. Journal of the American College of Cardiology 63 (2014): 636-646.
- Townsend RR, Wilkinson IB, Schiffrin EL, et al. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension 66 (2015): 698-722.
- Mattace-Raso FU, van der Cammen TJ, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke. Circulation 113 (2006): 657-663.
- Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 37 (2001): 1236-1241.
- Laurent S, Katsahian S, Fassot C, et al. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke 34 (2003): 1203-1206.
- Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. Journal of the American College of Cardiology 55 (2010): 1318-1327.
- O’Rourke MF, Staessen JA, Vlachopoulos C, et al. Clinical applications of arterial stiffness; definitions and reference values. American Journal of Hypertension 15 (2002): 426-444.
- Reference Values for Arterial Stiffness' Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors:‘establishing normal and reference values’. European Heart Journal 31 (2010): 2338-2350.
- Lehmann ED, Parker JR, Hopkins KD, et al. Validation and reproducibility of pressure-corrected aortic distensibility measurements using pulse-wave-velocity Doppler ultrasound. Journal of Biomedical Engineering 15 (1993): 221-228.
- Asmar RG, Topouchian JA, Benetos A, et al. Non-invasive evaluation of arterial abnormalities in hypertensive patients. Journal of Hypertension 15 (1997): S99-S107.
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). European Heart Journal 39 (2018): 3021-3104.
- Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. European Heart Journal 27 (2006): 2588-2605.
- Sugawara J, Hayashi K, Yokoi T, et al. Brachial–ankle pulse wave velocity: an index of central arterial stiffness?. Journal of Human Hypertension 19 (2005): 401-406.
- Gedikli O, Altinbas A, Orucoglu A, et al. Elastic Properties of the Ascending Aorta in Patients with β-Thalassemia Major. Echocardiography 24 (2007): 830-836.
- Vitarelli A, Conde Y, Cimino E, e al. Assessment of ascending aorta distensibility after successful coarctation repair by strain Doppler echocardiography. Journal of the American Society of Echocardiography 21 (2008): 729-736.
- Herment A, Kachenoura N, Lefort M, et al. Automated segmentation of the aorta from phase contrast MR images: validation against expert tracing in healthy volunteers and in patients with a dilated aorta. Journal of Magnetic Resonance Imaging: An Official Journal of the International Society for Magnetic Resonance in Medicine 31 (2010): 881-888.ed
- Weintraub A, Schwartz S, Pandian NG, et al. Evaluation of acute aortic dissection by intravascular ultrasonography. The New England Journal of Medicine 323 (1990): 1566-1567.
- Alfonso F, Goicolea J, Aragoncillo P, et al. Diagnosis of aortic intramural hematoma by intravascular ultrasound imaging. The American Journal of Cardiology 76 (1995): 735-738.
- Mileva N, Vassilev D, Gil R, et al. Misdiagnosed Aortic Intramural Hematoma and the Role of Intravascular Ultrasound Imaging in Detection of Acute Aortic Syndrome: A Case Report. Cardiovascular Innovations and Applications 2 (2018): 447-449.
- Wei H, Schiele F, Meneveau N, et al. The value of intravascular ultrasound imaging in diagnosis of aortic penetrating atherosclerotic ulcer. EuroIntervention: journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology 1 (2006): 432-437.
- Hughes DJ, Fearnot NE, Babbs CF, et al. Continuous measurement of aortic radius changein vivo with an intra-aortic ultrasonic catheter. Medical and Biological Engineering and Computing 23 (1985): 197-202.
- Hansen ME, Yucel EK, Megerman J, et al. In vivo determination of human arterial compliance: preliminary investigation of a new technique. Cardiovascular and Interventional Radiology 17 (1994): 22-26.
- Latham RD, Westerhof N, Sipkema P, et al. Regional wave travel and reflections along the human aorta: a study with six simultaneous micromanometric pressures. Circulation 72 (1985): 1257-1269.
- Boutouyrie PI, Lacolley PA, Girerd XA, et al. Sympathetic activation decreases medium-sized arterial compliance in humans. American Journal of Physiology-Heart and Circulatory Physiology 267 (1994): H1368-H1376.
- Giannattasio C, Failla M, Lucchina S, et al. Arterial stiffening influence of sympathetic nerve activity: evidence from hand transplantation in humans. Hypertension 45 (2005): 608-611.
- Giannattasio C, Failla M, Stella ML, et al. Angiotensin-converting enzyme inhibition and radial artery compliance in patients with congestive heart failure. Hypertension 26 (1995): 491-496.


 Impact Factor: * 5.6
Impact Factor: * 5.6 Acceptance Rate: 74.36%
Acceptance Rate: 74.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 