Management of Residual False Lumen Flow After Thoracic Endovascular Repair in Chronic Type B Aortic Dissection
Hervé Rousseau1*, Paul Revel-Mouroz1, Charline Zadro1, Camille Dambrin2, Christophe Cron2, Marion Jaffro1, Pierre Marek1, Adrien Vavasseur1, Fatima-Zohra Mokrane1
1Department of Radiology, Hôpital Rangueil, Centre Hospitalier Universitaire de Toulouse, France
2Department of Cardiovascular surgery, Hôpital Rangueil, Centre Hospitalier Universitaire de Toulouse, France
*Corresponding Authors: Hervé Rousseau, Department of Radiology, Hôpital Rangueil, Centre Hospitalier Universitaire de Toulouse, 1, avenue du Pr. Jean Poulhès, TSA 50032, 31059 Toulouse Cedex 9, France
Received: 16 September 2020; Accepted: 24 September 2020; Published: 30 September 2020
Article Information
Citation: Hervé Rousseau, Paul Revel-Mouroz, Charline Zadro, Camille Dambrin, Christophe Cron, Marion Jaffro, Pierre Marek, Adrien Vavasseur, Fatima-Zohra Mokrane. Management of Residual False Lumen Flow After Thoracic Endovascular Repair in Chronic Type B Aortic Dissection. Cardiology and Cardiovascular Medicine 4 (2020): 591-610.
View / Download Pdf Share at FacebookKeywords
<p>Aortic dissection; Type B aortic dissection; TEVAR; Endovascular therapy; Aorta; False lumen embolisation</p>
Article Details
1. Introduction
Complicated type B aortic dissection (aTBAD) has undergone a complete paradigm shift from open surgery to thoracic endovascular repair (TEVAR) in the past decade, and TEVAR has now become standard of care for patients who have suitable anatomy. Despite the clear superiority of TEVAR over conventional open surgery for aTBAD, considerable debate exists regarding the optimal treatment for chronic type B aortic dissection (cTBAD). Traditionally, uncomplicated type B dissection are treated with optimal medical therapy (OMT), and lifestyle modification to minimize risk of progression. Patients were serially followed with cross-sectional imaging and typically only considered for invasive therapy when traditional aneurysmal size thresholds were met or complications from the chronic dissection arose.
Some debate exists regarding the efficacy of TEVAR for chronic type B aortic dissection (cTBAD), however endovascular strategies have been increasingly used, offering better outcomes in terms of mortality and morbidity compared to open surgical repair. But, in contrast to the acute setting, where dissection flaps are extremely soft and aortic wall reapposition is easily accomplished, aortic remodeling after standard TEVAR is less likely in cTBAD [1, 2]. A commonly cited concern is the stiff intimal flap present in chronic aortic dissections [3]. The thickened intimal flap encountered in the chronic setting may also tear following TEVAR, precipitating stent-graft-induced new entry tears (SINEs). Another limition of endovascular therapy is continued retrograde false lumen perfusion with back-flow from distal entry tears, from the aorta or distal branches [4]. For these reasons, complete false lumen thrombosis is rarely observed after TEVAR alone in chronic type B aortic dissection and is independently associated with poor long-term survival [4].
In order to prevent these challenging problems, endovascular treatment strategies should not only target on occlusion of the proximal entry-tear but should focus in addition on the prevention of false lumen back-flow as only a thrombosed false lumen promotes sufficient aortic remodeling. Potential solutions to achieve false lumen thrombosis are open surgical repair or the use of fenestrated or branched stent-grafts covering long segments of the aorta, occluding more entry points and reducing pressure and flow transmission into the false lumen [5-7]. However, these techniques are complex and associated with significative risk of morbidity and endoleaks. False lumen embolization techniques offer less invasive treatment strategies with promising early results. Moreover, this approach avoids coverage of segmental arteries at the thoraco-abdominal level and lowers the risk for spinal cord ischemia and adverse events with a less complex procedure. This article describes technical aspects and results of these new endovascular techniques to occlude the false lumen and prevent retrograde flow in chronic aortic dissection.
2. The problem of persistent false lumen perfusion
The primary rationale for TEVAR in chronic type B aortic dissection revolves around the issue of aortic remodeling. TEVAR in cTBAD is typically performed from the left subclavian artery to the celiac artery, thus facilitating reverse remodeling in the entire descending thoracic aorta. It remains unclear whether this alters the natural history of chronic type B dissection, with specific regard to aneurysm progression in the visceral segment and ultimate need for open aortic replacement, branched endograft within the perivisceral aorta or over endovascular techniques to promote false lumen thrombosis.
After standard TEVAR complete false lumen thrombosis can be achieved only in around 40% of the patients by covering the proximal entry tear alone [8-10]. More than one third of patients treated by TEVAR alone shows false lumen progression with a mortality of 36% after three years [11-12]. The theory behind this is the persistent perfusion from proximal or distal entry-tears and continuous run-off through collaterals with pressurization of the false lumen [13].
