The Relation Between ACEI/ARB use and COVID-19 Severity in RTPCR- Confirmed Cases: A Retrospective Case-Control Study
Sabrina Amaouche1#, Ziad Letaief1#, Nico Buls1,*, Sabine Allard2, Johan de Mey1
1Department of Radiology, Universitair Ziekenhuis Brussel, Brussel, 1090, Belgium
2Department of Infectiology, Universitair Ziekenhuis Brussel, Brussel, 1090, Belgium
*Corresponding author: Ziad Letaief, Department of Radiology, Universitair Ziekenhuis Brussel, Brussel, 1090, Belgium.
Received: 14 June 2022; Accepted: 21 June 2022; Published: 30 June 2022
Article Information
Citation: Sabrina Amaouche, Ziad Letaief, Nico Buls, Sabine Allard, Johan de Mey. The Relation Between ACEI/ARB use and COVID-19 Severity in RT- PCR -Confirmed Cases: A Retrospective Case- Control Study. Cardiology and Cardiovascular Medicine 6 (2022): 374-378.
View / Download Pdf Share at FacebookAbstract
One hypothesis suggests that patients undergoing Angiotensin Converting Enzyme Inhibitor (ACEI) or Angitensin Receptor Blocker (ARB) treatment might be at greater risk for severe COVID-19 disease. This retrospective study aims to elucidate whether patients with Reverse Transcription-Polymerase Chain Reaction (RT-PCR) -confirmed Severe Acute Respiratory Syndrome Coronavirus 2 (SARSCoV- 2) infection undergoing ACEI or ARB treatment present with a more severe clinical presentation or more severe lung injury than other patients, as evaluated by CT thorax scans. Comorbidities related to ACEI or ARB use, including Arterial Hypertension (AHT), Heart Disease (HD), and Diabetes Mellitus (DM), were found to be more frequent (p < 0.05) in the ACEI or ARB users’ group. The odds ratio of ACEI or ARB users for having a more severe clinical presentation was 1.12 (95% [CI] 0.59 -2.13, p = 0.741). For having a severe CT severity score (with a cut-off of 12.5 on a total score of 25), the odds ratio was 1.46 (95% [CI] 0.73 -2.94, p = 0.287). Furthermore, the odds ratio of the mortality outcome was 1.1 (95% [CI] 0.49 -2.48, p = 0.824). Although the group of ACEI or ARB users had more comorbidities, we found no significant association between CT severity, clinical severity, or mortality and the use of these drugs. These findings bolster the argument against ACEI or ARB withdrawal in COVID-19 patients.
Keywords
<p>COVID-19; RTPCR-</p>
Article Details
1. Introduction
At the time of writing, COVID-19-caused by SARS-CoV-2 infection-has claimed more than 280,000 lives. So far, no effective treatment or vaccine has been approved for use against this strain of coronavirus. Its diagnosis relies upon the clinical presentation and biological markers and is confirmed by RT-PCR, which has a reported pooled sensitivity of 89% (95% CI: 81%, 94%; I2 = 90%). The specificity of RT-PCR should be 100% as per the definition of a reference standard.1 The role of CT thorax as a screening tool for COVID-19 is a conundrum; the pooled sensitivity and specificity of CT thorax were 94% (95% CI: 91%, 96%; I2 = 95%) and 37% (95% CI: 26%, 50%; I2 = 83%), respectively [1]. As to sensitivity, CT thorax outperforms RT-PCR and has thus been used as a diagnostic tool for the early detection of COVID-19 in some centers [2]. Belgian guidelines therefore recommend performing CT scans 48 hours after the onset of symptoms on patients with negative RT-PCR and highly suspicious clinical manifestation. According to experts’ opinions, false negative results of RT-PCR can arise from testing that is conducted too early or too late during the course of the disease, [3] as well as from swabs that have been collected or processed improperly [4]. At the Universitair Ziekenhuis Brussel (UZ Brussel), CT scans have been used alongside RT-PCR to gather more information on the extent of pulmonary abnormalities. It has been reported that SARS-CoV-2 enters host cells through Angiotensin-Converting Enzyme 2 (ACE-2) protein receptor binding [5]. The expression of this protein is believed to be upregulated in diabetic and hypertensive patients treated with ACEIs or ARBs [6, 7]. It is important to note that no substantial data corroborate this hypothesis to date. Nevertheless, following the discovery of the entering mechanism of SARS-CoV-2, it was contended that treatment with ACEIs and ARBs could clinically result in more severe presentations of COVID-19. Unsupported by evidence, this hypothesis made headlines immediately; the European and American Societies of Cardiology thus felt compelled to reaffirm the safety of ACEIs and ARBs and argued that treatment should not be discontinued