Stem Cell Therapy for the Treatment of Chronic Ischemic Heart Disease
Roberto G Carbone, Simone Negrini, Giuseppe Murdaca, Francesco Puppo*
Department of Internal Medicine, University of Genoa, Genoa, Italy
*Corresponding author: Francesco Puppo, Department of Internal Medicine, University of Genoa, Genoa, Italy
Received: 12 April 2021; Accepted: 20 April 2021; Published: 05 May 2021
Article Information
Citation: Roberto G Carbone, Simone Negrini, Giuseppe Murdaca, Francesco Puppo. Stem Cell Therapy for the Treatment of Chronic Ischemic Heart Disease. Cardiology and Cardiovascular Medicine 5 (2021): 277-284.
View / Download Pdf Share at FacebookAbstract
Chronic ischemic heart disease remains a major cause of morbidity and mortality worldwide. Several trials have been performed to evaluate benefit of stem cells transplantation to restore cardiac function in short- and long-term period after myocardial infarction. This concise review analyzes 15 clinical trials between 2005 and 2020 comprising 1372 patients (608 treated) and is aimed to: 1): assess percent increase in left ventricular ejection fraction (LVEF) and decrease in New York Heart Association (NYHA) class at 12 months after stem cells transplantation after acute myocardial infarction and 2) correlate LVEF percent increase with number of transfused stem cells.
Nine trials reported a significant percent LVEF increase and NYHA class decrease at 12 months after bone marrow and peripheral blood stem cells transplantation correlating with transplanted cells number. Caution should be exercised in the evaluation of these results due to the different stem cells utilized, transplant protocols and endpoints, and the small number of patients treated in some studies.
Keywords
<p>Stem cells; Chronic ischemic heart disease; Transplantation; Clinical trials</p>
Article Details
1. Introduction
Chronic ischemic heart disease remains a major cause of morbidity and mortality worldwide and 50% of diagnosed cases dies within 5 years [1]. In this context stem cells transplantation has been proposed as potential useful procedure and one of clinical options to reduce ischemic damage and restore cardiac function after acute myocardial infarction [2]. Different autologous or allogeneic stem cell types derived from bone marrow, peripheral blood, mesenchymal and cardiac cells have been utilized [2]. Notably, conflicting outcome results have been reported. Moreover, optimal stem cell type and dose as well as transplantation regimen have not been identified.
In addition, little is known about the potential benefits of stem cells transplantation in chronic ischemic heart disease. Aim of this concise review is to report the present knowledge about the potential benefit of stem cells therapy in chronic ischemic heart disease.
2. Methods
Clinical trials were selected applying as search terms “bone marrow cells”, “stem cells”, “mesenchymal cells”, and “chronic ischemic heart disease” in www.pubmed.gov and Cochrane library from 2005 to 2020. Notably, studies were included if they were randomized blinded trials, randomized unblinded trials, non-randomized trials with a follow up ≥ 6 months.
Clinical trials including patients suffering from ischemic and non-ischemic heart failure, chronic heart failure, and acute and chronic angina were criteria of exclusion as well as clinical trials with incomplete outcome data (attrition bias).
Left ventricular ejection fraction (LVEF) increase and New York Heart Association (NYHA) class decrease were identified as the outcome parameters in long term follow up. LVEF was recorded before stem cells transplantation and then after 6 and 12 months in all studies, and over 12 months in selected studies, after stem cells transplantation. Statistical significance of percent LVEF increase at 12 months was recorded. NYHA class at baseline and 12 months after stem cells transplantation was also recorded.
2.1 End points
2.1.1 Primary: Assess percent increase in LVEF and
decrease in NYHA class at 12 months after stem cells transplantation for acute myocardial infarction.