The importance of false channel thrombosis has been demonstrated by numerous studies. Sueyoshi et al. demonstrated that false lumen thrombosis prevents aortic aneurysmal expansion in chronic AD [14]. Similarly, Tsai et al. reported that a partially closed false lumen is a predictor of mortality and Akutsu et al. have proved that patency of the false lumen is a risk factor for dissection-related death [15, 16]. Tolenaar et al. reported that expansion of the true lumen is associated with a decreased aortic growth rate [17]. Finally, the long-term results from the INSTEAD-XL trial, highlights not only the positive aortic remodeling effects of TEVAR in cTBAD but also a survival benefit that is only realized in the long term [18].
Several other studies have specifically examined aortic remodeling following TEVAR in cTBAD. Significant expansion of the true lumen post TEVAR and complete thrombosis of the false lumen within the descending thoracic aorta (DTA) with a concomitant regression of false lumen diameter was found in more than 80% of patients [3, 19, 20]. Results were further stratified by extent of the dissection; patients with DeBakey IIIa achieved 100% false lumen thrombosis, as opposed to only 68% in the DeBakey IIIb group. In another trial from Mani et al., the rate of aortic remodeling was dependent on the extent of the aortic dissection and the presence of false lumen thrombosis. Although total false lumen thrombosis was achieved in 83% of the patients with the dissection confined to the thoracic aorta, this was the case for only 23% of the patients with dissection extending into the abdomen. Patients with less than total false lumen thrombosis were more likely to experience aortic expansion over time, which was the strongest predictor of midterm mortality [11].
Other, predicting factors regarding false lumen thrombosis in patients with chronic TBAD have been outlined in different papers [21, 22]. Thus, patients presented with TBAD and branch vessel involvement or a patent entry tear after TEVAR are less likely to develop FL thrombosis during follow-up [13].
3. Options to manage persistent false lumen perfusion
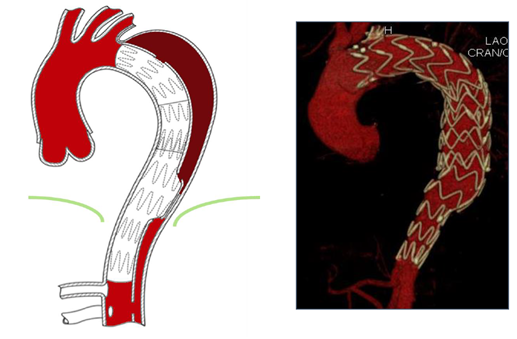
A significant proportion of patients with post-dissection aneurysms develop aneurysmal dilatation in the distal aortic arch and the mid descending thoracic aorta alone, while the abdominal segment remains relatively normal not requiring treatment [23]. In case of aneurysmal expansion of the descending aorta, a more extensive procedure is needed to promote false lumen thrombosis by using different endovascular techniques. Proper planning and individualized selection of patients that are good candidates for false lumen thrombosis may be crucial (Figure 1). Moreover, precise analysis of the anatomy of each particular case is mandatory to consider “patient specific”, suitable therapeutic options. It is of note, that in the majority of cases, the false lumen thrombosis was achieved with intervention to the distal part of the dissection; however, in some cases a proximal or a mid-aortic embolization may be needed. These procedures could be undertaken either initially after TEVAR placement, when residual flow in the thoracic FL is observed, or in chronic dissections cases after TEVAR or ascending thoracic aorta repair in which the false lumen remains patent increasing the risk of an adverse event. Moreover, in emergency cases of an acute rupture of false lumen, inducing the thrombosis of the false lumen may represent the only endovascular effective treatment to stop aortic bleeding. After stent-grafts have been deployed into the true lumen usually with extension to the coeliac artery, occlusion of the false lumen can be achieved by embolizing the false lumen itself at the level of the distal descending thoracic aorta sparing the abdominal aortic segment, or by exclusion of the different reentries or collaterals.
Procedures can be classified into 4 groups:
- Direct embolization of the false aortic lumen to promote false lumen thrombosis when persistent perfusion of the false lumen raised from retrograde abdominal re-entries or intercostal or lombar arteries. A large variety of devices as well as liquid embolization materials have been used to occlude the false lumen. Initially to reduce the flow and avoid distal migration of the materials, coils and plugs (Amplatzer Vascular Plug (AVP) Abbott Vascular) are deployed in the FL, generally at the level of the coeliac trunk, completed by glue injection.
- PETTICOAT & STABILISE Techniques
- Covered stents in the true lumen of a collateral artery to close re-entry tears.
- Embolization of a collateral artery from the false lumen to occlude type 2 endoleak.
Moreover, frequently, combined procedures are performed to achieve complete false lumen thrombosis (Figure 2).
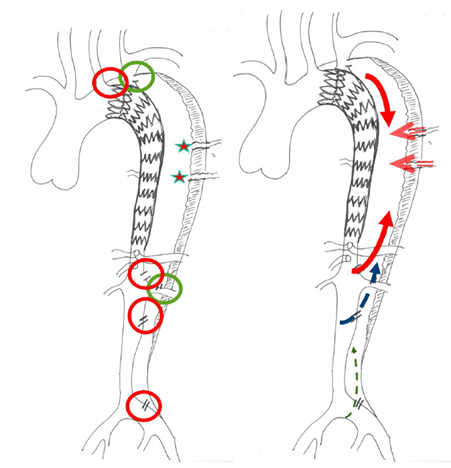
Figure 1: Schematic of the principle of false lumen back-flow: The theory behind the formation of false lumen aneurysms in cTBAD is the persistent perfusion from distal entry-tears and continuous run-off through collaterals with pressurization of the false lumen. Before selecting the more adapted therapeutic strategy, it is crucial to detect all the reentries (red) and collateral orgins (green) and the direction of the false lumen flow (antegrade or retrograde).
