The Role of Thrombosis and Vessel Injury in Acute Myocardial Infarction: Current Standard of Care and Therapeutic Options
Ian Vargas, Samuel A Wickline, Hua Pan*
The USF Health Heart Institute, Morsani College of Medicine, University of South Florida, FL, USA
*Corresponding author: Hua Pan, Assistant Professor of Medicine, The USF Health Heart Institute, Morsani College of Medicine, 560 Channelside Dr. Tampa, FL, 33602, USA
Received: 20 August 2021; Accepted: 30 August 2021; Published: 15 September 2021
Article Information
Citation: Ian Vargas, Samuel A Wickline, Hua Pan. The Role of Thrombosis and Vessel Injury in Acute Myocardial Infarction: Current Standard of Care and Therapeutic Options. Cardiology and Cardiovascular Medicine 5 (2021): 502-529.
View / Download Pdf Share at FacebookAbstract
Despite recent advances in therapy, acute myocardial infarction (AMI) remains a major cause of morbidity and mortality. Among the most significant pathologic mechanisms contributing to AMI are vessel injury and thrombosis, which are prime targets for therapies in the clinical setting. Endothelial dysfunction precedes atherosclerotic plaque rupture and/or erosion, which promote platelet plug formation and the activation of the coagulation cascade and thrombin that leads to coronary occlusion. In current clinical practice, two general strategies have been developed for treating this acute, initial presenting event: mechanical and pharmacologic intervention. The former involves physical disruption of the clot through percutaneous coronary intervention (PCI), while the latter involves administration of various thrombolytic and anticlotting agents. Prior clinical trials have solidified primary PCI and stenting as the definitive method of treatment of AMI. Unfortunately, primary PCI is limited by relatively low availability, which has prompted the study of alternative forms of PCI and pharmacologic therapy such as fibrinolysis. However, many patients fail to achieve adequate revascularization and suffer various morbidities in the days to weeks following AMI. To this end, recent studies have examined adjunctive interventions aimed at inducing vessel and myocardial repair such as angiogenic factors and stem cell injections, among others. This review will describe the landscape of thrombosis and vessel injury in AMI, from current clinical practice for targeting these mechanisms to more recent studies advancing basic knowledge in the field.
Keywords
<p>AMI, Thrombosis, Vessel injury, Reperfusion, Angiogenesis</p>
Article Details
1. Introduction
According to the CDC, heart disease remains the leading cause of death in the U.S., contributing to over 600,000 deaths per year. Among the most common complications associated with heart disease is acute myocardial infarction (AMI), with an approximate annual incidence of over one million cases, about one-third of which are re-infarctions according to the AHA. In addition, patients who experience AMI manifest a 30% higher risk of all-cause death within five years after the ischemic event despite recent advances in therapeutic options for managing and treating AMI [1]. Although therapeutic advances have reduced the overall mortality rate after AMI over the past several decades, the incidence of heart failure post-MI has increased over the same time frame [2].
Among the most significant pathologic mechanisms that contributes to the morbidity and mortality of AMI are thrombosis and vessel injury. These processes are preceded by the development of an atherosclerotic plaque, which over time can become vulnerable to rupture or erosion and initiate the formation of a coronary thrombus that can lead to AMI. Current clinical guidelines have established mechanical revascularization via primary precut-aneous coronary intervention (PCI) and stenting as the preferred treatment for ST-segment elevation myocardial infarction (STEMI), which yields a 2% absolute risk reduction for lowering mortality versus fibrinolysis alone [3]. Unfortunately, primary PCI remains limited by relatively scarce availability, as only about 25% of STEMI patients in the U.S. receive acute primary PCI [3].
Thus, many thrombolytic and fibrinolytic therapies serve as alternative strategies to reestablish coronary patency and blood flow. Importantly, not all patients who undergo PCI achieve adequate revascularization, and suffer greater risk of deleterious ventricular remodeling [4]. Indeed, incomplete revascularization may occur in as many as one-third of patients receiving PCI or CABG [4]. While conventional revascularization methods can reduce the extent of myocardial infarctions, they do little to preserve global left ventricular function [5]. Herein, we examine the role that vessel injury plays in the pathogenesis of AMI, assess current standards of care for managing and treating vessel injury, and review proposed novel therapeutic strategies.
2. Pathogenesis of Thrombosis & Vessel Injury in AMI
2.1 Atherosclerotic plaque formation and disruption
The dominant underlying factor in the acute onset of coronary thrombosis is atherosclerosis whose risk factors and evolution have been well-described [6-8]. Endothelial activation and inflammation is an early phenomenon promoted by disturbed flow in high prevalence vascular territories that involves upregulation of NF-kB and other chemotactic factors and cytokines. The expression of cell adhesion receptors such as VCAM-1 and ICAM-1 together with the associated endothelial dysfunction facilitates adherence and transmigration of monocytes, which become activated to foamy macrophages after uptake of oxidized LDL by upregulated scavenger receptors. Thickening of the vessel intima occurs over time culminating in the formation of a complex atheroma consisting of a fibrous cap covering a core of lipids, migrated smooth muscle cells, and inflammatory cells [9]. The mechanical disadvantage of a lipid-rich and necrotic core of the atheroma pre-disposes to eventual rupture and subsequent thrombus formation due to cap thinning from destruction of supporting structural fibrous tissues such as collagen and elastin [10]. Following plaque cap rupture or erosion from endothelial sloughing, exposure of blood clotting factors to the prothrombotic plaque milieu leads to coronary artery occlusion.
2.2 Atherosclerotic plaque disruption
Once the atheroma has formed, thrombus can form via plaque rupture or erosion. The more common of these is plaque rupture, in which a fibrous cap tear promotes luminal thrombus formation [6]. This process is often asymptomatic, but given the appropriate contents of the thrombus, can increase in size to occlude luminal blood flow [11]. Many mechanisms have been postulated for the initial events that lead to atherosclerotic plaque rupture, among which is the notion that vessel stenosis creates hemodynamic conditions predisposing to focal collapse of the arterial wall, which in turn degrades the integrity of the plaque, leading to rupture [12].
Additional theories have invoked mechanical shear stress and rupture of the vasa vasorum as potential mechanisms [13]. Strong evidence also exists for the role that macrophage infiltration plays in plaque rupture, which activates several different inflammatory signaling pathways that promote further damage [14, 15]. Macrophages also produce matrix-degrading metalloproteinases (MMPs) that degrade collagen and weaken the fibrous cap, priming it for rupture [15]. Ultimately, all of these mechanisms contribute to some degree to the rupture, and a clearer understanding of the process has been essential in developing appropriate therapy in managing ischemic heart disease and myocardial infarction. Interestingly, it has been suggested that the contents of coronary thrombus are correlated with ischemic time, namely platelets and fibrin, which carries implications for treatment options [16]. Less commonly, erosion of the plaque accounts for the formation of coronary thrombosis. Unlike rupture, erosion is not associated with hyperlipidemia and is more common in pre-menopausal women [17].
In addition, erosion is thought to occur with intact fibrous cap, is driven more by smooth muscle cells versus macrophages, is less often occlusive, and appears to be more frequent in non-STEMI [18]. In this process, a thrombus forms on a defect in the endothelial layer that covers the plaque. Despite being less common, it is still clinically significant, as up to 30% of ST-segment elevation myocardial infarctions are thought to be related to plaque erosion [19].
2.3 Role of platelets in thrombus formation
In the presence of healthy endothelium, atherothrombosis is inhibited by various antiplatelet and anticoagulant molecules [20]. However, upon plaque rupture, the exposure of damaged endothelial cells to circulating platelets initiates a cascade of hemostatic events that ultimately promotes the formation of a platelet-rich thrombus that can lead to vessel occlusion and ischemic damage [21]. This cascade begins by the binding of von Willebrand factor (vWF) to exposed collagen in the endothelial defect. Circulating platelets bind to this complex via interaction of the glycoprotein (Gp) Ib-alpha receptor to the A1 domain of vWF [20]. This complex is strengthened by the binding of the platelet receptors GpIa/IIa and GpVI to collagen. These interactions promote a conformational change in the platelets that induces degranulation of various substances, including ADP, calcium, and thromboxane A2. ADP is of particular interest, as its binding to its receptor stimulates the expression of Gp IIb/IIIa on the platelet surface. Fibrinogen binding to this receptor promotes platelet aggregation and cross-linking, which concludes the events of primary hemostasis, the initial event leading to thrombus formation after plaque rupture.
