Leadless Pacemaker Implantation in Prosthetic Valve Endocarditis Patient: Lesson with the Micra Transcatheter Pacemaker
Santomauro Maurizio1, Santomauro Andrea2, Rapacciuolo Antonio3, Riganti Carla4, Francesco Maffettone4, Fiore Francesco4, Iengo Martina4, Chiappetti Rosaria4, Cacciatore Francesco1*,
1Department of Cardiovascular Emergency, Internal Medicine and Geriatric, Medical School, Federico II University of Naples, Italy
2Department of Medicine, Surgey, Dentistry, Salernitana Medical School, Italy
3Department of Advanced Biomedical Sciences, Federico II University of Naples, Italy
4University of Naples, "Federico II", Italy
5Cotugno Hospital, cardiology department, Naples, Italy
*Corresponding author: Francesco Cacciatore, Department of Cardiovascular Emergency, Internal Medicine and Geriatric, Medical School, Federico II University of Naples, Italy.
Received: 22 November 2021; Accepted: 29 November 2021; Published: 13 December 2021
Article Information
Citation: Santomauro M, Santomauro A, Rapacciuolo A, Riganti C, Maffettone F, Fiore F, Iengo M, Chiappetti R, Cacciatore F. Leadless Pacemaker Implantation in Prosthetic Valve Endocarditis patient: lesson with the Micra Transcatheter Pacemaker. Cardiology and Cardiovascular Medicine 5 (2021): 704-707.
View / Download Pdf Share at FacebookAbstract
Leadless Pacemakers (L-PM) are an effective alternative for patients requiring only ventricular pacing, with a low major complication rates and long-term complications given the absence of leads and a device pocket. Successful implantation from the septal location has been described using the Micra Transcatheter Pacing System (TPS) (Medtronic Inc, Minneapolis, MN) introducer sheath, a Micra delivery catheter, or a steerable sheath that allows better alignment with the device and a loop snare. We report the case of a patient with a leadless pacemaker implantation because of atrial fibrillation with low ventricular response with an endocarditis on prosthetic mechanical mitral valve.
Keywords
<p>Prosthetic Valve Endocarditis; Micra Transcatheter Pacemaker</p>
Article Details
1. Case report
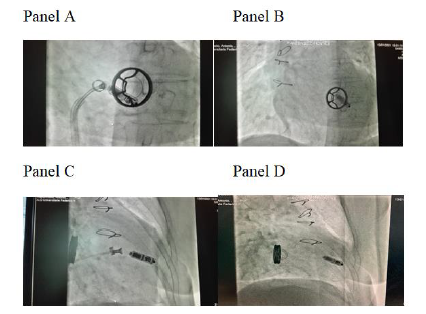
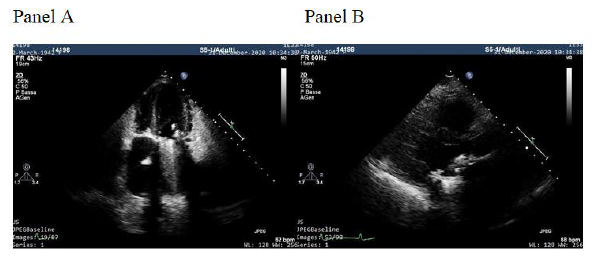
A 79-year-old woman was referred from a periferical hospital of our Region (Campania, Italy) for a single-chamber pacemaker implant because of recurrent syncope in permanent slow rate atrial fibrillation. The patient implanted in 1984 a Sorin 27 monoleaflet Prostetic Mitral Valve (PMV). After 6 months a prolonged antibiotic treatment (3 months) was administred because of endocarditis on prosthetic mitral valve. The blood culture performed in the periferical hospital was positive for Staphylococcus aureus sensitive to tigecycline and levofloxacin that were initiated soon after blood culture result. We decided to further evaluate the PMV as a potential source of infection, and transthoracic (Figure 1, Panel A and B) and then transesophageal echo showed a vegetation of 13 x 7 mm on the ventricular face adhered partly to the annulus of the prosthesis and partly to the ventricular wall frond-like material on the surface of the PMV. PET-TC (after 10 days of antibiotic therapy) showed an increase in metabolic FDG activity around the prostetic valve with a SUVmax of 3.8. All the subsequent 5 blood-culture performed during hospitalization resulted negative. The patient received a Micra VR leadless cardiac pacemaker (Medtronic Inc) to try to minimize the risk of possible future infection. (Figure 2A, 2B, 2C, 2D) (Figure 3).