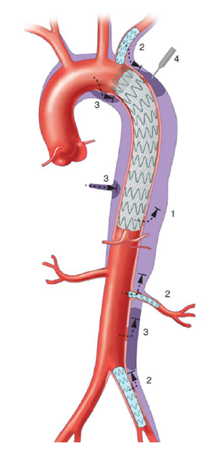
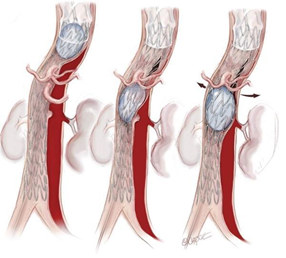
Figure 2: Different options to occlude the false lumen, after TEVAR insertion: (1) Stent-graft proximal or distal extension, to close a secondary entry tear of the dissection flap; (2) Close re-entry tears either arising from collateral arteries, by embolization or using covered stents; (3) Direct embolization of the false aortic lumen combine with embolization of collaterals from false lumen to promote false lumen thrombosis when persistent perfusion of the false lumen raised from retrograde abdominal re-entries or collaterals; (4) Direct puncture of the aorta, in the absence of FL access, allowing injection of coils or glue.
3.1 False lumen embolization
Three main techniques are described for direct false lumen embolisation: the “cork in the bottle neck” Technique, the Candy-Plug Technique, the Knickerbocker Technique.
3.1.1 The “cork in the bottle neck” Technique: To prevent false lumen back flow at the level of the distal descending aorta embolization of the false lumen in chronic aortic dissection was first described by Loubert et al. in 2003 as the “cork in the bottle neck” strategy, using cava filters, detachable balloons, thrombin and Talent occluders into the false lumen to interrupt back-flow at the level of the distal descending thoracic aorta [24]. However, most of these materials are limited by the diameter of the false lumen resulting in using more than one device or a lot of adjunctive embolization material in many cases. A second occluder or several large plugs (18-22 mm) or glue to fill the false lumen need to be placed using the same technique [25]. There are only small case series demonstrating good experiences with occluders, coils or Amplatzer Vascular Plugs [26-28].
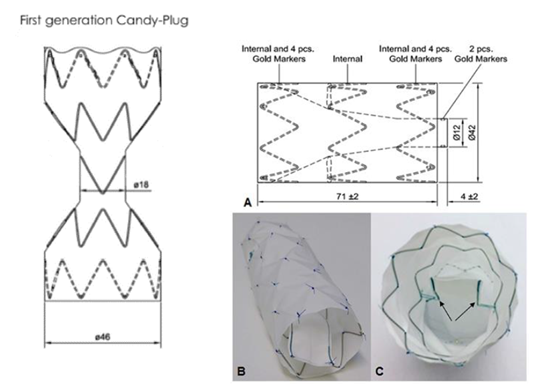
3.1.2 The Candy-Plug Technique: To overcome the problem of large false lumen diameters, the Candy-Plug Technique was first introduced by Kölbel et al. in 2013 [29]. This technique consists of the placement of a large coccluder device (the Candy-Plug) into the false lumen distally aligned to a true lumen stent-graft ending above the celiac trunk (Figure 3). Initially, the Candy-Plug consist in a back-table modification of a 42 mm diameter Zenith TX2 ProForm thoracic stent-graft (Cook Medical, Bjaeverskov, Denmark) by placing a central diameter reducing suture in the middle of the graft giving it a candy-wrapper shaped appearance. The Candy-Plug required a central opening to allow retrieval of the nose-cone of the introduction system, which could be closed using commercially available Amplatzer vascular plug (AVP; St. Jude Medical, St. Paul, MN, USA) or ZIP Occluder (Cook Medical, Bjaeverskov, Denmark) (Figure 4). Oversizing of the Candy-Plug should be 10-30% of the false lumen diameter at the specific area above the celiac trunk. Suitability for the use of the Candy-Plug Technique includes available access to the false lumen at the iliac or infrarenal level and sufficient extension of the dissection above the celiac trunk to accommodate the introduction system.
This first generation Candy-Plug has been successfully applied as a less invasive endovascular strategy to treat chronic aortic dissection showing a good remodelling rate in mid-term follow-up with low mortality and morbidity [30].
A problem of the first-generation Candy-Plug was the need to plug the central midsection. This is an additional procedural step and a potential risk for insufficient sealing with persistent false lumen back-flow through the Candy-Plug itself. To overcome this problem a second-generation Candy-Plug II was recently introduced with a self-closing central channel. Different than the previous generation this Candy-Plug has a tubular appearance thereby increasing its sealing capability (Figure 4).