The crucial final steps of this process have become targets of pharmacotherapies directed at inhibiting this platelet plug formation and subsequent downstream activation of the clotting cascade [22]. Not only does ADP release promote fibrinogen receptor expression, but it also plays a critical role in platelet responsiveness to thromboxane A2 and thrombin, as in the absence of ADP receptors, platelet aggregation is decreased [20]. As such, most of the anti-platelet therapies that are commonplace in clinical practice are directed at ADP receptors or Gp IIb/IIIa.
2.4 Thrombin and the coagulation cascade
Following the formation of the platelet plug, activation of the coagulation cascade contributes to further thrombogenicity. This process is largely initiated by tissue factor (TF) and terminated with the conversion of prothrombin to thrombin, which forms the fibrin clot. TF is expressed both on subendothelial tissues and on endothelial cells during inflammatory conditions, and as such is abundant during conditions leading to AMI [23]. It is also expressed by macrophage-derived foam cells in atherosclerotic plaques, an additional source that becomes exposed during plaque rupture [23].
It activates the extrinsic pathway of the coagulation cascade, a key player in the development of coronary thrombi. Thrombin, upon its activation by factor Xa, not only promotes the formation of the fibrin monolayer that covers the damaged endothelial surface, but also activates PAR-1 and PAR-4, which stimulates conformational changes in platelets, dense granule release, formation of thromboxane A2 that promotes vasoconstriction, and GpIIb/IIIa activation [20]. Due to the many pathogenic roles of thrombin in the process of thrombus formation and endothelial dysfunction, it has become an attractive target in pharmacotherapies in AMI.
Figure 1: The pathophysiology of thrombus formation in AMI. Atherosclerotic plaques underlie most coronary thrombi, the formation of which has been well described. Once an atheroma is formed, the plaque is at risk of disruption via rupture or erosion. The former is more common and occurs when a cap tear promotes luminal thrombus formation. This is often asymptomatic but can increase in size to occlude luminal blood flow given the appropriate contents. The resulting endothelial dysfunction promotes thrombus formation through platelet aggregation and ultimately, the activation of the clotting cascade and thrombin. Many of the pharmacologic therapies directed at coronary thrombosis in AMI target one or several of these mechanisms.
3. Current Standard of Care in Managing Thrombosis in AMI
3.1 Percutaneous coronary intervention (PCI)
In the management of an acute ischemic event as AMI, there are two broad strategies used to target the developed thrombosis- mechanical and pharmacological intervention. Both have been studied extensively, and the former has become the favored strategy. More specifically, percutaneous coronary intervention (PCI) has long been recognized as the preferred first-line treatment for AMI [23-25]. Primary PCI, the urgent use of coronary stenting or angioplasty without previous fibrinolytic or anti-GpIIb/IIIa agents, is the currently recommended reperfusion therapy by the European Society of Cardiology provided it can be performed within 120 minutes of first medical contact [24]. However, timely administration of primary PCI is not always feasible, suggesting the need for additional options in targeting thrombotic clots. In these cases, pharmacologic intervention with thrombolytics and GpIIb/IIIa inhibitors in conjunction with immediate PCI, termed facilitated PCI, is another option [26]. Yet another is fibrinolytic agents followed by transfer to a PCI capable hospital or rescue PCI, called pharmaco-invasive PCI [26]. Although primary PCI remains the gold standard, the aforementioned sub-types of PCI have been compared, the efficacy of which will be summarized.
3.1.1 Facilitated PCI: The promise of facilitated PCI was recognized upon the validation of thrombolysis and primary PCI as effective treatments for STEMI [27]. Combined pharmacologic and mechanical disruption of clots was thought to act synergistically to enhance clinical outcomes. However, studies since have not shown the benefits that were hoped for. Among the landmark studies in the comparison of primary versus tenecteplase facilitated PCI was the ASSENT-4 trial, which examined STEMI patients presenting with symptoms of less than 6 hours [28]. Unfortunately, this was prematurely terminated due to significantly higher mortality rates in the facilitated PCI group, with stroke and ischemic cardiac complications accounting for the majority of these deaths [28]. In the LIPSIA-STEMI clinical trial conducted five years later, STEMI patients with less than 3 hours of symptom onset were found to have greater infarct sizes and a trend toward higher adverse events with facilitated PCI with tenecteplase vs primary PCI, despite better pre-interventional TIMI scores [29]. No difference was observed in ST-segment resolution.
In the FINESSE trial, facilitated PCI with abciximab or a combination of reteplase and abciximab also failed to demonstrate clinical improvement in adverse events or mortality rates versus primary PCI [30]. The only benefit to the combination facilitated PCI group was an earlier ST-segment resolution [30]. The STEPP-AMI trial found an increase in the primary end point of death, cardiogenic shock, reinfarction, recurrent revascularization, and congestive heart failure at 30 days in Indian patients treated with tenecteplase facilitated PCI, although infarct-related artery patency was enhanced significantly in the same group [31]. The finding of greater artery patency rates with facilitated PCI is not unusual, as several other studies have demonstrated this trend despite not demonstrating functional improvement or acceptable safety profiles [32].
The general inferiority of facilitated PCI may be related to variations in door to balloon times, as some time points indicate its safety and efficacy. One study has shown that in STEMI patients treated with facilitated PCI with a door to balloon time of greater than 90 but less than 150 minutes, major adverse clinical events were actually reduced compared to primary PCI [33]. Likewise, the results of the FINESSE trial also suggested potential benefits with an intermediate door-to-balloon time [30, 34]. Still, the composite data is enough to contra-indicate facilitated PCI when the option for primary PCI is available, but additional research regarding specific time points may be warranted to elicit any concealed benefit. The use of different thrombolytic agents or platelet inhibitors in these contexts may also be a cause of future investigation.
3.1.2 Pharmacoinvasive PCI: Though clinical benefits of facilitated PCI have yet to be shown over primary PCI, early recanalization with pharmacologic agents with subsequent rapid transfer to a PCI capable center is still a viable option in centers where primary PCI is not available. While the differences between facilitated and pharmacoinvasive PCI may seem vague, the key difference is the decision to perform PCI is already planned before administering thrombolytics in the former, while the latter represents a sort of invasive back-up plan implying transportation to a PCI capable hospital [35]. An observational study has demonstrated the superiority of the pharmacoinvasive approach over fibrinolysis alone, with an absolute risk reduction in hospital mortality of 5.0% [36]. Trials comparing its feasibility to standard care have also been conducted. The TRANSFER-AMI trial investigated STEMI patients receiving fibrinolytic therapy in hospitals without PCI performing capability to compare the pharmacoinvasive strategy versus standard care [37]. At 30 days, the former group demonstrated a lower risk of death, reinfarction, recurrent ischemia, congestive heart failure, and cardiogenic shock, with no difference in incidences of major bleeding [37]. These results suggest the benefit of immediate transfer for PCI within 6 hours of giving fibrinolytic agents. The CARESS-AMI trial likewise indicated benefits for immediate transfer. In STEMI patients treated with reteplase, abciximab, heparin, and aspirin, immediate transfer for PCI was found to reduce rates of death, reinfarction, or refractory ischemia at 30 days [38]. Incidence of major bleeding was found to be insignificant between the two groups [38].
