Pulmonary Artery Catheter Induced Ventricular Fibrillation In A Patient Undergoing Retroperitoneal Mass Excision: Report of a rare case
Vishnu Datt*, Priyanka Dahiya, Simran Yadav, Priyanka Kaushik, Sakshi Dhingra,Samya Agarwal, Shreya Khatri, Parth Gangwar, Arpan Sonkar, Shivam Singla, Aanchal Tyagi
Department of anaesthesiology and critical care and Department of Radiodiagnosis, SGT Medical college, Budhera, Gurugram, Haryana, India.122505
*Corresponding author: Dr Vishnu Datt, Professor & HOD of Anaesthesiology and critical care, SGT Medical College, Bhudera,Gurugram, Haryana,India.
Received: 06 Nov 2025; Accepted: 12 Nov 2025; Published: 21 Nov 2025
Article Information
Citation: Vishnu Datt, Priyanka Dahiya, Simran Yadav, Priyanka Kaushik, Sakshi Dhingra, Samya Agarwal, Shreya Khatri, Parth Gangwar, Arpan Sonkar, Shivam Singla, Aanchal Tyagi. Pulmonary Artery Catheter Induced Ventricular Fibrillation In A Patient Undergoing Retroperitoneal Mass Excision: Report of a rare case. Cardiology and Cardiovascular Medicine. 9 (2025): 464-470.
View / Download Pdf Share at FacebookAbstract
Pulmonary artery catheter (PAC) is a vital monitor in cardiac patients undergoing cardiac as well as noncardiac surgery. PAC remains a gold standard for monitoring several hemodynamic parameters like continuous cardiac output measurements, right ventricular end-diastolic volume, right heart pressures, pulmonary artery pressures, and pulmonary capillary wedge pressure (PCWP), and “a” wave on wedging to diagnose the significant mitral regurgitation, and mixed venous saturation, and right atrial and ventricular pacing. The PCWP helps in maintaining the adequate LV filling and avoids volume overload. Additional derived calculations from these measurements, like pulmonary and systemic vascular resistance (PVR, SVR), cardiac index (CI), stroke volume (SV), LV and RV stroke work index, oxygen delivery, and oxygen consumption, help in diagnosing the type of shock (cardiogenic vs. septic) and selection of vasoactive drugs, vasodilators, and fluid administration. Therefore, PAC utilization could be valuable in guiding the management in high-risk surgical patients. Moreover, PAC provides several hemodynamic parameters and helps in the perioperative hemodynamic management. However, the indications for PA catheterization remain controversial due to the precipitation of complications, including fatal arrhythmia. PA catheterization can be associated with arrhythmias in approximately 12.5% to 70% of the patients. The most common arrhythmia is multiple premature ventricular contractions (PVCs) and rarely VF /VT in 1% and persistent VF in 4.7% as a fatal arrhythmia. Herein, we report a case of a 53-year-old male with a massive retroperitoneal mass with severe LV dysfunction and atrial fibrillation (AF) posted for mass excision after obtaining the informed high-risk consent, who experienced multiple PVCs during PA catheterization upon reaching the RV, resulting in severe hypotension, and converted to VF during manipulation for advancing from RV to PA. After external cardiac massage, DC shock (biphasic 200 J), and administration of lidocaine (75 mg) and magnesium sulfate (2 gm), a rhythm (95 bpm) and systolic BP of 90/60 mmHg returned on the first defibrillation; however, AF persisted. The patient's hemodynamics were optimized and maintained with milrinone, dobutamine, and nitroglycerin (NTG) infusions. After resuscitation, the surgery was completed without any complications, and the patient was extubated on the table with stable hemodynamics.