2.1.2 Secondary: Correlate LVEF percent increase and number of transfused stem cells.
3. Results
Selection data searched 15 clinical trials [3-17] evaluating the efficacy of stem cells transplantation in chronic ischemic heart disease from 2005 to 2020 and included: 5 randomized blinded trials, 6 randomized unblinded trials, and 4 non-randomized trials. A total of 1372 patients were enrolled of which 608 (551 males - median age 61 years) were treated. At baseline mean LVEF was 35.9% (range 26 – 46%). Bone marrow (BMC), peripheral blood (PBC), cardiac (CDC) or mesenchymal (MES) stem cells were utilized in 8, 3, 2 and 1 trials, respectively; both PBC and MES were utilized in 1 study. Total number of transplanted cells ranged from 5 x 106 to 1,500 x 106 (median 70 x 106) depending on cell type, cell population purification, and transplantation route. Transplant procedure was intracoronary in 8 trials and intramyocardial in 6 trials; both procedures were utilized in one trial.
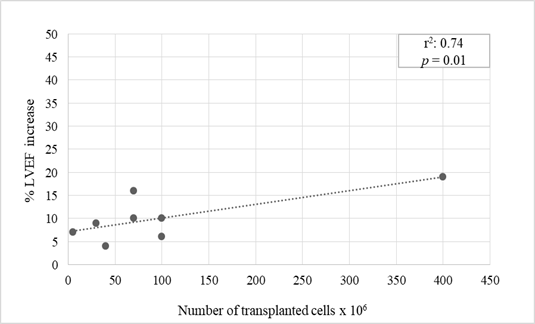
Nine trials reported a statistically significant percent LVEF increase between 4% to 19% (mean 9.6%) at 12 months after stem cells transplantation (p values ranging from 0.05 to 0.001) [3-11]. BMC and PBC stem cells were utilized for transplantation in all these studies (Table 2). A significant correlation was found between number of transplanted cells and percent LVEF increase (Pearson correlation coefficient 0.74, p=0.01) (Figure 1). Of interest, the highest LVEF percent increase was observed in patients transplanted with an elevated amount (400 x 106) of purified cells expressing CXCR4 molecule that regulates cardiac stem cells migration [11]. Transplant of either BMC and PBC unselected stem cells or CD133+ purified stem cells, that represent the most immature cell population which has shown to efficiently regenerate ischemic myocardium in pre-clinical models, attained analogous results (mean percent LVEF increase 7.8% and 8.2%, respectively).
Similarly, no significative differences in percent LVEF increase were observed between patients who underwent intracoronary or intramyocardial transplantation (8.1% and 9.4%, respectively). NYHA class decrease from severe or moderate to mild was also detectable in all these trials at 12 months after stem cells transplantation (Table 1). By contrast, neither LVEF amelioration nor NYHA class decrease was reported in 6 studies [12-17] (Table 1). BMC, PBC, MES and CDC stem cells were transplanted in these trials (Table 2).
N/A: not available
Table 1: Clinical trials evaluating stem cells transplantation in chronic ischemic heart disease.
|
Trials [Ref. N.] |
Cells |
Transplantation route |
||
|
Source* |
Cells number |
Intracoronary |
Intramyocardial |
|
|
Erbs 2005 [3] |
PBC(133+) |
69 ± 14 x 106 |
+ |
|
|
Stamm 2007 [4] |
BMC(133+) |
25 - 34 x 106 |
+ |
|
|
Gyongyosi 2009 [5] |
BMC |
1300 ± 1.64 x 106 |
+ |
|
|
Gyongyosi 2009 [5] |
BMC |
200 x 106 |
+ |
|
|
Pokushalov 2009 [6] |
BMC |
41 ± 16 x 106 |
+ |
|
|
Flores-Ramìrez 2010 [8] |
PBC(133+) |
103 ± 164 x 106 |
+ |
|
|
Turan 2011 [7] |
BMC(133+) |
99 ± 25 x 106 |
+ |
|
|
Makkar 2012 [12] |
CDC |
12-17-25 x 106 |
+ |
|
|
Hare 2012 [13] |
MES |
20-100-200 x 106 |
+ |
|
|
Honold 2013 [14] |
PBC |
183 ± 101 x 106 |
+ |
|
|
Malliaras 2014 [15] |
BMC |
12.5- 25 x 106 |
+ |
|
|
Heldman 2014 [16] |
PBC |
N/A |
+ |
|
|
Heldman 2014 [16] |
MES |
N/A |
+ |
|
|
Nassseri 2014 [9] |
BMC(133+) |
5 x 106 |
+ |
|
|
Trifunovic 2015 [10] |
BMC |
70.7 ± 32.4 × 106 |
+ |
|
|
Aceves 2020 [11] |
PBC(133+) |
400 x 106 |
+ |
|
|
Makkar 2020 [17] |
CDC |
12-17-25 x 106 |
+ |
|
*BMC (bone marrow cells), PBC (peripheral blood cells), CDC (cardiosphere-derived cells), MES (mesenchymal cells)
N/A: not available
Table 2: Stem cells source and transplantation route.