3.1.3 Rescue PCI: In the event that fibrinolysis fails, mechanical reperfusion can still be attempted, which is defined as rescue PCI. This practice was originally considered controversial due to high arterial re-occlusion rates and increased mortality, but it was unclear whether these occurrences were related to rescue PCI itself, as these patients often had poor prognostic factors regardless [39]. There are now a few studies that suggest its possible benefit, though overall body of literature is more scare than for other types of PCI. The MERLIN trial randomized STEMI patients who had failed to respond to fibrinolysis to emergency rescue PCI or conservative treatment [40]. Although rescue PCI did not improve 30-day all-cause mortality, the secondary end-point of death, re-infarction, stroke, and subsequent revascularization was found to be reduced, mostly entirely due to major reductions in subsequent revascularization [40]. There was no observed difference in LV systolic function at 30 days, and more strokes and transfusions were found in the rescue group. Several problems were noted with this study, including too early randomization, a low use of stents and Gp IIb/IIIa inhibitors, and an abnormally high absolute mortality rate [39]. Noting this, the REACT trial was conducted comparing rescue PCI in STEMI patients who failed to achieve reperfusion within 90 minutes of thrombolytics [41]. These were randomly assigned to repeated thrombolysis, conservative treatment, or rescue PCI. The primary end-point of death, reinfarction, stroke, or severe heart failure within 6 months was lowest in the PCI group, with the most significant hazard ratio being observed between rescue PCI and repeated thrombolysis. No differences were found in all-cause mortality. The only adverse event increased in the rescue PCI group was nonfatal bleeding. Due to the superior design of this study, rescue PCI is now recommended in more scenarios [39]. Future studies will be needed to examine the viability of rescue PCI in specific sub-populations of patients, as well as its efficacy beyond certain time points.
|
Characteristics |
Primary PCI |
Facilitated PCI |
Pharmaco-invasive PCI |
Rescue PCI |
|
Advantages |
· Recommended by AHA for STEMI patients who present with symptoms <12 hours in onset and door-to-balloon of 120 minutes · Compared to fibrinolysis alone, significantly redu-ces short-term mortality, reinfarction, stroke, and bleeding events · Compared to other forms of PCI, reduced risk of death, intracranial |
· Improved coronary artery patency rates versus primary PCI · May reduce adverse clinical events in specific door-to-balloon time windows |
· Superior to fibrinoly-sis alone in reducing hospital mortality · Lower risk of death, reinfarction, or recurrent ischemia versus standard care · Greater ST-segment resolution versus primary PCI |
· May improve death, reinfar-ction, Stroke, or severe heart failure versus thrombolys · May improve post-MI revascularization |
|
Limitations |
· Geographical barriers: not readily available in many locations · Logistical barriers: scarce resources compli-cate timely delivery |
· Higher adverse bleeding events · No mortality benefit versus primary PCI |
· Indicated only when delay to primary PCI exists · Most studies com-paring pharmaco-invasive and primary PCI are observa-tional |
· High arterial reocciusion rates · High absolute mortality rate · Increased risk of stroke and transfusions |
Table 1: Summary of the advantages and limitations of various types of PCI. Primary PCI is currently recommended as the treatment of choice in STEMI patients provided it can be administered in a timely fashion, beyond which its efficacy is reduced. However, recent studies have highlighted the advantages of the pharmaco-invasive approach, which involves a conjunction of mechanical and pharmacologic intervention. Early trials failed to show any benefit for this approach, mainly due to higher rates of death and adverse clinical events such as bleeding and ischemic events. More recent data has challenged this notion, demonstrating such benefits as higher coronary artery patency and similar clinical outcomes. Data on rescue PCI is relatively scarce and is confounded by the fact that patients who undergo this often have predisposing poor prognostic factors. Nevertheless, it may provide a benefit versus conservative management alone.
3.2 Thrombolytic therapy
PCI is rarely performed alone, as the administration of various anti-platelet and anti-thrombotic agents often accompanies the procedure. Direct thrombin inhibitors have been attractive options in treating AMI due to the multi-faceted role thrombin plays in the atherothrombosis. Activated by factor Xa and its cofactor factor Va, thrombin cleaves fibrinogen to fibrin and activates factor XIII, which stabilizes platetlet-rich thrombi and promotes fibrin cross-linking [20]. Among the thrombin inhibitors used during PCI, bivalirudin has emerged as a drug of extensive study and its use has become an accepted standard of care in primary PCI [42]. However, there remains ambiguity about whether bivalirudin is superior to the more conventional agent heparin [43]. Owing to the HORIZONS-AMI trial in 2011, which demonstrated greater efficacy and safety of bivalirudin monotherapy versus heparin, the use of bivalirudin became standard clinical guidelines for treating STEMI patients [44, 45]. The definitive superiority of bivalirudin has been questioned in several trials since.
In a clinical trial comparing the use of bivalirudin alone to heparin and glycoprotein IIb/IIIa inhibitors, bivalirudin was found to reduce 30-day adverse clinical events by 2.9%, mainly due to its less frequent rate of major bleeding [45]. The 30-day cardiac and all-cause mortality was also significantly decreased in the bivalirudin group, and no significant difference was observed in risk of acute stent thrombosis at the same time point [45]. However, the HEAT-PPCI study suggests the superiority of unfractionated heparin versus bivalirudin [42]. In patients undergoing primary PCI, patients receiving heparin experienced reduced rates of major cardiac adverse events at 28 days and no difference in bleeding complications versus bivalirudin, which may indicate the superiority of unfractionated heparin given its much lower cost [42]. These findings have been challenged by the BRIGHT trial, which likewise compared bivalirudin vs heparin in primary PCI, this time with or without tirofiban, a Gp IIb/IIIa antagonist [46]. The rate of adverse clinical events at 30 days was significantly lower in the bivalirudin group, with an even greater reduction being observed when compared to heparin plus tirofiban [46]. The 30-day bleeding rate was also significantly lower in the bivalirudin group, but no difference was found in major adverse cardiac or cerebral events. The BRAVE-4 trial additionally compared bivalirudin plus prasugrel versus heparin plus clopidogrel administration in primary PCI, but was terminated prematurely upon finding no significant difference in mortality, stent thrombosis, or ischemic or hemorrhagic complications at 30 days [47]. The most recent trial comparing the two, the VALIDATE-SWEDEHEART trial, also established no appreciable difference in all-cause mortality, MI, or major bleeding complications [48]. The BRIGHT-4 trial comparing bivalirudin and heparin in emergency PCI is currently underway and results are pending.
Various anti-platelet drugs are often co-administered with these agents, including glycoprotein IIb/IIIa inhibitors and ADP receptor antagonists. Among the Gp IIb/IIIa inhibitors gaining attention is Abciximab, a monoclonal antibody against the fibrinogen receptor on platelets, which has been compared to lower weight Gp IIb/IIIa agents tirofiban and eptifibatide in a previous meta-analysis [49]. Among the randomized trials included in the study, Abciximab was not found to improve TIMI flow grade 3, ST-segment resolution, 30 day mortality, reinfarction, or bleeding complications after primary PCI. Though Abciximab had previously shown impressive benefits when used in primary PCI, the lower weight agents are less expensive and only reversibly inhibit platelet aggregation, which suggests a reduced risk for adverse clinical events [49]. This combined with the demonstrated lack of significant differences between the two may indicate the use of small molecules in the setting of primary PCI.
Figure 2: A comparison of the mechanism of action of heparin and bivalirudin in thrombin inhibition. Inhibition of thrombin occurs through the interaction of three different binding sites – two allosteric exosites and the active site. Heparin inhibits thrombin by binding to the second exosite, stabilizing the interaction between antithrombin III and thrombin. Additionally, it can inhibit factor Xa directly, preventing thrombin activation. Conversely, bivalirudin employs a two-fold mechanism of inhibition. It binds to the first exosite, the fibrinogen binding site, via its carboxyl-terminal domain which promotes a change to competitive inhibition of the active site.