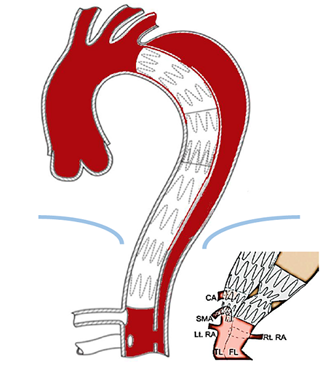
3.1.3 The Knickerbocker Technique: Different to the Candy-Plug, the Knickerbocker technique is an endovascular fenestration technique and does not require access to the false lumen nor additional materials to occlude the false lumen. Its application consists of expanding a compliant balloon at the level of a large diameter stent-graft segment in the true lumen to rupture the dissection membrane into the false lumen at a limited segment of the aorta shortly above the celiac trunk [31] (Figure 5). After an initial experience with using oversized standard tubular stent-grafts, the Knickerbocker-graft has been used as a custom-made unilateral double-tapered stent-grafts with a marked preformed bulbous section. Gold-markers allow direction of the bulbous section towards the false lumen. After orienting the bulbous section, which is marked with gold-markers, towards the false lumen the graft is deployed and a compliant balloon is used to dilate the bulbous section of the stent-graft until the dissection membrane ruptures at the targeted segment of the aorta. Now the oversized stent-graft expands into the false lumen sealing off false lumen backflow. Proximally the graft should be positioned with sufficient overlap to a proximal stent-graft if required. The shape of the deployed stent-graft is similar to knickerbocker-trousers, which explains the denomination. The Knickerbocker-Technique has at yet been applied in small series with favourable outcomes [32, 33].
3.2 PETTICOAT & STABILISE Techniques
The PETTICOAT (provisional extension to induce complete attachment) technique was described in 2006 by Nienaber et al. to treat sustained abdominal FL back flow and pressurization despite successful stent-graft sealing of the thoracic entry tear in patients with complicated type B aortic dissection [34]. In this technique, a scaffolding bare metal stent is placed for distal extension of a previously implanted thoracic stent-graft. Even though the technique was firstly used in the acute setting to promote aortic remodeling [35], some authors have used it as an adjunctive treatment in chronic dissections. In the latest review published in 2017 by Bertoglio et al. including 439 cases treated with the Petticoat technique, the results showed that this technique was safe and feasible and could enhance re-expansion of the TL at the distal thoracoabdominal aorta possibly improving end-organ perfusion [36]. Nevertheless, no evidence of improved short and mid-term survival or positive remodelling of FL in the distal aorta could be demonstrated when compared to a simple proximal stent-grafting. The authors concluded that the Petticoat procedure should be limited as a bailout adjunctive tool to cases complicated by dynamic malperfusion.
As a whole, in the cochrane meta analysis of the PETTICOAT technique, the authors concluded that no randomised controlled trials were identified and therefore cannot draw any definite conclusion on this topic. Evidence from non-randomised studies appears to be favourable in the short-term, for combined proximal descending aortic endografting plus distal bare metal stenting to solve the problem of unfavourable distal aortic remodeling. Randomised controlled trials are warranted to provide solid evidence on this topic [37].
In 2014, the group of Mossop et al. proposed the STABILISE concept (Stent-Assisted Balloon-Induced Intimal Disruption and Relamination in Aortic Dissection Repair) using a proximal thoracic aortic stent-graft and a distal bare aortic stent (as in the PETTICOAT technique) with additional balloon-induced intimal disruption (Figure 6) [38]. Aim of this technique is to create immediate intimal reapposition inducing complete FL obliteration. The technique was reported in a total of 11 patients with thoracoabdominal dissections. No intraprocedural complications were noticed, and the thirty-day mortality was 9%. During follow-up (median: 18 months, range, 4-54 months) two patients (18%) required reintervention. No late adverse events or aortic-related deaths occurred. Complete FL obliteration occurred in all surviving patients (n = 10) at latest follow-up.
Implantation of balloon expandable covered stents through pre-existing entries and struts of dissection stents into FL originating arteries can be added if needed (The Deeb Petticoat or Distal extended branched Petticoat technique). With this technique, blood flow is re-established to FL originating target vessels, while achieving sealing of the corresponding tear in the dissection membrane with the use of a covered stent.
Recently, Melissano et al. reported their experience with the STABILISE technique in 10 patients with acute complicated type B dissections [39]. The 30-day technical and clinical success rates were 100%, with complete thrombosis of the thoracic FL and no type I endoleak. No aortic ruptures were recorded, and no open conversion was required. Postoperative computed tomography showed complete thrombosis of the thoracic FL in all cases. In two cases, some degree of patency of the abdominal FL was observed, while at short-term follow-up, the overall aortic diameters remained stable with no further dilation.
Faure et al. has also presented the use of STABILISE technique for the treatment of Type A (acute and chronic) and acute type B dissections [40]. In a total of 17 patients, the authors reported no major in-hospital morbidity and mortality. No rupture was induced by the procedure, but 39% of the patients required additional stenting of a target vessel. During a median follow-up of 17 months (mean 5-28.5 months) complete aortic remodelling down to infrarenal level was noticed in 88% of the patients. There was no late mortality.
3.3 Oclusions of collaterals and reentries
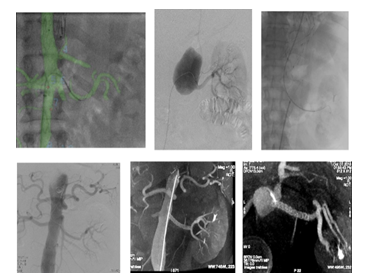
Occlusion of collaterals from the false lumen and exclusion of the reentries from the distal or proximal aorta, the supraortic or visceral branches, could be a useful alternative (Figure 7 and 8) [41]. By placing stent grafts on a visceral artery the objective is twofold: to exclude the re-entry and to improve the downstream flow (Figure 9). As a whole, in most of the cases, combined procedures are performed to achieve complete false lumen thrombosis.