Keywords
<p>Arrhythmias, CPR, General anaesthesia, PA catheter, PVCs, retroperitoneal mass, resuscitation, VF.</p>
Article Details
1. Introduction
The indications for pulmonary artery catheterization remain controversial, and the routine use of a PAC is not recommended because of the data from the large trials have suggested no benefit on the major outcome and rather increased mortality. However, a PAC placement can aid in both diagnosis and guide the therapy for shock, pulmonary edema, heart failure, congenital heart diseases, and pulmonary arterial hypertension (PAH). Several direct parameters and derived calculations obtained from the PAC such as PVR, SVR, and CI helps in the management of variety of the patients. The right interpretation of these hemodynamic parameters and derived calculations and its clinical implications is of the utmost essence in order to guide a specific therapy i.e. fluid and drug therapy. Even though clinical trials have not shown a reduction in mortality with the use of the PAC, it remains a valuable tool in a wide variety of clinical settings. On the contrary, PA catheterization can lead to significant arrhythmias particularly during migration through the RV due to endocardial irritation. The nature of arrhythmias varies from a common multiple PVCs to a rare fatal VT/VF particularly in patients with ventricular dysfunction with enlarged chambers. We report a case of a patient with low EF and AF, who developed multiple PVCs, converted to the VF during migration of the catheter from RV to PA and destination PCWP position. The CPR with external cardiac massage and DC shock (biphasic 200 J) and lignocaine, and magnesium sulphate and administration of milrinone, dobutamine and NTG restored the heart rhythm, and hemodynamic stabilization. Finally, the surgery was completed, and patient was extubated on the table in stable condition.
2. OR Preparation
All essential drugs and equipment were arranged like defibrillator with biphasic mode, levosimendan, epinephrine, nor-epinephrine, vasopressin, Dobutamine, Milrinone, NTG, magnesium sulphate, calcium gluconate, metoprolol, verapamil, TEE Machine & PA Catheter, diltiazem, insulin and temporary pacemaker. In addition, cardiac catheterization lab backup support was also kept ready to deal with any major cardiac event.
3. Case presentation
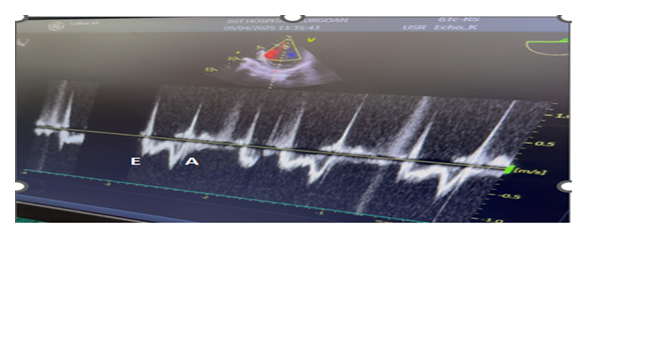
A 53 yr-male, weighing 55kg presented with occasional chest pain and palpitations and dyspnoea of NYHA class III since one year. In addition, he had progressive abdominal swelling and Intermittent dull aching pain for the last one month. Patient did not have any history of weight loss and tuberculosis. He did not consult physician for the cardiac symptoms but admitted in the surgical ward for the abdominal swelling. His biochemical and haematological values were with in normal limits. 2-D echocardiography revealed EF of 35% suggestive of severe LV systolic dysfunction, enlarged LA(4.3cm), LVEDD(5.9cms), LVESD(4.3cms), Hypokinesia of IVS and inferior wall. Mitral inflow velocity showed E<A and TDI of mitral annulus revealed a E/e’ ratio of 14 suggestive of diastolic dysfunction. ECG suggestive of AF with HR of 90 bpm and occasional PVCs. Chest X- ray was suggestive of mild to moderate cardiomegaly.