3.3 Fibrinolytic therapy
Various factors make the conductance of PCI infeasible in many circumstances, which necessitates the need for alternative methods of reperfusion. Fibrinolytic therapy is another method of inducing reperfusion that is not preferred but is indicated in some instances. Under normal physiologic condi-tions, plasminogen is released by the liver into the circulation and activated via tissue plasminogen activator (tPA), which is one of the natural mechanisms by which fibrin clots gets lysed [20]. This process is inhibited in stable thrombi however, as fibrin cross-links mask the binding sites for tPA, impairing a key method of fibrinolysis in AMI [20]. Additionally, plasminogen activator inhibitor-1 functions to inhibit tPA, and high levels of this enzyme during AMI have been associated with higher mortality rates [20, 50]. With impaired natural fibrinolytic activity, the need for pharmacologic intervention to induce fibrin clot dissolution was recognized and met by various fibrinolytic agents, the benefits and drawbacks of which will be discussed below.
Current clinical guidelines indicate the use of fibrinolysis if PCI cannot be performed within 90 minutes of door to balloon time [3]. Prior studies have demonstrated that timely administration of fibrinolysis leads to absolute reductions in 35-day mortality of up to 37 per 1000 patients experiencing anterior STEMI [51]. Several fibrinolytic agents have been approved and studied in the management of AMI, including alteplase, reteplase, tenecteplase, and streptokinase [51]. Most of these act through fibrin binding and the subsequent activation of plasminogen to plasmin. Alteplase is a fibrin specific enzyme with an increased affinity for plasminogen that has no associated allergic or hypotensive effects, the potential of which for use in AMI was recognized early on [52]. The use of alteplase has been compared to tenecteplase, a genetic variation of the former possessing higher fibrin specificity and slower plasma clearance [28]. Additionally, due to being genetically engineered, tenecteplase demonstrates a much higher resistance to inhibition by plasminogen activator inhibitor-1, which negates one of the means by which natural fibrinolysis is impaired in AMI [53]. In the ASSENT-2 trial, tenecteplase treated patients were found to have fewer non-cerebral bleeding events and reduced need for blood transfusions, but no difference in 30-day mortality rates and intracranial hemorrhages were observed [28]. Additionally, no significant difference was observed between the two groups at one-year follow-up [52]. A separate study corroborated these findings, showing no difference in 30-day mortality or hemorrhagic events in Chinese STEMI patients [54]. Overall, both these agents have been found to have similar efficacy, with tenecteplase possessing a slightly lower bleeding risk. Alteplase has also been compared to reteplase, a mutant tissue plasminogen activator (tPA) that has a longer half-life [55]. A clinical trial showed no significant difference in 30-day mortality rate or stroke occurrence between the two when given to STEMI patients within 6 hours [55]. These results again suggest that there is no apparent benefit to one particular fibrinolytic agent over another, but that there is likewise no major drawback either. The exception is streptokinase, which has generally not been preferred to alteplase, but is still indicated in some cases [56]. This is partly due to its adverse drug reactions [56, 57]. Streptokinase is another inducer of plasmin, the viability of which as a fibrinolytic therapy was established in the 1980s [58, 59]. Clinical trials have since demonstrated the improvements of coronary blood flow and microvascular resistance upon intracoronary streptokinase infusion [60]. It has also been shown to limit infarct size and ventricular function at 6 months compared to no additional therapy [60]. However, because of adverse reactions relating to antistreptococcal antibodies, streptokinase is generally not preferred over other fibrinolytic agents [52]. A more in-depth review of fibrinolytic therapy has been conducted by Jinatongthai et al., which demonstrates no clear advantages of alteplase, reteplase, and tenecteplase for reperfusion in fibrinolysis, as well as the inferiority of streptokinase [61].
Furthermore, most recent guidelines indicate the addition of the anti-platelet agents aspirin and clopidogrel with fibrinolytic therapy [22]. Once metabolized, clopidogrel irreversibly prevents ADP binding to the platelet receptor P2Y12, thus inhibiting the activation of the fibrinogen receptor GpIIb/IIIa and blocking later steps of primary hemostasis. The efficacy of clopidogrel was established in the COMMIT clinical trial that demonstrated a significant reduction in all-cause mortality, reinfarction, or stroke at two weeks in STEMI patients [22]. The CLARITY-TIMI 28 trial likewise showed a reduced presence of an infarct-related artery as well as lower death or recurrent MI in clopidogrel treated patients [62]. Dual anti-platelet therapy with aspirin and clopidogrel to complement fibrinolysis has since become routine practice, but several other anti-platelet agents have also been examined. Clopidogrel has since been compared with ticagrelor, another P2Y12 inhibitor that provides faster and more consistent inhibition of the receptor [63]. Despite these advantages, no significant reduction in bleeding events was observed in a clinical trial of STEMI patients under the age of 75 receiving clopidogrel or ticagrelor, respectively [63]. Results were similar for major bleeding events, fatal and intracranial bleeding events, and death from vascular causes in both groups, which at least demonstrated the noninferiority of ticagrelor [63]. Prasugrel is another agent with similar mechanism of action to clopidogrel. In a randomized trial, platelet reactivity was found to be lower in STEMI patients treated with prasugrel versus clopidogrel, suggesting a faster and stronger platelet inhibition with the former [64]. Future studies will be needed to determine if this greater potency will lead to improved mortality or complications. Lastly, though still commonly used in other methods of reperfusion, GpIIb/IIIa inhibitors have been shown to provide no benefit in fibrinolysis and actually may be contra-indicated due to their increased bleeding risk [61].
|
Fibrinolytic Agent |
Alteplase |
Reteplase |
Tenecteplase |
Streptokinase |
|
Features |
Serine protease specific to fibrin |
Non-glycosylated form of tPA |
Genetic variation of alteplase |
Generally inferior to other fibrinolytic agents, but effective compared to no additional therapy |
|
Enhances activation of plasminogen |
Less fibrin specific than alteplase, which facilitates clot penetration |
Higher fibrin specifi-city and slower plasma clearance |
Higher incidence of aller-gic reactions |
|
|
Half-life of 3-4 minutes |
Half-life of 13-16 minutes |
Not as readily inhi-bited by plasminogen activator inhibitor-1 |
Antistreptococcal anti-bodies necessitate higher doses |
|
|
Most common fibrinolytic agent given in AMI |
Clinical efficacy similar to alteplase |
Clinical efficacy simi-lar to alteplase, but fewer bleeding comp-lications |
Bleeding side effects fairly common |
Table 2: Various fibrinolytic agents commonly used in AMI treatment [53]. Clinical trials have demonstrated no clear advantages or disadvantages to alteplase, reteplase, or tenecteplase in clinical outcomes or reperfusion. Streptokinase is generally not preferred due to its side effect profile but is beneficial versus conservative management.
4. Current Areas of Research in Managing Thrombosis in AMI
Unfortunately, a sizable minority of patients do not respond well to conventional revascularization methods. The phenomenon of incomplete revascul-arization is estimated to occur in as many as one-third of patients receiving PCI or CABG [4]. Among those who have undergone PCI, an increase in major adverse cardiac events as high as 10% has been observed [4]. In fact, most cases of mortality following AMI do not involve the initial event itself, but from the complications that develop in the subsequent days to weeks, including arrhythmias, pericarditis, or interventricular or free wall rupture due to weakening of the myocardial wall [65-66]. In addition to ischemic damage, recent studies have suggests that AMI events induce endothelial dysfunction, inflammation, and neointimal progress-sion that drive these pathologic processes [67].
Due to the occasional insufficiency of traditional revascularization methods in achieving adequate myocardial reperfusion, alternative treatments have emerged that target the complications of thrombosis in different ways. Clinical guidelines regarding these treatments are not as well-defined those of PCI and fibrinolytic therapy, mainly owing to relatively scarce literature in this area. Nevertheless, an emerging body of research is attempting to address these issues with alternative treatments. Among these treatments that have generated recent interest are the administration of therapeutic angiogenic factors that function to induce the formation of collateral blood flow [68]. As endothelial dysfunction is one of the defining features pre-disposing to thrombus formation [69] and of complications following AMI [67] the generation of new healthy vasculature may prove an effective method of achieving revascularization in patients refractory to traditional anti-thrombotic treatment. The two most investigated methods over the past two decades for inducing this angiogenesis have been pro-angiogenic factors such as VEGF and FGF and various types of progenitor stem cells. We will now discuss the current findings related to these methods below.