Figure 4: The Candy-Plug technique. Draft of the two generations of Candy-Plugs: The first generation of Candy-Plug on the left is constructed with a thoracic stent graft with a diameter-restricting suture in the middle of the graft. Placement of a Vascular Plug in the central channel is needed to close the Candy-Plug; On the right, the tubular shape of the second generation of Candy-Plug allows a longer sealing segment. In addition the self-closing fabric channel obviates the previously needed placement of a Vascular Plug in the central channel. It is covered at the top and has a small fabric channel fixed to the middle nitinol stent inside the graft by two opposed sutures. When the graft is constrained within its sheath, there is no tension on these sutures and the central channel is loose around the pusher allowing smooth retraction. Release of the graft tenses the sutures by expansion of the nitinol stent and closes the channel from each side.
A double tapered stent-graft is implanted in the true lumen distally landing above the celiac trunk. The bulbous section expands through the dissection membrane to the outer aortic wall, preventing false lumen back-flow into the thoracic segment.
The Stent-Assisted Balloon-Induced Intimal Disruption and Relamination in Aortic Dissection Repair (STABILISE concept) is a combination of a proximal thoracic aortic stent-graft and a distal bare aortic stent, as in the PETTICOAT technique, but with additional balloon-induced intimal disruption of all the distal aorta [40].
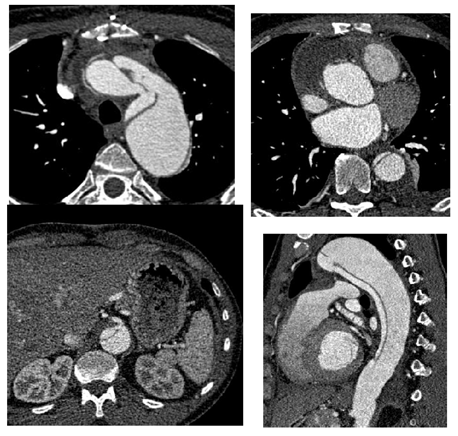
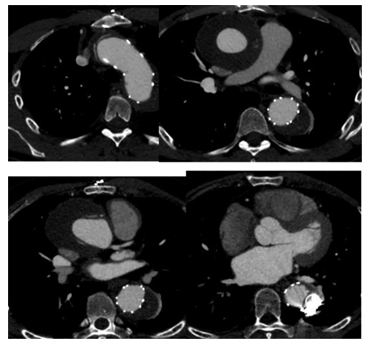
Figure 7A: Patient presenting with an initial type A aortic dissection, was treated by surgical replacement of the ascending aorta. CTA follow-up at 6 months shows persistent false lumen patency due to a re-entry located in the origin of the left subclavian artery and a secondary entry tear in the descending thoracic aorta.
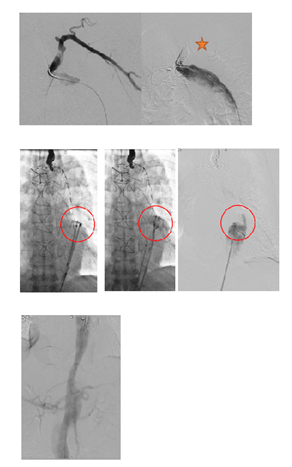
Figure 7C: An ancillary endovascular procedure is performed in order to treat this FL flow. Selective angiography of the false lumen confirm that the false lumen is perfused by a LSA reentry and by distal reentries. False lumen embolization of the aortic arch with multiple coils (*) and distal false lumen by a combination of large plugs and glue (red circles) is achieved and the completion angiography shows a complete exclusion of the FL.
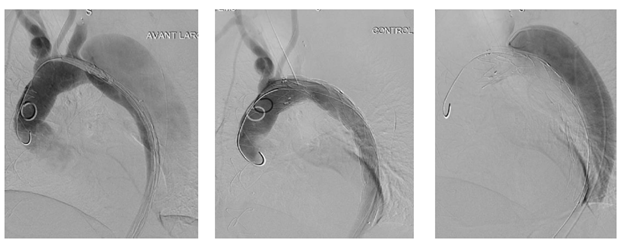
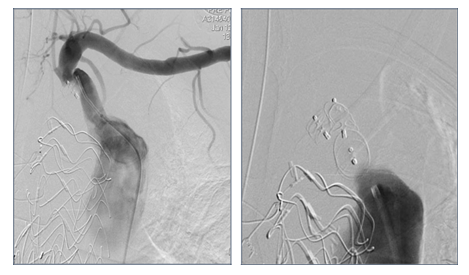
Figure 8: Patient presenting a false lumen expansion 10 months after TEVAR insertion for an initial type B aortic dissection. Selective catheterism of the false abdominal aortic lumen shows the persistance of a false lumen patency, arising from LSA. False lumen embolization is performed with the deployment of a type II, 16 mm diameter Amplatzer plug.