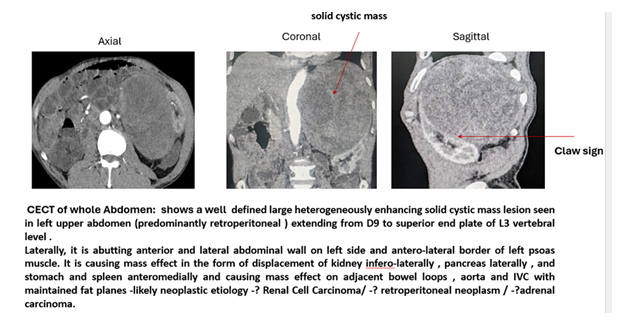
USG Abdomen was suggestive of a well-defined heterogeneously hypoechoic lump filled of fluid in the left hypochondrium region. CECT whole abdomen was suggestive of a large heterogeneously enhancing solid cystic mass lesion in left upper abdomen (predominantly retroperitoneal) extending from D9 to superior end plate of L3 vertebral level. Laterally, it is abutting anterior and lateral abdominal wall on left side and antero-lateral border of left psoas muscle. It is causing mass effect in the form of displacement of kidney infero-laterally, pancreas laterally, and stomach and spleen anteromedially and causing mass effect on adjacent bowel loops, aorta and IVC with maintained fat planes -likely neoplastic aetiology as Renal Cell Carcinoma or retroperitoneal neoplasm i.e. adrenal carcinoma. [Figure-1]. A high-risk consent was obtained, and patient was scheduled for urgent excision of retroperitoneal mass. The Clinical trial number: not applicable. As it belongs to the case report ’The multidisciplinary team included cardiologist decided to perform the mass excision first and then coronary angiography and PCI in view of fast growing tumour, and requirement of dual antiplatelet therapy and delaying the non-cardiac surgery at least for 3 to 6 months post drug eluting stenting as per ACC/ AHA guidelines.
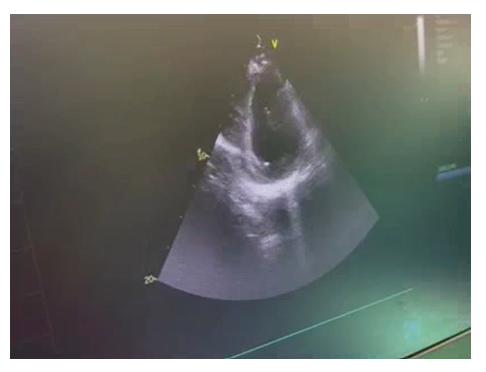
In OR, the anaesthesia technique was planned considering the patient with chronic coronary disease. Accordingly, all standard ASA monitors were attached (ECG, non-invasive BP, SPO2, EtCO2, temperature). His HR was 104bpm, BP -144/86 mmHg, arterial saturation -99% on room air, and ECG revealed AF and occasional PVCs. In view of the severe ventricular dysfunctions, infusions of levosimendan (0.1mcg/kg/min), dobutamine(2.5mcg/kg/min) and NTG(0.5mcg/kg/min) were started for cardiac support to stabilize the hemodynamic during anaesthetic and surgical manipulations. General anaesthesia was induced and maintained with etomidate (18mg), sevoflurane (1 MAC), midazolam (2mg), fentanyl (100mcg), acetaminophen (15mg/kg), and vecuronium (8mg) was used to facilitate the endotracheal intubation with 8.5 mm, ID cuffed tube. Transesophageal echocardiography (TEE) probe [GE,6TC-RS, Vivid T9 V205, Norway] was inserted after tracheal intubation. It also revealed severe LV systolic dysfunction, diastolic dysfunction, with global hypokinesia, mild MR with first order chordae rupture that is free floating in LA and LVEF of 20-25%. (Figure 2 & Video 1,2). A 20G, arterial catheter was placed in the right radial artery to measure beat to beat blood pressure and serial arterial blood gas analysis. A 7.5 F Swan Ganz, thermodilution, PA catheter was inserted via the right internal jugular vein. After the catheter was withdrawn and reinserted, it reached the RV at the second insertion. However, multiple premature ventricular contractions (PVCs) occurred while it was migrating from RV to PA, thus resulting in hypotension, which converted to VF during final manipulation for the PCWP position.