4.1 VEGF
Due to the limitations of conventional treatment methods, attention has turned to other means by which to induce revascularization. Among these are administration of angiogenic growth factors, including VEGF and FGF. Several different isotypes of VEGF have been studied, as well as various means of delivery. One such preclinical study used recombinant adenoviral vectors to deliver VEGF165 into human umbilical vein endothelial cells (HUVEC) in vitro, which showed viable VEGF165 mRNA [70]. When administered subcutaneously in mice, revascularization and increased hemoglobin count was demonstrated two weeks post-injection in the experimental group [70]. The ability of plasmid encoded VEGF165 to induce angiogenesis in the myocardium of rats was further established by Schwarz et al in 2000 [71]. Subsequent preclinical trials went a step further in demonstrating the viability of plasmid VEGF as a therapeutic option, reducing the infarct size when injected intramyocardially in sheep hearts one hour post coronary artery ligation [72]. When given in conjunction with PDGF-BB, alginate gels capable of sequentially delivering VEGF-A165 have been found to increase vessel density and improve cardiac function in rat models of AMI more than each factor individually [73]. When given with angiopoietin-1 in porcine AMI models, higher vascular density and greater proliferating cardiomyocytes were observed in the infarct and peri-infarct zones [74]. This was partially due to angiopoietin-1’s priming of endothelial cells to more strongly respond to angiogenic factors [74]. Furthermore, the specific role of the protective effects of VEGF post-MI is suggested by decreased infarct sizes and improved angiogenesis found in mice with exercise-induced elevations in VEGF levels [75]. Taken together, these studies provide a foundation for clinical investigation, and many have since sought to examine the efficacy of such a method for revascularization in ischemic hearts in clinical trials.
Clinical trials of VEGF so far have mainly focused on generalized ischemic conditions such as coronary artery disease and angina, which have produced mixed results. However, the preclinical basis for use in AMI warrants further investigation, with more trials specific to AMI models being needed to draw clearer conclusions about its efficacy in this setting. One of the current landmark trials is the phase II KAT trial using the same adenoviral VEGF165 as previously described, which showed improved myocardial perfusion at 6 months, but no significant difference in percent stenosis at the same time point in CAD patients [76]. Recombinant VEGF165 protein (rhVEGF) has also been tested in Phase II clinical trials, which showed an improvement versus placebo in exercise treadmill test, angina class, and quality of life at 120 days in angina patients [77]. However, unlike the KAT study, no difference was observed in myocardial perfusion at the same time point [77]. It has been hypothesized that one of the reasons for the shortcomings in several of these clinical trials is the short half-life of VEGF, which necessitates higher doses [78]. A pre-clinical study addressing this problem administered a higher dose of VEGF in the form of an immunoliposome conjugated to anti-P-selectin, which successfully improved revascul-arization and myocardial function post-MI in rats [78]. It is unknown how these results would translate in a clinical trial, but the phase I Genesis trial may provide a glimpse. The administration of higher dose plasmid VEGF165 in patients with severe coronary artery disease unsuitable for conventional revas-cularization proved to be without serious adverse events much like the previous trials, but additionally demonstrated a decrease in angina class, an increase in myocardial perfusion and quality of life, and no change in stress ejection fraction [79]. Although promising, the lack of a control arm is cause for discretion [79]. Nevertheless, these provide a basis for future direction in VEGF therapy.
4.2 Fibroblast growth factor
In addition to VEGF, other proangiogenic factors such as fibroblast growth factor (FGF) have been studied as a means of inducing revascularization in AMI. The promotion of angiogenesis by FGF in animal models was established by Schumacher et al., in which isolated and purified FGF-1 from Escherichia coli was found to successfully promote new vessel formation in the damaged myocardium of animal models via angiographic imaging [80]. This was met with additional preclinical studies that demonstrated improvements in myocardial perfusion and ventricular function in pigs injected intrapericardially with basic fibroblast growth factor (bFGF-2), which also showed evidence of neoangiogenesis in the myocardium [81]. One of the first studies to demonstrate the viability of FGF as a therapeutic option in AMI involved the intrapericardial injection of basic fibroblast growth factor (bFGF) and heparin sulfate in canine models of AMI. A significant reduction in infarcted weight and an increase in vascular number in the infarcted area was observed in groups who received bFGF versus saline or heparin sulfate alone, with even greater improvements being observed in the group receiving both bFGF and heparin sulfate, suggesting a synergistic protective effect [82]. In a separate study, intramyocardial injection of FGF-2 into rat models post-MI exhibited attenuation of both acute and chronic damage, with treated groups displaying a reduced infarct size and reduced troponin T at 24 hours, as well as functional improvement at 6 weeks [83].
Adenoviral vectors as a form of delivery of FGF have also been examined. Intramyocardial injection has been hypothesized to be a more effective form of delivery than intravenous or intracoronary due to enhanced retention and localization of the protein of interest [84]. Preclinical studies using these two methods of delivery to administer FGF-4 in pigs found improvement in myocardial perfusion and a preservation of ventricular wall motion compared to control groups [84]. Interestingly, a signal peptide was included with the viral vector to enhance protein secretion from the cell, which was absent from one of the control groups. This control group did not demonstrate the improvements of the experimental group, suggesting a vital role for the signal peptide in facilitating lower therapeutic doses [84]. Additional preclinical studies likewise found that intracoronary injection of Ad5FGF-4 improved cardiac contraction and restored regional blood flow in pig models, with histologic evidence of new capillary beds [85].
Much like VEGF, clinical trials for FGF have produced mixed results. The basis for use in human subjects was established when purified FGF-1 was injected intramyocardially into patients who had undergone CABG, upon which a newly formed capillary network connecting the proximal coronary artery with the post-stenotic area was observed, providing an impetus for further research [80]. In one phase I trial, intracoronary injection of the same recombinant FGF-2 used in the porcine models described earlier demonstrated improved exercise tolerance, regional thickening of the myocardial wall, and reduced size of the ischemic area, with the most pronounced changes occurring in 180 days post-treatment [86]. However, limitations to the study design include a lack of control arm as well as the open-label dose escalation design, which led to some patients being given differing doses depending on the dose-limiting toxicity [81]. Nevertheless, the FIRST phase II trial of this same rFGF-2, also administered intracoronary, was conducted this time with a placebo control group. Although increased overall in both groups, exercise tolerance and myocardial perfusion were not significantly different at 90 days [87]. However, angina frequency was found to be improved in the rFGF2 group at the same time point, and although no differences were noted across groups among any of the metrics at 180 days, continued improvement was still noted in both groups over this time [87]. Still, these results leave much to be desired from an efficacy standpoint.
Subsequent clinical trials of adenoviral vector delivered FGF have also been conducted. In a phase II trial, Ad5FGF-4 delivered via intracoronary injection in patients with stable angina and reversible ischemia have demonstrated more encouraging results compared to the aforementioned clinical trials, with treated patients experiencing a notable reduction the size of the ischemic defect versus placebo [88]. A reduction of perfusion defect size was also observed upon exclusion of a single outlier. The phase III AFFIRM trial for Ad5FGF-4 in patients with refractory angina is currently underway, with an estimated completion date of 2022 (NCT02928094).