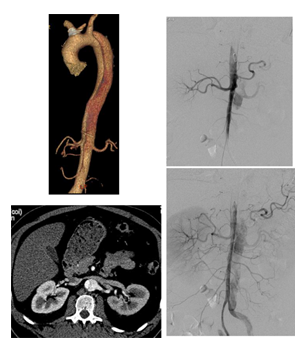
Figure 9B: Successive ancillary endovascular procedures are conducted in order to close the secondary sources of false lumen perfusion. Selective catheterism of the left renal artery from the true lumen, with fusion imaging, is performed. The initial angiography confirms the false lumen perfusion through the renal artery desinsertion. A covered stent is deployed in the left renal artery from the true aortic lumen. The completion angiography with 3D reconstruction, confirms the sealing of the entry tear and the normal flow in the distal branches.
4. Indications
- Aneurysmal dilatation with persistent FL retrograde flow after TEVAR,
- Symptomatic chronic aortic dissections with pain or rapid growth of aortic diameter (> 5 mm/y) requiring rapid exclusion of the aneurysm,
- Emergency cases
- Simultaneous TEVAR and embolisation, if retrograde thoracic FL flow is maintained on post-TEVAR angiography. Kim and al. compared on 73 patients with type B dissections 2 groups. One group A, with TEVAR plus embolisation (41 patients), to 32 patients in group B, treated by TEVAR alone. The whole thoracic aorta FL thrombosis rate was significantly higher in group A (83% vs 56%; P = 0.002) [42].
5. Results
Several small retrospective studies have specifically examined aortic remodeling following endovascular procedures to promote the false lumen thrombosis, after TEVAR in cTBAD [42-44]. Pellenc et al. has presented there experience in 27 patients with chronic aortic dissection. Twenty patients presented with type B chronic aortic dissections (74.1%) and seven presented a residual type A chronic aortic dissections (25.9%). Embolization devices included coils and vascular plugs. The technical success rate was 100%. Complete spinal cord ischemia was observed in one patient (3.7%). There was one hospital death from pneumonia. FL thrombosis was observed in 81.5% of patients. Five patients required two or more embolization procedures, leading to a high rate of complete FL thrombosis (92.6%). The maximum thoracic aortic diameter significantly decreased [45]. Miletic K et al. published also there results on 51 patients with chronic dissection. Devices included a combination of devices. Significative mean maximum aortic diameter and FL diameter decrease, was observed with a true lumen expansion. FL thrombosis with ³ 10% decrease in diameter and ³ 10% increase in true lumen diameter was achieved in 39.2%. Nine patients progressed after the first embolisation: persistent FL flow with increase in aortic diameter and underwent repeat embolization with complete thrombosis (n=4) or open thoracoabdominal completion (n=5). 51% had indeterminate response: FL thrombosis without change in maximum diameter. 12% had complete obliteration of the entire FL. At last follow-up, 42 (82%) patients were alive [46].
In our experience, 59 ancillary endovascular procedures were performed, using different techniques, for aneurysmal expansion in 93.2% of cases or symptomatic dissection in 6.8% of cases (respectively aortic rupture (3.4%), malperfusion syndrome (1.7%), or recurrent pain, (1.7%)). Technical success was observed in all cases with a mean of 1,7 interventions by patient. No stroke or spinal cord ischemia was observed. At the end of the follow-up with a median follow-up time of 60.7 months [44,4-76,8], positive remodeling was obtained in 85.7% of cases with a complete false lumen thrombosis in 25,7%, and diameter reduction or stability in 93.2% of cases at the thoracic aortic level [47].
Finally, a systematic review was published by Spanos et al. [48]. One hundred one patients either after open repair of a TAAD (n = 40) or after cTBAD (n = 61) underwent an endovascular procedure for intentional FL occlusion. Thirty-one (78%) patients with TAAD and fifty-one (83%) with cTBAD were treated electively. Four main techniques were used: (1) the candy-plug (19/101), (2) the knickerbocker (3/91), (3) the "cork in the bottle neck" technique (2/101), and (4) FL embolization with combined use of coils, onyx, plugs, and glue (77/101). The technical success rate was 100%, with a 30-day mortality rate of 2.5% in TAAD and 0% in cTBAD patients. During follow-up (ranging: 2 to 63 months), the mortality rate was 0% and 7.1% in TAAD and cTBAD patients, respectively. The FL remained completely thrombosed in 78% of TAAD and 62% of cTBAD patients, whereas it was partially thrombosed in 3 and 2 patients, respectively.
6. Conclusions
Aortic dissection is a diverse pathology with various patterns of complications, anatomical extension, and chronic development. The current literature clearly underlines the need for a tailored therapeutic approach to this disease, based on the characteristics of each patient and their pathology. The principle in chronic aortic dissection should be to exclude the false lumen from flow and pressure and to achieve thrombosis of the false lumen. For patients with aortic dissection extending into the abdomen, endovascular treatment of the thoracic segment can be a first step, but seems rarely to offer a final solution. Open surgery and complex aortic endografting are limited by either high technical and logistic demands or high rates of mortality and morbidity. False lumen endovascular techniques offer less invasive therapy strategies with promising early results. A staged aortic repair strategy with timely successive treatment of persistent flow within the thoracic false lumen causing complications are mandatory. Improved imaging techniques for assessment of false lumen flow and distal entry tears have the potential to further guide the need for reintervention in these patients.
References
- Cheng D, Martin J, Shennib H, et al. Endovascular aortic repair versus open surgical repair for descending thoracic aortic disease a systematic review and meta-analysis of comparative studies. J Am Coll Cardiol 55 (2010): 986-1001.