4. Discussion
Between 2000 and 2020 many clinical trials evaluating the efficacy of stem cells transplantation after acute myocardial infarction have been reported in the literature with conflicting results [10,18]. Jeevanantham et al. [19] published a systematic review and meta-analysis reporting 2,635 patients treated with BMC for ischemic heart disease. Authors concluded that BMC transplantation improved LVEF and that beneficial effects persisted for long-term with a median follow up of 6 months (range 3 months to 60 months) reducing mortality and recurrence of ischemic myocardial infarction. Cut-off number of transplanted BMC cells to obtain efficient response was 40 x 106cells.
Recently, we analytically reviewed 34 randomized trials in the period 2000 to 2020 that recruited 3142 patients (the largest number to date) evaluating the efficacy of stem cells transplantation on LVEF increase at 6 months after acute myocardial infarction. Despite the large number of patients evaluated, results demonstrated uncertain efficacy of this therapeutic approach. In fact, 20 trials showed a significant LVEF increase while 14 trials did not show LVEF improvement [20]. These previous controversies have stimulated this concise review aimed to identify a possible benefit of stem cells transplantation at 12 or more months after myocardial infarction in patients affected by chronic ischemic heart disease. To this end we searched clinical trials on this topic and selected 15 trials published from 2005 to 2020.
Nine trials reported a statistically significant LVEF increase and NYHA class improvement at 12 months after stem cells transplantation whereas 6 studies did not report clinical benefit. Of interest, BMC and PBC stem cells were utilized for transplantation in all studies showing positive results and a statistically significant correlation was found between number of transplanted cells and percent LVEF increase. At variance with Jeevanantham et al. [19], positive results were observed in two trials in which less than 40 x 106 stem cells were transplanted. In agreement with literature data [2] myocardial function of transplanted patients was severely impaired with baseline ejection fraction between 26% and 46%. No significative differences were observed between patients who received either unselected BMC and PBC cells or 133+ BMC and PBC selected cells as well as between those that were transplanted either via intracoronary or intramyocardial route. In addition, MSC and CDC cells transplantation did not lead to ejection fraction improvement.
Results of current literature search seem to favor a moderate positive effect of stem cells transplantation on chronic ischemic heart disease at 12 or more months follow up. The positive effect was correlated with the number of transplanted cells. However, caution should be exercised in the evaluation of clinical trials due to the different stem cells utilized, transplant protocols and endpoints. Moreover, statistical significance of data analysis is limited by the small number of patients treated in some studies which affects the value of the results.
Therefore, large multicenter clinical trials aimed at the identification of optimal type and dose of cells as well as at the determination of the better transplantation protocol are needed to achieve a consistent response regarding the long-term effectiveness of stem cells therapy to improve cardiac function and quality of life of patients affected by chronic ischemic cardiomyopathy after acute myocardial infarction.
References
- Roger VL, Go AS, Lloyd-Jones DM, et al. Heart diseases and strokes statistics-2012 update: a report from the American Heart Association. Circulation 125 (2012): e2-e220.
- Donndorf P, Strauer BE, Haverich A, et al. Stem Cell Therapy for the Treatment of Acute Myocardial Infarction and Chronic Ischemic Heart Disease. Curr Pharm Biotechnol 14 (2013): 12-19.
- Erbs S, Linke A, Adams V, et al. Transplantation of Blood-Derived Progenitor Cells After Recanalization of Chronic Coronary Artery Occlusion First Randomized and Placebo-Controlled Study. Circ Res 97 (2005): 756-762.
- Stamm C, Kleine HD, Choi YH, et al. Bone marrow cells and coronary artery bypass grafting for chronic ischemic heart disease: Safety and efficacy studies. J Thorac Cardiovasc Surg 133 (2007): 717-725.