2. Discussion
L-PM have shown resistance to infection even when inserted at the time of or shortly after conventional system extraction [1-6]. A 2019 e-mail advertisement from Medtronic reports that a total of 50,000 Micras have been implanted worldwide. To date there has been only 1 other case report of documented Micra infection. L-PM are felt to be resistant to infection owing to the lower surface area, no device pocket, turbulent right ventricular flow, and subsequent device encapsulation. The Micra transcatheter pacemaker is largely encased titanium with a parylene coating. A recent study by El-Chami and colleagues [7] documented that the perylene coating on titanium provided bacterial resistance to Stafilococcus aureus and Pseudomonas aeruginosa compared to bare titanium and postulated this to be a potential mechanism of the devices bacterial resistance. A substudy of the Micra Transcatheter Pacing study reviewed the incidence and outcomes of patients who developed serious infectious events (bacteremia or endocarditis) after Micra implantation [8-11]. Among the 720 patients implanted in the investigational trial, 15 patients had 21 serious infectious events. All events were adjudicated and determined to be unrelated to Micra device or implant procedure, and no persistent bacteremia was seen after antibiotic treatment.
3. Conclusion
Leadless pacemakers were recently introduced to address lead and pocket-related complications. In cases such as the presented one, a leadless pacemaker avoid possible source of prosthetic valve endocarditis in somebody already susceptible to it.
References
- Boveda S, Lenarczyk R, Haugaa KH, et al. Use of leadless pacemakers in Europe: results of the European Heart Rhythm Association survey. EP Europace 20 (2018): 555-559.
- Chew DS, Kuriachan V. Leadless cardiac pacemaker:Present and the future. Curr Opin Cardiol 33 (2018): 7-13.
- Roberts PR, Clementy N, Al Samadi F, et al. A Leadless Pacemaker in the Real-World Setting: The Micra Transcatheter Pacin System Post-Approval Registry. Heart Rhythm 14 (2017): 1375-1379.
- El-Chami MF, Al-Samadi F, Clementy N, et al. Updated performance of the Micra transcatheter pacemaker in the real-world setting: A comparison to the investigational study and a transvenous historical control Heart Rhytm 15 (2018): 1800-1807.
- Kiani S, Black GB, Rao B, et al. Outcomes of Micra leadless pacemaker implantation with uninterrupted anticoagulation. J Cardiovasc Electrophysiol 30 (2019): 1313-1318.
- El-Chami MF, Mayotte J, Bonner M, et al. Reduced bacterial adhesion with parylene coating: Potential implications for Micra transcatheter pacemakers. J Cardiovasc Electrophysiol 31 (2020): 712-717.
- El-Chami MF, Soejima K, Piccini JP, et al. Incidence and outcomes of systemic infections in patients with leadless pacemakers: Data from the Micra IDE study. Pacing Clin Electrophysiol 42 (2019): 1105-1110.
- Bhatia N, El-Chami M. Leadless pacemakers: A contemporary review. J Geriatr Cardiol 2018;15(4):249–253. doi: 10.11909/j.issn.1671-5411.2018.04.002
- Beurskens NEG, Tjong FVY, Dasselaar KJ, et al. Leadless pacemaker implantation after explantation of infected conventional pacemaker systems: A viable solution? Heart Rhythm 16 (2019): 66-71.
- El-Chami MF, Johansen JB, Zaidi A, et al. Leadless pacemaker implant in patients with pre-existing infections: Results from the Micra postapproval registry. J Cardiovasc Electrophysiol 30 (2019): 569-574.
- Fiorelli A, Messina G, Capaccio D, Santini M. Recurrent spontaneous pneumomediastinum: a rare but possible event! J Thorac Dis 4 (2012): 431-433.


 Impact Factor: * 5.6
Impact Factor: * 5.6 Acceptance Rate: 74.36%
Acceptance Rate: 74.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 