- Fattori R, Tsai TT, Myrmel T, et al. Complicated acute type B dissection: is surgery still the best option?: a report from the International Registry of Acute Aortic Dissection. JACC Cardiovasc Interv 1 (2008): 395-402.
- Yang CP, Hsu CP, Chen WY, et al. Aortic remodeling after endovascular repair with stainless steel-based stent graft in acute and chronic type B aortic dissection. J Vasc Surg 55 (2012): 1600-1610.
- Li D, Ye L, He Y, et al. False Lumen Status in Patients With Acute Aortic Dissection: A Systematic Review and Meta-Analysis. J Am Heart Assoc 5 (2016).
- Verhoeven EL, Paraskevas KI, Oikonomou K, et al. Fenestrated and branched stent-grafts to treat post-dissection chronic aortic aneurysms after initial treatment in the acute setting. J Endovasc Ther 19 (2012): 343-349.
- Estrera AL, Sandhu H, Afifi RO, et al. Open repair of chronic complicated type B aortic dissection using the open distal technique. Ann Cardiothorac Surg 3 (2014): 375-384.
- Simring D, Raja J, Morgan-Rowe L, et al. Placement of a branched stent graft into the false lumen of a chronic type B aortic dissection. J Vasc Surg 54 (2011): 1784-1787.
- Nienaber CA, Rousseau H, Eggebrecht H, et al. Randomized comparison of strategies for type B aortic dissection: the INvestigation of STEnt Grafts in Aortic Dissection (INSTEAD) trial. Circulation 120 (2009): 2519-2528.
- Tanaka A, Sakakibara M, Ishii H, et al. Influence of the false lumen status on clinical outcomes in patients with acute type B aortic dissection. J Vasc Surg 59 (2014): 321-326.
- Kusagawa H, Shimono T, Ishida M, et al. Changes in false lumen after transluminal stent-graft placement in aortic dissections: six years' experience. Circulation 111 (2005): 2951-2957.
- Mani K, Clough RE, Taylor PR. Regarding "patient outcomes and thoracic aortic volume and morphologic changes following thoracic endovascular aortic repair in patients with complicated chronic type B aortic dissection". J Vasc Surg 57 (2013): 898.
- Scali ST, Feezor RJ, Chang CK, et al. Efficacy of thoracic endovascular stent repair for chronic type B aortic dissection with aneurysmal degeneration. J Vasc Surg 58 (2013): 10-17.
- Liu F, Ge YY, Guo W, et al. Preoperative thoracic false lumen branches are predictors of aortic enlargement after stent grafting for DeBakey IIIb aortic dissection. J Thorac Cardiovasc Surg 155 (2018): 21-29.
- Sueyoshi E, Sakamoto I, Uetani M. Growth rate of affected aorta in patients with type B partially closed aortic dissection. Ann Thorac Surg 88 (2009): 1251-1257.
- Tsai TT, Evangelista A, Nienaber CA, et al. Partial thrombosis of the false lumen in patients with acute type B aortic dissection. N Engl J Med 357 (2007): 349-359.
- Akutsu K, Nejima J, Kiuchi K, et al. Effects of the patent false lumen on the long-term outcome of type B acute aortic dissection. Eur J Cardiothorac Surg 26 (2004): 359-366.
- Tolenaar JL, van Keulen JW, Jonker FH, et al. Morphologic predictors of aortic dilatation in type B aortic dissection. J Vasc Surg 58 (2013): 1220-1225.
- Nienaber CA, Kische S, Rousseau H, et al. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv 6 (2013): 407-416.
- Andacheh ID, Donayre C, Othman F, et al. Patient outcomes and thoracic aortic volume and morphologic changes following thoracic endovascular aortic repair in patients with complicated chronic type B aortic dissection. J Vasc Surg 56 (2012): 644-650.
- Leshnower BG, Szeto WY, Pochettino A, et al. Thoracic endografting reduces morbidity and remodels the thoracic aorta in DeBakey III aneurysms. Ann Thorac Surg 95 (2013): 914-921.
- Ge YY, Guo W, Cheshire N, et al. Preoperative thoracic false lumen branches relate to aortic remodeling after thoracic endovascular aortic repair for DeBakey IIIb aortic dissection. J Vasc Surg 65 (2017): 659-668.
- Schwartz SI, Durham C, Clouse WD, et al. Predictors of late aortic intervention in patients with medically treated type B aortic dissection. J Vasc Surg 67 (2018): 78-84.
- Song JM, Kim SD, Kim JH, et al. Long-term predictors of descending aorta aneurysmal change in patients with aortic dissection. J Am Coll Cardiol 50 (2007): 799-804.
- Loubert MC, van der Hulst VP, De Vries C, et al. How to exclude the dilated false lumen in patients after a type B aortic dissection? The cork in the bottleneck. J Endovasc Ther 10 (2003): 244-248.
- Hofferberth SC, Nixon IK, Mossop PJ. Aortic false lumen thrombosis induction by embolotherapy (AFTER) following endovascular repair of aortic dissection. J Endovasc Ther 19 (2012): 538-545.
- Idrees J, Roselli EE, Shafii S, et al. Outcomes after false lumen embolization with covered stent devices in chronic dissection. J Vasc Surg 60 (2014): 1507-1513.