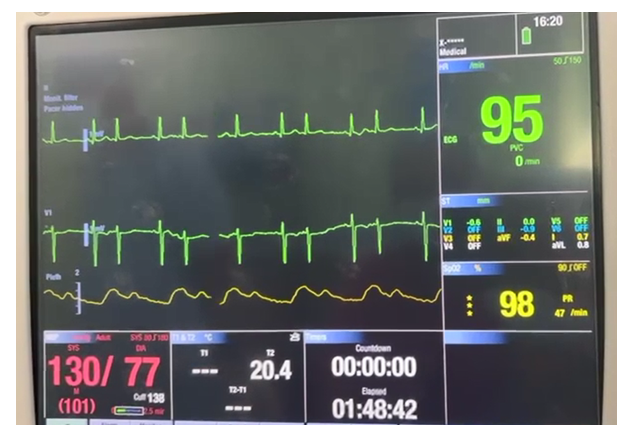
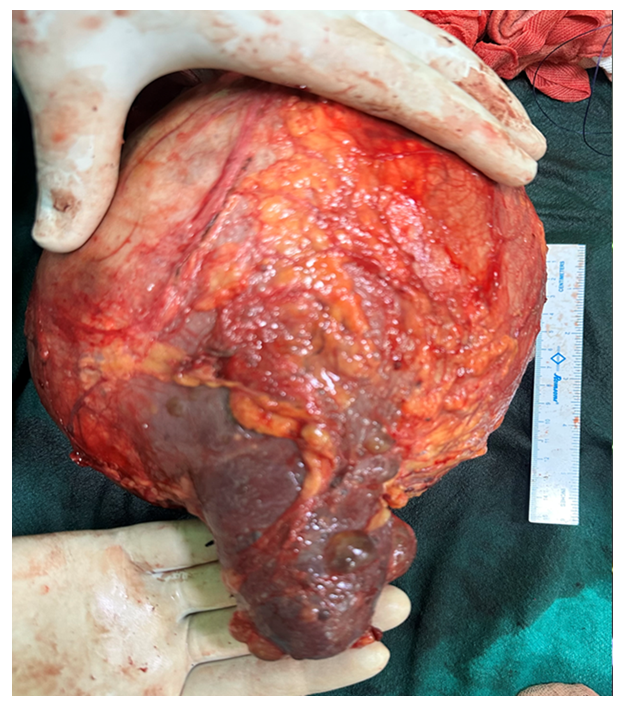
The CPR with external cardiac massage and DC shock (biphasic 200 J), and administration of 75 mg of lidocaine and magnesium sulphate (1 gm) and hydrocortisone (200mg ) restored the heart rhythm, and BP of 90/60 mmHg, and further increased to 130/70 mmHg with the administration of milrinone, dobutamine and NTG and titrated volume infusion. The rhythm returned after the first electrical defibrillation with in one minute. The duration of PAC placement time was 3 min. We finally advanced the PAC into the distal pulmonary artery and initiated the surgery. Perioperative ECG monitoring revealed AF with HR of 95bpm and BP of 130/70 mmHg. [Figure 3] The surgery was continued after proper counselling to the family members by the multidisciplinary team, and big retroperitoneal mass was excised. (Figure 4) The patient was extubated on the table after reversal of neuromuscular block, in stable hemodynamic without any residual effects and shifted to the ICU for extended monitoring, and rest of the course was uneventful. The histopathological evaluation of the excised mass was suggestive of Papillary renal cell carcinoma. He was discharged from the hospital on 10th postoperative day with instructions for regular follow-up including cardiology.