in patients diagnosed with COVID-19 [8]. Conversely, earlier studies reported the beneficial effects of these drugs in Acute Respiratory Distress Syndrome (ARDS). For example, the administration of angiotensin-(1-7) (Ang [1-7]) appeared to prevent Lipopolysaccharide (LPS)-induced ARDS and lung fibrosis in rats [9 Furthermore, SARS-CoV-1 has been documented to downregulate ACE-2 expression in murine lung alveolar type II epithelial cells and thereby compromise the anti-inflammatory activity of the ACE-2 -Ang (1-7) -MasR pathway [10]. The activation of the ACE-2-Ang (1-7)-AT2R and ACE-2-Ang (1-7)-MasR pathways has been proven to counteract the detrimental effects of the ACE-1 -Ang II -AT1R pathway in the lungs. Moreover, LPS-induced ARDS has been demonstrated to reduce the ACE/ACE-2 ratio in rats, which in turn is prevented by the administration of Ang (1-7) or losartan (angiotensin receptor antagonist) [11]. This indicates that ACEIs and ARBs could be beneficial by impeding the ACE-1 -Ang II -AT1R pathway and counteracting the ACE-2 downregulation. In other words, ACEIs and ARBs are likely doing more good than harm in patients with COVID-19. In addition, a pilot clinical trial confirmed that the administration of recombinant human ACE-2 (rh-ACE2) to subjects with ARDS was associated with an increase in surfactant protein D concentration and a decrease in interleukin-6, although no significant changes in the acute physiology or clinical outcomes have been noted [12]. Similarly, a small study on 12 COVID-19 patients reported elevated plasma levels of angiotensin II, which in turn was correlated with the viral load and the degree of lung injury [13]. A phase I clinical trial is currently underway to evaluate the safety of losartan in respiratory failure due to COVID-19 (NCT04335123). A randomized controlled trial is also testing whether stopping/replacing chronic treatment with ACEIs or ARBs improves outcomes in SARS-CoV2-infected patients (NCT04353596).
2. Results
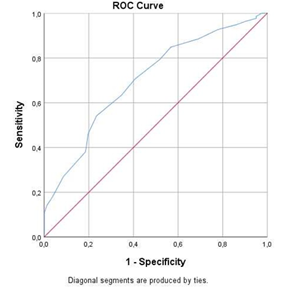
The study population of this retrospective case-control study included 114 men and 185 women. The mean age of the selected patients was 61.9 ± 17.0 years. In terms of ethnicity, the population comprised 199 (66.6%) Caucasians, 43 (14.4%) Black Africans, and 57 (19.1%) Arabs. Of the total population, 68 (27.7%) patients used ACEIs or ARBs; among these patients, 36 (12.0%) were undergoing ACEI treatment, while 32 (10.7%) were undergoing ARB treatment. The overall mortality was 13.4%. Table 3 depicts the frequency distribution of the symptoms and comorbidities among ACEI or ARB users and non-users. No statistically significant differences were found between the ACEI or ARB users and non-users according to age, gender, and ethnicity or the clinical symptoms, including cough, dyspnea, thoracic pain, anosmia, dysgeusia, fever, myalgia, fatigue, rhinitis, sore throat, headache, anorexia, diarrhea, confusion, and sudden fall. Comorbidities related to ACEI or ARB use, such as AHT, CVD, and DM, were relatively more frequent (p < 0.05) in the ACEI or ARB users’ group than the other group. Moreover, Chronic obstructive pulmonary disease (COPD) (p = 0.608) and malignancy (p = 0.439) prevalence did not differ significantly between the two groups. The accuracy of the CT severity score to distinguish between clinical mild-common and severe-critical patients was determined using an ROC analysis (Figure 1). The Area Under the Curve (AUC) was 0.70 (95% [CI] 0.64 -0.77; p < 0.05). The optimal cut-off value was 12.5, with a corresponding sensitivity and specificity of 54.1% and 76.5%, respectively, and an associated Youden’s J index of 0.307. From the AUC, we concluded that the CT severity score is merely an acceptable discriminator between the clinical mild-common type and the severe- critical type.
The cumulative logistic regression conducted to determine the odds ratio of ACEI or ARB users for having a higher clinical severity was 1.12 (95% [CI] 0.59 -2.13, p = 0.741) when adjusted for the confounding factors (age, gender, ethnicity, and the considered comorbidities). The odds ratio of ACEI or ARB users for being in the category of severe CT score versus non-severe CT score was 1.46 (95% [CI] 0.73 -2.94, p = 0.287), which implies that the use of ACEIs or ARBs was not accompanied by higher odds of having either a severe CT score or a higher clinical severity. The adjusted odds ratio of mortality for ACEI or ARB users was 1.1 (95% [CI] 0.49 -2.48, p = 0.824) and thus insignificant.