5. Current Preclinical Research
5.1 Bone marrow mononuclear cell
One explanation for the unremarkable results that have been found so far in angiogenic growth factors is the delivery of a single growth factor or cell type being insufficient to support adequate cardiac repair [89]. It has been hypothesized that optimization of future angiogenic therapy could involve the simultaneous stimulation of angiogenesis and vessel maturation, or the addition of progenitor stem cells to enhance myocardial repair [89]. Indeed, the use of various types of stem cells for this purpose have been studied extensively [64]. Among the most studied of these are bone marrow mononuclear stem cells (BMNCs), which offer the advantage of being easily harvested and readily available to administer in a relatively short-time frame [64]. Much like the pro-angiogenic factors, these stem cells demonstrated promising pre-clinical results that have not always translated to clinical improvement. We will now summarize the pertinent trials in more detail. Among the first clinical trials to report the efficacy of BMNCs on inducing regeneration of ischemic myocardium after AMI was the BOOST trial. STEMI patients who had undergone successful PCI were randomized to receive an intracoronary injection of BMNCs or optimum postinfarct medical therapy [90]. The former group experienced enhanced global LV ejection fraction at 6 months with no elevated risk of adverse clinical events, with the greatest enhancement being observed in segments of myocardium adjacent to the infarct [90]. These findings were seemingly affirmed two years later in the phase II REPAIR-AMI trial, which demonstrated improved left ventricular contractile function at 4 months in patients receiving intracoronary BMNCs [5]. A corollary was noted between lower baseline LV ejection fractions and functional improvement, suggesting their viability in patients with severe LV impairment [5].
However, these findings were challenged by two separate trials published that same year, the ASTAMI trial and the BELGIUM trial [91, 92]. In the former, STEMI patients were randomized to BMNCs or control, with no benefit being observed in LV end diastolic volume or infarct size at 6 months [92]. It is possible that the extra two months of study time contributed to this discrepancy, though unlikely. The BELGIUM trial assessed BMNC administration in STEMI patients who had undergone PCI. Although a significant reduction of myocardial infarct size and improved regional systolic function was noted after BMNC treatment, there was no observed difference in global LV ejection fraction at 4 months, nor was there a difference in myocardial perfusion [91]. The SWISS-AMI trial published years later seemed to support these two trials. Attempting to test for optimal time point of administration, BMNCs were given at 5-7 days or at 3-4 weeks in two different groups [93]. Despite this, BMNCs failed to improve global LV ejection fraction in STEMI patients at 4 months in both groups, which was consistent with the earlier TIME trial that had also attempted to test different time points of delivery [93, 94]. Needless to say, these conflicting findings have done little to elucidate tangible benefits to mononuclear cell therapy.
5.2 Mesenchymal stem cell
Less studied than the mononuclear stem cells but still pertinent are mesenchymal stem cells (MSCs). These are thought to play a role in tissue engineering due to their expression of numerous chemokine receptors and adhesion molecules, which gives them migratory potential [95]. Additionally, allogeneic MSCs negate the need for bone marrow aspiration and tissue culture delays, allowing for quicker treatment [96]. The safety of MSC therapy in the context of AMI was established in the PROCHYMAL trial, in which human MSCs were delivered intravenously to patients post-MI [97]. Left ventricular ejection fraction and subsequent reverse remodeling was found to be increased in the treatment group, as well as less adverse clinical events. The follow up, Prochymal II, is currently underway with results pending (NCT00877903). A variant of MSC called Wharton’s jelly-derived mesenchymal stem cells, hypothesized to be effective due to their nature as a primitive stromal population, have also been studied in STEMI patients [98]. Likewise, LV ejection fraction was significantly increased at 18 months in the MSC group, while LV end systolic and end diastolic volumes saw greater decreases [98].
5.3 Hematopoietic stem cells
Yet another type of stem cell that has been studied
for revascularization in AMI is hematopoietic stem cells. These can be isolated from larger pools of bone marrow stem cells due to their specific surface markers, and their expression of surface stem cell factor receptors allows them to play a role in repairing damaged myocardium [99]. The COMPARE-AMI trial involved the intracoronary injection of CD133+ hematopoietic stem cells in patients following AMI [100]. This was determined to be safe, with no increase in adverse events, and effective, with a significantly improved LV ejection fraction at 4 months follow up. Both measurements were relatively unchanged at one year [100]. A much larger study was the REGENT trial, which compared intracoronary infusion of bone marrow derived mononuclear cells, CD34+/CXCR4+ hematopoietic stem cells, and control in AMI patients with reduced ejection fraction (<40%) [101]. Left ventricular ejection fraction was found to be increased by 3% in each stem cell group, with no change observed in the control. Overall, absolute changes in ejection fraction, LV end systolic volumes, or LV end diastolic volumes were not noted, but both forms of stem cell therapy trended toward functional improvements in patients with severely impaired LV ejection fraction or longer delay between symptom onset and revascularization [101]. Knowledge remains limited by the scarce amount of research in this area, and more data is needed to draw substantial conclusions.
5.4 Serotonin receptor antagonists
Yet another area of research that is relatively less explored but has yielded promise in preclinical studies is the use of serotonin receptor antagonists. These have been shown to have both anti-thrombotic properties and a use in AMI in animal models, and as such could be a lucrative source of progress in this area [102-104]. In a study evaluating the effect of a novel 5-HT2A receptor antagonist called AR246686, the agent was found to inhibit serotonin induced amplification of ADP stimulated platelet aggregation in human embryonic kidney cells [102]. It also reduced bleeding time and time to occlusion in the femoral artery of rats when given orally, the latter of which required a lower dose than clopidogrel to achieve the same effect. The effect on platelets and vascular smooth muscle cells proved hopeful in the translation of this drug to the setting of coronary thrombosis and AMI. In a study that administered the selective 5-HT2A receptor antagonist APD791 to canines with recurrent coronary thrombosis mimicking unstable angina, coronary patency was improved even when given after the onset of thrombosis versus saline [104], suggesting its viability in vivo. This supported an earlier study that demonstrated that the blockade of platelet 5-HT2 receptors successfully disrupted coronary artery thrombi in beagles [105]. Additionally, it has been shown to be viable as pre-treatment in a rat model of AMI [103]. When given a 5-HT2A receptor blocker three days before surgically induced AMI, rats in the experimental group showed reduced mortality, decreased infarct size, and reduced LV end diastolic pressure versus control. It has since been hypo-thesized that these protective effects stem from downregulation of the antiapoptotic Bcl-2, which mitigates ischemia-reperfusion injury [103, 106]. Nevertheless, these findings remain encouraging, but whether similar results can be achieved when given post-injury remains unknown. Moreover, as with the angiogenic factors and stem cell therapies, it is uncertain if any of these results can translate to successful clinical trials, which is a point for future monitoring.
5.5 Novel drug delivery platforms
Among the current challenges of developing novel
therapeutics in treating AMI is enhancing the specificity of drug interactions, which allows both for more localized targeting of the drug to the site of interest as well as reduced unwanted systemic side effects. As such, recent attention has been turned to novel mechanisms of delivery for anti-thrombotic agents and attenuating vessel injury. One of the fields that meet this criteria is nanotherapy, which involves the delivery of particles of nanometer scale that possess unique structural and chemical properties that allow a high degree of reactivity and localization [107]. Although many types of nanoparticle delivery involve targeting inflammatory mechanisms, many also target thrombosis and vessel injury, which offers additional protection [108]. The potential role of these agents in treating AMI is yet to be fully elucidated, but existing preclinical studies suggest their potential viability. Some of these studies utilize revascularization methods as previously described, such as the delivery of bone marrow mesenchymal stem cells (BMMSCs) via hybrid hollow mesoporous organosilica nanoparticles (HMONs) [109]. This method enabled more effective gene transfection of the BMMSCs in rat models of AMI, which demonstrated reduced infarct size and myocyte apoptosis as well as increased myocardial angiogenesis [109]. One of the limitations of stem cell delivery has been its low cell retention and engraftment due to wash-out from coronary blood flow [110]. Vandergriff et al. sought to enhance this retention through cardiosphere-derived stem cells labeled with ferumoxytol nanoparticles in the presence of heparin and protamine (FHP). This magnetic targeting technique enhanced acute cellular retention in rat models of AMI, which correlated with reduced left ventricular remodeling and a greater ejection fraction 3 weeks post-treatment, with greater angiogenesis being observed histologically [110].