- Roselli EE, Idrees J, Reside J, et al. Use of covered stent devices for false lumen embolization in chronic dissection: a novel approach. Ann Thorac Surg 98 (2014): 737-739.
- Mendes BC, Oderich GS, Erben Y, et al. False Lumen Embolization to Treat Disseminated Intravascular Coagulation After Thoracic Endovascular Aortic Repair of Type B Aortic Dissection. J Endovasc Ther 22 (2015): 938-941.
- Kolbel T, Lohrenz C, Kieback A, et al. Distal false lumen occlusion in aortic dissection with a homemade extra-large vascular plug: the candy-plug technique. J Endovasc Ther 20 (2013): 484-489.
- Rohlffs F, Tsilimparis N, Fiorucci B, et al. The Candy-Plug Technique: Technical Aspects and Early Results of a New Endovascular Method for False Lumen Occlusion in Chronic Aortic Dissection. J Endovasc Ther 24 (2017): 549-555.
- Kolbel T, Carpenter SW, Lohrenz C, et al. Addressing persistent false lumen flow in chronic aortic dissection: the knickerbocker technique. J Endovasc Ther 21 (2014): 117-122.
- Rohlffs F, Spanos K, Tsilimparis N, Debus ES, Kolbel T. Techniques and outcomes of false lumen embolization in chronic type B aortic dissection. J Cardiovasc Surg (Torino) 59 (2018): 784-788.
- Marques de Marino P, Oikonomou K, Verhoeven EL, et al. Techniques and outcomes of secondary endovascular repair for postdissection TAA/TAAA. J Cardiovasc Surg (Torino) 59 (2018): 767-774.
- Nienaber CA, Kische S, Zeller T, et al. Provisional extension to induce complete attachment after stent-graft placement in type B aortic dissection: the PETTICOAT concept. J Endovasc Ther 13 (2006): 738-746.
- Lombardi JV, Cambria RP, Nienaber CA, et al. Aortic remodeling after endovascular treatment of complicated type B aortic dissection with the use of a composite device design. J Vasc Surg 59 (2014): 1544-1554.
- Bertoglio L, Rinaldi E, Melissano G, et al. The PETTICOAT concept for endovascular treatment of type B aortic dissection. J Cardiovasc Surg (Torino) 60 (2019): 91-99.
- Rong D, Ge Y, Liu J, et al. Combined proximal descending aortic endografting plus distal bare metal stenting (PETTICOAT technique) versus conventional proximal descending aortic stent graft repair for complicated type B aortic dissections. Cochrane Database Syst Rev 10 (2019).
- Hofferberth SC, Nixon IK, Boston RC, et al. Stent-assisted balloon-induced intimal disruption and relamination in aortic dissection repair: the STABILISE concept. J Thorac Cardiovasc Surg 147 (2014): 1240-1245.
- Melissano G, Bertoglio L, Rinaldi E, et al. Satisfactory short-term outcomes of the STABILISE technique for type B aortic dissection. J Vasc Surg 68 (2018): 966-975.
- Faure EM, El Batti S, Sutter W, et al. Stent-assisted balloon dilatation of chronic aortic dissection. J Thorac Cardiovasc Surg (2020).
- Wojtaszek M, Wnuk E, Maciag R, et al. Promoting False-Lumen Thrombosis after Thoracic Endovascular Aneurysm Repair in Type B Aortic Dissection by Selectively Excluding False-Lumen Distal Entry Tears. J Vasc Interv Radiol 28 (2017): 168-175.
- Kim TH, Song SW, Lee KH, et al. The effect of false lumen procedures during thoracic endovascular aortic repair in patients with chronic DeBakey type IIIB dissections. J Vasc Surg 68 (2018): 976-984.
- Rakestraw S, Feghali A, Nguyen K, et al. False lumen embolization as a rescue technique in the setting of acute and chronic dissecting aneurysms as adjunct to thoracic endovascular aortic repair. J Vasc Surg Cases Innov Tech 6 (2020): 110-117.
- Zhang H, Ge YY, Lu K, et al. Coil Embolization for Persistent Thoracic False Lumen of Type B Aortic Dissection after Thoracic Endovascular Aortic Repair. Ann Vasc Surg 57 (2019): 60-68.
- Pellenc Q, Roussel A, De Blic R, et al. False lumen embolization in chronic aortic dissection promotes thoracic aortic remodeling at midterm follow-up. J Vasc Surg 70 (2019): 710-717.
- Miletic KG, Kindzelski BA, Hodges KE, et al. Impact of endovascular false lumen embolization on thoracic aortic remodeling in chronic dissection. Ann Thorac Surg (2020).
- Paul Revel-Mouroz F-ZM, Camille Dambrin, Christophe Cron, et al. Complementary Endovascular Procedures Improve Aortic Remodeling in Progressive Chronic Aortic Dissection. Clinics in Surgery 5 (2020).
- Spanos K, Kolbel T, Rohlffs F, et al. Intentional Targeted False Lumen Occlusion after Aortic Dissection: A Systematic Review of the Literature. Ann Vasc Surg 56 (2019): 317-329.


 Impact Factor: * 5.6
Impact Factor: * 5.6 Acceptance Rate: 74.36%
Acceptance Rate: 74.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 