3. Discussion and Conclusion
To the best of our knowledge, no study has thus far evaluated the potential harmful effects of ACEI or ARB use in COVID-19 patients or the causal relationship between ACEI or ARB use and severe COVID-19. In contrast, the beneficial effects of ACEIs and ARBs in diabetic nephropathy, AHT, and heart failure have been demonstrated [14]. We found that the use of ACEIs or ARBs is not associated with a higher clinical severity or a more severe lung injury in RT-PCR -confirmed COVID-19 patients, as assessed by symptoms and CT thorax scans, respectively. More importantly, ACEIs or ARBs did not seem to affect the mortality outcome of the same patients. However, the following limitations merit consideration: First, our study was limited by a relatively small subpopulation size (N = 68) of ACEI or ARB users. Second, the included patients presented to the emergency department with suspected COVID-19, which suggests that most of them have advanced respiratory symptoms requiring emergency department consultation, which could have created a selection bias of more severe-critical patients in our study population when compared to the general population, impeding the appropriate extrapolation of the results obtained from this study to the general population. This is further confirmed by the high mortality rate (13.4%) in this population. We therefore can neither exclude nor confirm whether individuals using ACEIs or ARBs are more likely to be affected by the mild disease.
Also of note is that patients using these drugs already have a greater cardiovascular burden, as represented by the significant differences in cardiovascular comorbidities frequencies, though ACEI or ARB use was not related to a higher severity in our population. Nonetheless, previous studies linked this cardiovascular burden to a more severe clinical presentation of COVID-19 [15]. In other words, the use of ACEIs or ARBs in the general population could have a blunting effect on these comorbidities, which may be due to the opposing effect of these drugs on the Sars-CoV-2-induced downregulation of the anti- inflammatory ACE-2 protein; however, the relation between ACEI or ARB treatment and Sars- CoV-2 must be further unfolded by larger cohort studies and robust clinical trials. Given the relatively low AUC of the CT severity score ROC analysis, we do not support screening COVID-19 severity using CT thorax scans in RT-PCR -confirmed patients. No current evidence attests that CT severity scoring systems are more accurate than clinical scoring in predicting outcomes. While our findings must be reinforced in future studies that incorporate larger population sizes, we underline the importance of continuing treatment with ACEIs or ARBs in patients with RT-PCR -confirmed COVID-19.
4. Material and Methods
For this retrospective case-control study, the medical records of 299 COVID-19 RT-PCR - positive patients who presented themselves to the emergency department of the UZ Brussel between March 7 and May 3, 2020, were extracted from the database of the radiology department. All emergency department symptomatic patients of all ages with positive RT-PCR results were included. Demographic and clinical differences between these two groups were evaluated. For each participant, the following registered variables were collected from medical records: age, gender, ethnicity, and symptoms. These symptoms included cough, dyspnea, thoracic pain, anosmia, dysgeusia, fever, muscle pain, fatigue, rhinitis, sore throat, headache, diarrhea, anorexia, confusion, and sudden fall. Comorbidities, including AHT, HD, DM, smoking, COPD, malignancy, and chronic kidney disease, were also considered. In addition, ACEI or ARB treatment, RT-PCR results, and CT findings were encoded appropriately. All patients were scanned on an Apex Revolution CT (GE Healthcare, Milwaukee, USA). The non-contrast CT thorax scan protocol consisted of a spiral acquisition (pitch = 1), a rotation time of 0.35 s, and an auto kVp and mA selection (average dose length product = 149 mGy.cm). Two radiologists analyzed the CT scan images, with one of them having more than a decade of experience in radiology. The CT severity score-as established in prior studies16-was based on the extent of lung abnormalities in each lobe. The CT severity score, with a maximum total score of 25, was calculated by totaling the individual scores for each lobe. Scores 0, 1, 2, 3, 4, and 5 correspond to 0 -4%, 5 -25%, 26 -49%, 50 -75%, and >75% of the lobe affected, respectively (Table 1).
|
Score |
1 |
2 |
3 |
4 |
5 |
|
The extent of lung abnormalities |
0 -4% |
5 -25% |
26 -49% |
50 -75% |
>75% |
Table 1: The CT severity score per lobe, with a maximum total score of 25.