Likewise, nanoparticles containing a core of lecithin containing VEGF and a shell of Pluronic F-127 have been shown to improve ejection fraction and cardiac output in rat models following subacute MI [111]. VEGF has also been encapsulated in polylactic coglycolic acid nanoparticles and administered in murine models of AMI, successfully increasing vascular density in the infarct region, reducing infarct size, and improving LV systolic function at 4 weeks post-treatment [112]. Additionally, ultrasound methods have been used to target plasmid delivery to the myocardium, particular a method called ultrasound-targeted microbubble destruction [113]. Plasmids containing VEGF and stem cell factor (SCF) incubated with perflutren lipid microbubbles were injected intravenously into mice 7 days post-infarction, which led to increased capillary and arteriolar density, myocardial perfusion, and cardiac function in both groups [114]. These studies suggest the capability of such mechanisms to deliver angiogenic factors to ischemic myocardium in a safe and effective manner.
Many studies have identified the role that thrombin plays in inducing vessel injury post-MI [114-116]. More specifically, myocardial reperfusion has demonstrated rapid, acute onset thrombin generation at the site of tissue injury [114, 117]. This suggests the potential role of thrombin inhibitors in the management of AMI. Delivery of perfluorocarbon (PFC) nanoparticles conjugated to thrombin inhibitors such as phenylalanine-proline-arginine-chloromethylketone (PPACK) have shown success in mitigating myocardial injury when administered as pre-treatment in mice and rat models of AMI [118-119]. In murine models, pre-treatment with PPACK PFC NPs 15 minutes prior to LAD occlusion preserved end diastolic and end systolic volume with no change in infarct size [118]. In rat models, pre-treatment preserved vascular integrity and reduced hemorrhage associated with endothelial disruption without prolonging bleeding times [119]. While these studies implicate anti-thrombin nanoparticles in minimizing vascular damage, its efficacy as a therapy post-injury remains a point of future study.
6. Concluding Remarks
The complex role of thrombosis and vessel injury in AMI provides a basis for future direction of therapy for AMI. While attention has so far mainly focused on management of the initial presenting ischemic event via physical and chemical disruption of coronary clots, future studies are becoming more oriented toward addressing endothelial regeneration to mitigate additional mechanisms of damage induced by these processes. The optimization of delivery through novel transport platforms will also allow more specific interactions of existing therapies that enhance functional improvement. The growing but sparse literature will hopefully provide an impetus for additional research in these areas, and the progression of more novel therapies to clinical trials.
Sources of Funding
This study was supported by grants from the NIH (DK125322 and HL154009 to HP).
References
- Johansson S, Rosengren A, Young K, Jennings E. Mortality and morbidity trends after the first year in survivors of acute myocardial infarction: a systematic review. BMC Cardiovasc Disord 17 (2017): 53.
- Hellermann J P, Goraya T Y, Jacobsen S J, Weston S A, Reeder G S, Gersh B J, et al. Incidence of heart failure after myocardial infarction: is it changing over time? Am J Epidemiol 157 (2003): 1101-1107.
- Al Shammeri O, Garcia L. Thrombolysis in the age of Primary Percutaneous Coronary Intervention: Mini-Review and Meta-analysis of Early PCI. Int J Health Sci (Qassim) 7 (2013): 91-100.
- Taggart D P. Incomplete revascularization: appropriate and inappropriate. Eur J Cardiothorac Surg 41 (2012): 542-543.
- Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med 355 (2006): 1210-1221.
- Saleh M, Ambrose J A. Understanding myocardial infarction. F1000Res 7 (2018).
- Ross R. Atherosclerosis—an inflammatory disease. New England journal of medicine 340 (1999): 115-126.
- Libby P, Ridker P M, Maseri A. Inflammation and atherosclerosis. Circulation 105 (2002): 1135-1143.
- Badimon L, Padro T, Vilahur G. Atherosclerosis, platelets and thrombosis in acute ischaemic heart disease. Eur Heart J Acute Cardiovasc Care 1 (2012): 60-74.
- Fernandez-Ortiz A, Badimon J J, Falk E, Fuster V, Meyer B, Mailhac A, et al. Characterization of the relative thrombo-genicity of atherosclerotic plaque compo-nents: implications for consequences of plaque rupture. J Am Coll Cardiol 23 (1994): 1562-1569.
- Ambrose J A, Singh M. Pathophysiology of coronary artery disease leading to acute coronary syndromes. F1000Prime Rep (2015).
- Binns R L, Ku D N. Effect of stenosis on wall motion. A possible mechanism of stroke and transient ischemic attack. Arteriosclerosis 9 (1989): 842-847.
- Arroyo L H, Lee R T. Mechanisms of plaque rupture: mechanical and biologic interactions. Cardiovasc Res 41 (1999): 369-375.
- Boyle J J. Macrophage activation in atherosclerosis: pathogenesis and pharmacology of plaque rupture. Curr Vasc Pharmacol 3 (2005): 63-68.
- Shah P K, Falk E, Badimon J J, Fernandez-Ortiz A, Mailhac A, Villareal-Levy G, et al. Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques. Potential role of matrix-degrading metalloproteinases and implications for plaque rupture. Circulation 92 (1995): 1565-1569.
- Silvain J, Collet J P, Nagaswami C, Beygui F, Edmondson K E, Bellemain-Appaix A, et al. Composition of coronary thrombus in acute myocardial infarction. J Am Coll Cardiol 57 (2011): 1359-1367.
- Virmani R, Burke A P, Farb A. Plaque rupture and plaque erosion. Thromb Haemost (1999).
- Quillard T, Franck G, Mawson T, Folco E, Libby P. Mechanisms of erosion of atherosclerotic plaques. Curr Opin Lipidol 28 (2017): 434-441.
- Chandran S, Watkins J, Abdul-Aziz A, Shafat M, Calvert P A, Bowles K M, et al. Inflammatory Differences in Plaque Erosion and Rupture in Patients With ST-Segment Elevation Myocardial Infarction. J Am Heart Assoc 6 (2017).
- Badimon L, Vilahur G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J Intern Med, 276 (2014): 618-632.
- Spaet T H, Zucker M B. Mechanism of Platelet Plug Formation and Role of Adenosine Diphosphate. Am J Physiol 206 (1964): 1267-1274.
- Chen Z M, Jiang L X, Chen Y P, Xie J X, Pan H C, Peto R, et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 366 (2005): 1607-1621.
- Tatsumi K, Mackman N. Tissue Factor and Atherothrombosis. J Atheroscler Thromb 22 (2015): 543-549.
- Ibanez B, James S, Agewall S, Antunes M J, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). European heart journal 39 (2018): 119-177.
- Armstrong P W, Gershlick A H, Goldstein P, Wilcox R, Danays T, Lambert Y, et al. Fibrinolysis or primary PCI in ST-segment elevation myocardial infarction. N Engl J Med, 368 (2013): 1379-1387.
- Tofield A. Pharmaco-invasive vs. facilitated percutaneous coronary intervention strategies for ST-segment-elevation acute myocardial infarction patients in the new ESC Guidelines. Eur Heart J 30 (2009): 2817.
- Zimarino M, Sacchetta D, Renda G, De Caterina R. Facilitated PCI: rationale, current evidence, open questions, and future directions. J Cardiovasc Pharmacol 51 (2008): 3-10.
- Van De Werf F, Adgey J, Ardissino D, Armstrong P W, Aylward P, Barbash G. Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: the ASSENT-2 double-blind randomised trial. Lancet 354 (1999): 716-722.
- Thiele H, Eitel I, Meinberg C, Desch S, Leuschner A, Pfeiffer D, et al. Randomized comparison of pre-hospital-initiated facilitated percutaneous coronary intervention versus primary percutaneous coronary intervention in acute myocardial infarction very early after symptom onset: the LIPSIA-STEMI trial (Leipzig immediate prehospital facilitated angioplasty in ST-segment myocardial infarction). JACC Cardiovasc Interv 4 (2011): 605-614.
- Ellis S G, Tendera M, de Belder M A, van Boven A J, Widimsky P, Janssens L, et al. Facilitated PCI in patients with ST-elevation myocardial infarction. N Engl J Med 358 (2008): 2205-2217.