Figure 1 - CECT of the whole abdomen revealed a Well defined large heterogeneously enhancing solid cystic mass lesion seen in left upper abdomen (predominantly retroperitoneal) extending from D9 to superior end plate of L3 vertebral level . Laterally, it is abutting anterior and lateral abdominal wall on left side and antero-lateral border of left psoas muscle. It is causing mass effect in the form of displacement of kidney infero-laterally , pancreas laterally , and stomach and spleen anteromedially and causing mass effect on adjacent bowel loops , aorta and IVC with maintained fat planes- likely neoplastic etiology; Renal Cell Carcinoma or retroperitoneal neoplasm or adrenal carcinoma. [Labelled]
Video 1.Mid-esophageal, Two- chamber view of TEE: the 2-D and color doppler showing a ruptured fist order chordae free floating in the LA and color doppler confirms a mild to moderate mitral regurgitation
Video 2. TG- SAX Mid -Papillary view of LV of TEE. The 2-D echocardiography revealed global hypokinesia. On Eyeballing the EF is around 20-25% suggestive of severe LV systolic dysfunction
TG-trans gastric, SAX- short axis, LV- left ventricle, TEE- transesophageal echocardiography, 2-D- two dimensional, EF- ejection fraction
4. Discussion
PAC is an advanced monitor, its placement in noncardiac surgery can aid in both diagnosis and guide the therapy for the management of the severe shock (septic vs cardiogenic) using mixed venous oxygen saturation(Sv02); the SvO2 is low in cardiogenic shock due to reduced oxygen supply as a result of low cardiac output, and higher in septic shock due to deceased oxygen extraction by the tissues. It is also utilized in acute or chronic pulmonary hypertension, pulmonary edema, heart failure, severe valvular or congenital heart disease, and monitoring fluid status in major surgical procedures. In addition, it plays a vital role in high-risk surgeries with a high liability of major hemodynamic changes, fluid shifts, or organ damage, and severe underlying cardio-respiratory diseases like severe cardiomyopathy(DCMP, hypertrophic), and coronary artery disease with low EF with significant hemodynamic effects like low cardiac output syndrome.[1,2,3] It can provide right atrial and ventricular pacing to counter the perioperative brady arrhythmias. The PCWP allows the assessment of filling pressures in the left side of the heart. So, using continuous PCWP monitoring it helps to optimize fluid administration and avoid fluid overload.
PAC remains a gold standard for monitoring several hemodynamic parameters like continuous cardiac output measurements, right ventricular end-diastolic volume, right heart pressures, pulmonary artery pressures, and “a” wave on wedging to diagnose the significant mitral regurgitation. PAC provides vital information on cardiac output (CO), mixed venous oxygen saturation (SmvO2), pulmonary artery pressure (PAP), and pulmonary capillary wedge pressure (PCWP). Additional derived calculations from these measurements, including pulmonary and systemic vascular resistance (PVR, SVR), cardiac index (CI), stroke volume (SV), right and left ventricular end-systolic and end-diastolic volume, right ventricular ejection fraction (RVEF), LV and RV stroke work index, oxygen delivery, and oxygen consumption. Thus, PAC utilization could be valuable in guiding management in high-risk surgical patients.[ 4,5,6,7,8,9,10] on the contrary, some authors have reported that PAC does not confer significant benefits with respect to decreased mortality, shorter ICU stays and hospital length of the stay, and lower medical costs in the ICU.[6,7,8,9,10]
Perioperative arrhythmias including VF can be triggered by reversible conditions, including electrolyte abnormalities (e.g., potassium or magnesium imbalances),acute myocardial ischemia, hypoxia, drug toxicity, various surgical and anaesthetic manipulations in high risk patients with severe systolic/ diastolic dysfunctions as a result of acute coronary syndrome, chronic coronary diseases, valvular heart disease, cardiomyopathies or even with severe PAH and RV dysfunctions.[11] It is important to identify the Patients with a history of heart disease, which puts them at higher risk of arrhythmias, and should be carefully evaluated and optimized before the noncardiac surgery. PA catheterisation in such patients is associated with an unacceptably high risk.