The clinical severity scoring of RT-PCR -positive patients was divided into four classes based on the Chinese guidelines that the National Health Commission and State Administration of Traditional Chinese Medicine introduced on March 3, 2020. Class I, or mild type, includes symptomatic patients with negative CT scans. Class II, or common type, consists of symptomatic patients with positive CT scans. Class III, or severe type, comprises patients who fulfilled at least one of the following criteria: respiratory distress, a respiratory rate ≥ 30 times per minute, an SaO2 ≤ 93%, or a PaO2/FiO2 < 300 mmHg at any given time during admission. Finally, Class IV, or critical type, includes patients with shock, respiratory failure requiring mechanical ventilation, and other organ failure requiring ICU monitoring and treatment (Table 2).
|
Class I Mild type |
Class II Common type |
Class III Severe type |
Class IV Critical type |
|
Symptomatic patients |
Symptomatic patients |
Respiratory distress and/or |
Shock and/or |
Table 2: The clinical severity scores of RT-PCR -positive patients.
|
|
ACEI/ARB |
P-value |
||||
|
Yes |
No |
|||||
|
Demographic data |
Gender |
Male |
42 (61.8%) |
143 (61.9%) |
0.983 |
|
|
Female |
26 (38.2%) |
88 (38.1%) |
||||
|
Ethnicity |
Caucasian |
47 (69.1%) |
153 (66.2%) |
0.788 |
||
|
Black African |
10 (14.7%) |
38 (13.9%) |
||||
|
Arab |
11 (16.2) |
46 (19.6%) |
||||
|
Comorbidities |
Smoking |
4 (5.9 %) |
18 (7.8%) |
0.793 |
||
|
AHT |
62 (91.2%) |
64 (27.7%) |
<0.05* |
|||
|
HD |
24 (35.3%) |
27 (16.0 %) |
<0.05* |
|||
|
DM |
34 (50.0%) |
41 (17.7%) |
<0.05* |
|||
|
CRD |
9 (13.2%) |
26 (11.3%) |
0.655 |
|||
|
COPD |
2 (2.9%) |
10 (4.3%) |
1 |
|||
|
Malignity |
2 (2.9%) |
12 (5.2%) |
0.744 |
|||
|
Symptoms |
Cough |
44 (64.7%) |
159 (68.8%) |
0.522 |
||
|
Dyspnea |
44 (64.7 %) |
173 (74.9%) |
0.098 |
|||
|
Thoracic pain |
11 (16.2%) |
56 (24.2%) |
0.161 |
|||
|
Anosmia |
2 (2.9%) |
11(4.8%) |
0.739 |
|||
|
Dysgeusia |
4 (5.9%) |
9 (3.9 %) |
0.501 |
|||
|
Fever |
42 (61.8%) |
158 (68.4%) |
0.307 |
|||
|
Myalgia |
19 (27.9%) |
63 (27.3%) |
0.914 |
|||
|
Fatigue |
29 (42.6%) |
88 (38.1 %) |
0.499 |
|||
|
Rhinitis |
4 (5.9%) |
17 (7.4%) |
0.739 |
|||
|
Sore throat |
6 (8.8%) |
30 (13.0%) |
0.354 |
|||
|
Headache |
11 (16.2%) |
51 (22.1%) |
0.291 |
|||
|
Anorexia |
23 (33.8%) |
64 (27.7%) |
0.329 |
|||
|
Diarrhea |
9 (13.2 %) |
48 (20.8%) |
0.164 |
|||
|
Confusion |
4 (5.9%) |
15 (6.5%) |
1 |
|||
|
Sudden fall |
7 (10.3%) |
13 (5.6%) |
0.176 |
|||
|
Mean (±SD) |
Mean (±SD) |
|||||
|
Age |
70.04 (± 12,951) |
63.09 (± 19,237 ) |
0.128 |
|||
|
Two tailed p-values of demographic variables, symptoms, and comorbidities were determined using Pearson’s Chi-square (N > 5) or Fisher’s exact (N ≤ 5) test. Age difference was assessed using the Mann-Whitney U test. AHT: arterial hypertension; HD: heart disease; DM: diabetes |
||||||
Table 3: Demographic variables, comorbidities, and symptoms: A comparison between ACEI or ARB users and non-users.