- Gyongyosi M, Lang I, Dettke M, et al. Combined delivery approach of bone marrow mononuclear stem cells early and late after myocardial infarction: the MYSTAR prospective randomzed study. Nature Clin Pract Cardiovasc Med 6 (2009): 70-81.
- Pokushalov E, Romanov A, Chernyavsky A, et al. Efficiency of Intramyocardial Injections of Autologous Bone Marrow Mononuclear Cells in Patients with Ischemic Heart Failure: A Randomized Study. J Cardiovasc Trans Res 3 (2010): 160-168.
- Turan RG, Bozdag TI, Ortak J, et al. Improved Functional Activity of Bone Marrow Derived Circulating Progenitor Cells After Intra Coronary Freshly Isolated Bone Marrow Cells Transplantation in Patients with Ischemic Heart Disease. Stem Cell Rev Rep 7 (2011): 646-656.
- Flores-Ramírez R, Uribe-Longoria A, Rangel-Fuentesa MM, et al. Intracoronary infusion of CD133+ endothelial progenitor cells improves heart function and quality of life in patients with chronic post-infarct heart insufficiency. Cardiovasc Revasc Med 11 (2010): 72-78.
- Nasseri BA, Ebell W, Dandel W. Autologous CD133+ bone marrow cells and bypass grafting for regeneration of ischaemic myocardium: the Cardio133 trial. Eur Heart J 35 (2014): 1263-1274.
- Trifunovic Z, Obradovic S, Balint B, et al. Functional recovery of patients with ischemic cardiomyopathy treated with artery coronary bypass surgery and concomitant intramyocardial bone marrow mononuclear cell transplantation. A long term follow up study. Vojnosanit Pregl 72 (2015): 225-232.
- Aceves JL,Vilchis Lopez R, Mondragon Teran P, et al. Autologous CXCR4+ Hematopoietic Stem Cells Injected into the Scar Tissue of Chronic Myocardial Infarction Patients Normalizes Tissue Contractility and Perfusion. Arch Med Res 51 (2020): 135-144.
- Makkar RR, Smith RR, Cheng K, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet 379 (2012): 895-904.
- Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of Allogeneic vs Autologous Bone Marrow–Derived Mesenchymal Stem Cells Delivered by Transendocardial Injection in Patients With Ischemic Cardiomyopathy: The POSEIDON Randomized Trial. JAMA 308 (2012): 2369-2379.
- Honold J, Fischer-Rasokat U, Seeger FH, et al. Impact of intracoronary reinfusion of bone marrow-derived mononuclear progenitor cells on cardiopulmonary exercise capacity in patients with chronic postinfarction heart failure. Clin Res Cardiol 102 (2013): 619-625.
- Malliaras K, Makkar RR, Smith RR, et al. Intracoronary Cardiosphere-Derived Cells After Myocardial Infarction. J Am Coll Cardiol 63 (2014): 110-122.
- Heldman AW, DiFede DL, Fishman JE, et al. Transendocardial Mesenchymal Stem Cells and Mononuclear Bone Marrow Cells for Ischemic Cardiomyopathy: The TAC-HFT Randomized Trial. JAMA 311 (2014): 62-73.
- Makkar RR, Kereiakes DJ, Aguirre F, et al. Intracoronary ALLogeneic heart STem cells to Achieve myocardial Regeneration (ALLSTAR): a randomized, placebo-controlled double-blinded trial. Eur Heart J 41 (2020): 3451-3458.
- Abdel Latif A, Bolli R, Theyjch IM, et al. Adult bone marrow-derived cells for cardiac repair. A systematic review and meta-analysis. Arch Intern Med 167 (2007): 989-997.
- Jeevanantham V, Butler M, Saad A, et al. Adult Bone Marrow Cell Therapy Improves Survival and Induces Long-Term Improvement in Cardiac Parameters. A Systematic Review and Meta-Analysis. Circulation 126 (2012): 551-568.
- Carbone RG, Monselise A, Bottino G, et al. Stem cells therapy in acute myocardial infarction: a new era?. Clin Exp Med (2021).


 Impact Factor: * 5.6
Impact Factor: * 5.6 Acceptance Rate: 74.36%
Acceptance Rate: 74.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 