Perioperative arrhythmias have been reported approximately 12.5% to 70% during PAC insertion. Among these PVCs occur in 52%-68% and very rarely a fatal VF /VT can occur in 1% patients either during insertion or perioperatively during PAC manipulations. [12,13]
PA catheter can induce multiple arrhythmias including VT/VF due to direct stimulation of the RV endocardium by the balloon tip, particularly when uninflated tip present in RV. The manipulations of the catheter can cause irritation or trauma to the heart muscles, which can trigger various arrhythmias. [12] Longer insertion time, large cardiac chambers, pre-existing MI with severe ventricular dysfunction and repeated attempts and manipulation, and long length with coiling in RV, previous liver transplantation, complicated myocardial infarction, sepsis and acidosis (pH < 7.2) can leads to a higher incidence of ventricular arrhythmia.[14,15] The presented patient also had untreated CAD with severe LV dysfunctions (EF 20-30%) and therefore, was likely a potential candidate for VF during PA catheterization.
The multifocal premature ventricular contraction (PVC) is the most common arrhythmia and the PVCs with a "R-on-T phenomenon can trigger the VF or VT. The presented patient also developed multifocal PVCs during insertion of the PA catheter from RA to RV, but these were corrected spontaneously on withdrawal of the PAC from RV to RA, and finally with second attempt the PAC was successfully inserted with few PVCs. However, he developed VF after final position most likely due to RV endocardial irritation.
The PA catheter should be inserted with extreme care to minimize direct contact with the heart muscle particularly in the patients with severe LV dysfunctions with already unstable hemodynamic. It is well known fact that severe LV dysfunction (EF< 30%) is one of the indications for PAC monitoring and arrhythmias are also more frequently reported in patients with Low EF. The primary goal is to restore regular heart rhythm with the proper utilization of the defibrillation; (Biphasic 150–200 joules) and CPR with external cardiac massage with a focus to generate a MAP of > 65mmHg and systolic pressure of 90 mm Hg to maintain vital organ perfusion and oxygenation. In addition, the medical team must immediately investigate the cause of the VF. Following initial defibrillation and CPR with external cardiac massage, if VF persists, then the following medications can be administered intravenously: Amiodarone (Class III antiarrhythmic) a bolus of 300mg is the agent of choice for treating persistent VF. It prolongs the action potential and refractory period in myocardial tissue that helps restore a normal rhythm. Amiodarone infusion is considered useful for survival according to the 2015 American Heart Association guidelines.[16] Lidocaine (1-1.5 mg/kg) is an alternative to amiodarone for shock-refractory VF, as it works by suppressing the automaticity of ventricular conduction tissue.in addition, intravenous epinephrine as a 1 mg dose is intravenously every 3 to 5 minutes during cardiac arrest can be administered to improve myocardial and cerebral blood flow during CPR by stimulating alpha and beta-adrenergic receptors.[ 17,18,19]
Intravenous Magnesium Sulphate(1-2gm) is the drug of choice for the management of polymorphic VT called torsades de pointes. [18]
Some authors have reported that Nifekalant (20mg) as a pure potassium channel blocker and amiodarone improved 24-h survival to a similar degree in a multicentre cohort study. In addition, the time from drug administration to successful defibrillation was significantly shorter with nifekalant than with amiodarone. [20]
If malignant arrhythmias resulting in the persisting hemodynamic instability despite the goal directed medical therapy, then the PAC should be removed leaving the sheath in situ for infusion of fluid, blood or blood products and various anaesthetic and inodilators therapy, or it may be replaced with the CVP line and further monitoring can be continued with TEE only. Now a days Transesophageal echocardiography (TEE) has been increasingly used even in non-cardiac surgery. TEE can detect acute myocardial ischemia through regional wall motion abnormalities as early signs of acute MI. Moreover, TEE also provide information regarding valvular lesions and function, and detect intracardiac air [21] However, TEE necessitates an expert and skilled cardiac anaesthetist, and the standard TEE probes cannot be kept in the patient for too long in the postoperative period. Some authors strongly believe that placing defibrillator pads before the procedure is essential in these high-risk patients for immediate conversion of new onset atrial fibrillation or VF.[14]
If the initial non cardiac surgery is elective, it can be delayed for a significant period to allow for complete cardiac recovery, however, time for rescheduling the surgery is not specified. Elective non-cardiac surgery after a major cardiac event often requires waiting months to years, depending on the intervention.