The institutional review board of UZ Brussel (ethics committee BUN 2020/106) approved this retrospective case-control study. Written informed consent was waived. IBM SPSS Statistics for Windows Version 23.0 (Armonk, NY: IBM Corp) was used for statistical analysis. The ACEI or ARB users were compared with the non-users according to age, gender, ethnicity, comorbidities, and symptom frequency using Pearson’s Chi-square test and Fisher’s exact test. The Mann-Whitney U test was performed to compare the age distribution between the two groups. The optimal cut-off value of the CT severity score to detect a clinical severe- critical type was defined by calculating Youden’s J index on the cut points that an ROC curve analysis yielded. For this purpose, we considered the mild and common patients one group and the severe and critical patients the other group. In addition, a cumulative logistic regression was conducted to create a model wherein the potential confounding factors of age, gender, ethnicity, and comorbidities were considered. In this model, the cumulative odds ratio of ACEI or ARB users for having a higher clinical severity score of COVID-19 infection was determined. A binomial logistic regression was also conducted to determine the association between ACEI or ARB use and having a severe CT score. To evaluate the association between ACEI or ARB use and the current mortality outcome, a Poisson logistic regression was performed, using the standard α = 0.05 cut-off and a 95% Confidence Interval (CI).
Data Availability
The data that support the findings of this study are available from the corresponding author on request.
Author Information
Sabrina Amaouche and Ziad Letaief contributed to this work equally.
Affiliations
Department of Radiology, Universitair Ziekenhuis Brussel, 1090, Brussel, Belgium
Sabrina Amaouche, Ziad Letaief, Nico Buls, and Johan de Mey
Department of Infectiology, Universitair Ziekenhuis Brussel, 1090, Brussel, Belgium
Sabine Allard
Author Contributions
Sabrina Amaouche and Ziad Letaief collected the data, analyzed the results, and drafted the article. Sabine Allard, Nico Buls, and Johan de Mey reviewed the manuscript. Nico Buls reviewed the statistical analysis. Finally, Sabine Allard, Nico Buls, and Johan de Mey approved the final version of the article.
Corresponding Author
Direct correspondence to Sabrina Amaouche, Ziad Letaief, and Nico Buls.
Additional Information
The authors declare no competing interests.
References
- Kim H, Hong H, Yoon SH. Diagnostic Performance of CT and Reverse Transcriptase- Polymerase Chain Reaction for Coronavirus Disease 2019: A Meta-Analysis. Radiology 201343 (2020).
- Esposito A, Anna P, Scotti GM, et al. Why is chest CT important for early diagnosis of COVID-19? Prevalence matters. medRxiv 20047985 (2020).
- Wikramaratna P, Paton RS, Ghafari M. et al. Estimating false-negative detection rate of SARS-CoV-2 by RT-PCR. medRxiv 20053355 (2020).
- Xiao AT, Tong YX, Gao C, et al. Dynamic profile of RT-PCR findings from 301 COVID-19 patients in Wuhan, China: A descriptive study. Journal of Clinical Virology 127 (2020).
- Lu R, Zhao X, Juan L, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. The Lancet 395 (2020): 565-574.
- Wan Y, Shang J, Graham R, et al. Receptor Recognition by the Novel Coronavirus from Wuhan: An Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J Virol 94 (2020).
- Li XC, Zhang J, Zhuo JL. The vasoprotective axes of the renin-angiotensin system: Physiological relevance and therapeutic implications in cardiovascular, hypertensive and kidney diseases. Pharmacol Res 125 (2017): 21-38.
- Sommerstein Rami, Kochen Michael M, Messerli Franz H, et al. Coronavirus Disease 2019 (COVID-19): Do Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers Have a Biphasic Effect? Journal of the American Heart Association 9 (2020): e016509.
- Cao Y, Liu Y, Shang J, et al. Ang-(1-7) treatment attenuates lipopolysaccharide-induced early pulmonary fibrosis. Lab Invest 99 (2019): 1770-1783.
- Imai Y, Kuba K, Penninger JM. The discovery of angiotensin-converting enzyme 2 and its role in acute lung injury in mice. Experimental Physiology 93 (2008): 543-548.
- Asperen RMW, Rene L, Bos AP, et al. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin-(1 -7) or an angiotensin II receptor antagonist. The Journal of Pathology 225 (2011): 618-627.
- Khan A, BenthinC, Zeno B, et al. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Critical Care 21 (2017): 234.
- Liu Y, Yang Y, Cong Z, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 63 (2020): 364-374.
- Jennings GL. COVID, ACE inhibitors/ARBs, and cardiovascular diseases. The Medical Journal of Australia 1 (2020).
- Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS- CoV-2: A systematic review and meta-analysis. International Journal of Infectious Diseases 94 (2020): 91-95.
- Pan F, Ye T, Sun P, et al. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19). Radiology 295 (2020): 715-721.


 Impact Factor: * 5.6
Impact Factor: * 5.6 Acceptance Rate: 74.36%
Acceptance Rate: 74.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 