If the initial surgery is urgent/ emergency then the surgical team, cardiac anaesthetist and cardiologist will weigh the risk of postponing the procedure against the patient's new, very high cardiac risk.[11] Patients after successful CPR with stable hemodynamic without any residual cardiac and neurological complications can safely undergo the non-cardiac procedure not only for the emergent surgery but elective surgery also. The elective surgical procedure should be continued, If VF is terminated immediately and cardiac rhythm and hemodynamic remain stable with reasonable inodilators support and risk of postponing the surgery are higher.
The presented patient was hemodynamically stabilized with intravenous inodilators( levosimendan, dobutamine and NTG) and surgery was continued after proper detailed counselling of the family members regarding manyfold increased risk of postponing the surgery in this fast-growing retroperitoneal mass and untreated CAD with low EF. And thus, detailed counselling of the family members also plays a big role.
VF requires urgent CPR with defibrillation and cardiac massage, and further diagnostic and therapeutic measures like cardiac catheterization should be considered thereafter. If VF cannot be terminated, then veno-arterial extracorporeal membrane oxygenation (VA-ECMO) can buy the time needed to identify the cause and initiate specific treatment. [22].
5. conclusion
We observed VF during PAC placement in a patient with a low EF and pre-existing PVCs and AF. This single case report suggests that VF can be treated appropriately with external cardiac massage and biphasic DC shock of 200J, and intravenous lignocaine, and magnesium sulphate, and hemodynamic can be stabilized with inotropic support, and the surgery can be successfully completed, and patient can be successfully extubated in the OR without any residual effects. Perioperative stable hemodynamic status can be achieved with the use of vasoactive drugs. The multidisciplinary team of surgeons, cardiologist and cardiac anaesthetist should assess the clinical status of the patient and should weigh the balance between continuation or postponing the surgical procedure. Here the risk of postponing the surgery is many folds due to fast growing tumour and the untreated CAD with severe LV dysfunction which is always a risk of sudden death. In persistent VF, the first step is to recognize the catheter as a culprit for the arrhythmia. Either repositioning or removing the catheter is essential to stop the mechanical irritation of the heart muscle.
6. Conflicts of Interest
There is no conflict of interest.
7. Funding
Nil.
References
- Rodrigues Ziccardi M, Khalid N. Pulmonary artery catheterisation. Stat Pearls Publishing (2025).
- Ahmed SS, Akhtar MI, Kamal R. Frequency, indications and complications of pulmonary artery catheter insertion in adult open-heart surgery patients of a tertiary care hospital. J Ayub Med Coll 28 (2016): 793–797.
- Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, et al. Consensus on circulatory shock and hemodynamic monitoring: Task force of the European Society of Intensive Care Medicine. Intensive Care Med 40 (2014): 1795–1815.
- Xie CM, He LX, Shen MQ, Yao YT. Association of pulmonary artery catheterization utilization and surgical patients’ outcomes: A PRISMA-compliant systematic review and meta-analysis. J Cardiothorac Surg 20 (2025): 227.
- Demiselle J, Mercat A, Asfar P. Is there still a place for the Swan–Ganz catheter? Yes. Intensive Care Med 44 (2018): 954–956.
- Mittnacht JC, Weiner M, London MJ, Kaplan JA. Anesthesia for myocardial revascularization. In: Kaplan JA, Reich DL, Savino JS, eds. Kaplan’s Cardiac Anaesthesia, 6th ed. Saunders Elsevier (2016): 522–569.
- Harvey S, Harrison DA, Singer M, Ashcroft J, Jones CM, et al. Clinical effectiveness of pulmonary artery catheters in intensive care (PAC-Man): a randomized controlled trial. Lancet 366 (2005): 472–477.
- Rajaram SS, Desai NK, Kalra A, Gajera M, Cavanaugh SK, et al. Pulmonary artery catheters for adult patients in intensive care. Cochrane Database Syst Rev (2013): CD003408.
- Pasquier V, Deletombe B, Bedague D, Albaladejo P, Durand M. Impact of pulmonary artery catheter hemodynamic monitoring on postoperative morbidity and mortality in elective Bentall procedures. J Cardiothorac Vasc Anesth 34 (2020).
- Brown JA, Aranda-Michel E, Kilic A, Serna-Gallegos D, Bianco V, et al. Impact of pulmonary artery catheter use in cardiac surgery. J Thorac Cardiovasc Surg 164 (2022): 1965–1973.
- Kochiyama T, Kusano Y, Yamaguchi A, Sakuraya S, Kawagoe I. Ventricular fibrillation during pulmonary artery catheter placement in a patient with Stanford type A aortic dissection: a case report. AME Med J 8 (2023).
- Evans DC, Doraiswamy VA, Prosciak MP, et al. Complications associated with pulmonary artery catheters: a comprehensive clinical review. Scand J Surg 98 (2009): 199–208.
- Shah KB, Rao TL, Laughlin S, El-Etrr AA. Pulmonary artery catheterization: review of 6,245 patients. Anesthesiology 61 (1984): 271–275.
- Bergmann L, Großwendt T, Kahlert P, Konorza T, Wendt D, et al. Arrhythmogenic risk of pulmonary artery catheterisation in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation. Anaesthesia 68 (2013): 46–51.
- Satoh H, Miyata Y, Hayasaka T, Wada T, Hayashi Y, et al. Factors producing multiple ventricular arrhythmias during pulmonary artery catheterization. Ann Card Anaesth 20 (2017): 141–144.
- Dorian P, Cass D, Schwartz B, Cooper R, Robert G et al. Amiodarone compared with lidocaine for shock-resistant ventricular fibrillation. N Engl J Med 346 (2002): 884–890.
- Thompson A, Fleischmann KE, Smilowitz NR, de las Fuentes L, Lisa DLF et al. 2024 AHA/ACC/ACS/ASNC/HRS/SCA/SCCT/SCMR/SVM guideline for perioperative cardiovascular management for noncardiac surgery. Circulation 150 (2024): e351–e442.
- Kim CJ, Lever N, Cooper JO. Antiarrhythmic drugs and anaesthesia: Part 2 pharmacotherapy. BJA Educ 23 (2023): 52–60.
- Smith W, Ramsay J. Antiarrhythmic drugs. In: Flood P, Rathmell JP, Irman RD, eds. Stoelting’s Pharmacology & Physiology in Anesthetic Practice, 6th ed. Wolters Kluwer (2021).
- SOS-KANTO 2012 Study Group. Nifekalant hydrochloride and amiodarone hydrochloride result in similar improvements for 24-hour survival in cardiopulmonary arrest patients. J Cardiovasc Pharmacol 66 (2015): 600–609.
- Youssef N, Whitlock RP. Routine use of the pulmonary artery catheter should be abandoned. Can J Cardiol 33 (2017): 135–141.
- Pecha S, Kirchhof P, Reissmann B. Perioperative arrhythmias. Dtsch Arztebl Int 120 (2023): 564–574.


 Impact Factor: * 5.6
Impact Factor: * 5.6 Acceptance Rate: 74.36%
Acceptance Rate: 74.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 