- Victor S M, Subban V, Alexander T, Srinivas A, Mullasari A S. A prospective, observational, multicentre study comparing tenecteplase facilitated PCI versus primary PCI in Indian patients with STEMI (STEPP-AMI). Open Heart 1 (2014): e000133.
- Brodie B R. Facilitated percutaneous coronary intervention. Heart 91 (2005): 1527-1529.
- McKay R G, Dada M R, Mather J F, Mennet R R, Murphy D J, Maloney K W, et al. Comparison of outcomes and safety of "facilitated" versus primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Am J Cardiol 103 (2009): 316-321.
- Huang W C, Chiang C H, Liu C P. Facilitated Percutaneous Coronary Intervention in STEMI Patients: Does It Work in Asian Patients?. Acta Cardiol Sin 30 (2014): 292-297.
- Capodanno D, Dangas G. Facilitated/pharmaco-invasive approaches in STEMI. Curr Cardiol Rev 8 (2012): 177-180.
- Patel N, Patel N, Thakkar B, Patel N, Arora S, Chothani A, et al. Pharmacoinvasive Strategy Versus Fibrinolytic Therapy in Patients with St-Segment Elevation Myocardial Infarction: A Propensity Score Matched Analysis from the Nationwide Inpatient Sample. Journal of the American College of Cardiology 65 (2015): A65-A65.
- Cantor W J, Fitchett D, Borgundvaag B, Ducas J, Heffernan M, Cohen E A, et al. Routine early angioplasty after fibrinolysis for acute myocardial infarction. N Engl J Med 360 (2009): 2705-2718.
- Di Mario C, Dudek D, Piscione F, Mielecki W, Savonitto S, Murena E, et al. Immediate angioplasty versus standard therapy with rescue angioplasty after thrombolysis in the Combined Abciximab REteplase Stent Study in Acute Myocardial Infarction (CARESS-in-AMI): an open, prospective, randomised, multicentre trial. Lancet 371 (2008): 559-568.
- Eeckhout E. Rescue percutaneous coronary intervention: does the concept make sense?. Heart 93 (2007): 632-638.
- Sutton A G, Campbell P G, Graham R, Price D J, Gray J C, Grech E D, et al. A randomized trial of rescue angioplasty versus a conservative approach for failed fibrinolysis in ST-segment elevation myocardial infarction: the Middlesbrough Early Revascularization to Limit INfarction (MERLIN) trial. J Am Coll Cardiol 44 (2004): 287-296.
- Gershlick A H, Stephens-Lloyd A, Hughes S, Abrams K R, Stevens S E, Uren N G, et al. Rescue angioplasty after failed thrombolytic therapy for acute myocardial infarction. N Engl J Med 353 (2005): 2758-2768.
- Shahzad A, Kemp I, Mars C, Wilson K, Roome C, Cooper R, et al. Unfractionated heparin versus bivalirudin in primary percutaneous coronary intervention (HEAT-PPCI): an open-label, single centre, randomised controlled trial. Lancet 384 (2014): 1849-1858.
- Erlinge D, Koul S, Eriksson P, Schersten F, Omerovic E, Linder R, et al. Bivalirudin versus heparin in non-ST and ST-segment elevation myocardial infarction-a registry-based randomized clinical trial in the SWEDEHEART registry (the VALIDATE-SWEDEHEART trial). Am Heart J 175 (2016): 36-46.
- O'Gara P T, Kushner F G, Ascheim D D, Casey D E, Chung M K, de Lemos J A, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 61 (2013): e78-e140.
- Stone G W, Witzenbichler B, Guagliumi G, Peruga J Z, Brodie B R, Dudek D, et al. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med 358 (2008): 2218-2230.
- Han Y, Guo J, Zheng Y, Zang H, Su X, Wang Y, et al. Bivalirudin vs heparin with or without tirofiban during primary percutaneous coronary intervention in acute myocardial infarction: the BRIGHT randomized clinical trial. JAMA 313 (2015): 1336-1346.
- Schulz S, Richardt G, Laugwitz K L, Morath T, Neudecker J, Hoppmann P, et al. Prasugrel plus bivalirudin vs. clopidogrel plus heparin in patients with ST-segment elevation myocardial infarction. Eur Heart J 35 (2014): 2285-2294.
- Erlinge D, Koul S, Omerovic E, Frobert O, Linder R, Danielewicz M, et al. Bivalirudin versus heparin monotherapy in non-ST-segment elevation myocardial infarction. Eur Heart J Acute Cardiovasc Care, 8 (2019): 492-501.
- De Luca G, Ucci G, Cassetti E, Marino P. Benefits from small molecule administration as compared with abciximab among patients with ST-segment elevation myocardial infarction treated with primary angioplasty: a meta-analysis. J Am Coll Cardiol 53 (2009): 1668-1673.
- Collet J P, Montalescot G, Vicaut E, Ankri A, Walylo F, Lesty C, et al. Acute release of plasminogen activator inhibitor-1 in ST-segment elevation myocardial infarction predicts mortality. Circulation 108 (2003): 391-394.
- Kumar A, Cannon C P. Acute coronary syndromes: Diagnosis and management, part II. Mayo Clin Proc 84 (2009): 1021-1036.
- Sinnaeve P, Alexander J, Belmans A, Bogaerts K, Langer A, Diaz R, et al. One-year follow-up of the ASSENT-2 trial: a double-blind, randomized comparison of single-bolus tenecteplase and front-loaded alteplase in 16,949 patients with ST-elevation acute myocardial infarction. Am Heart J 146 (2003): 27-32.
- Go J S. Reperfusion Therapy for Acute Myocardial Infarction. Interventional Cardiology and Peripheral Vascular Intervention (2002).
- Liang F, Wang L Z, Hu D Y, Shi X B, Wei J P, Zhao H, et al. An angiographic trial to evaluate the efficacy and safety of tenecteplase in Chinese patients with acute myocardial infarction. Zhonghua Xin Xue Guan Bing Za Zhi 37 (2009): 514-517.
- Global Use of Strategies to Open Occluded Coronary Arteries I. A comparison of reteplase with alteplase for acute myocardial infarction. N Engl J Med 337 (1997): 1118-1123.
- GISSI-2: a factorial randomised trial of alteplase versus streptokinase and heparin versus no heparin among 12,490 patients with acute myocardial infarction. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico. Lancet 336 (1990): 65-71.
- Aslanabadi N, Safaie N, Talebi F, Dousti S, Entezari-Maleki T. The Streptokinase Therapy Complications and its Associated Risk Factors in Patients with Acute ST Elevation Myocardial Infarction. Iran J Pharm Res 17 (2018): 53-63.
- Group I S A M S. A prospective trial of intravenous streptokinase in acute myocardial infarction (I.S.A.M.). Mortality, morbidity, and infarct size at 21 days. N Engl J Med 314 (1986): 1465-1471.
- Kennedy J W, Ritchie J L, Davis K B, Stadius M L, Maynard C, Fritz J K. The western Washington randomized trial of intracoronary streptokinase in acute myocardial infarction. A 12-month follow-up report. N Engl J Med 312 (1985): 1073-1078.
- Sezer M, Cimen A, Aslanger E, Elitok A, Umman B, Bugra Z, et al. Effect of intracoronary streptokinase administered immediately after primary percutaneous coronary intervention on long-term left ventricular infarct size, volumes, and function. J Am Coll Cardiol 54 (2009): 1065-1071.
- Jinatongthai P, Kongwatcharapong J, Foo C Y, Phrommintikul A, Nathisuwan S, Thakkinstian A, et al. Comparative efficacy and safety of reperfusion therapy with fibrinolytic agents in patients with ST-segment elevation myocardial infarction: a systematic review and network meta-analysis. Lancet 390 (2017): 747-759.
- Ferguson J J. Clopidogrel plus aspirin in patients with acute myocardial infarction treated with fibrinolytic therapy--CLARITY-TIMI 28. Future Cardiol 1 (2005): 605-610.
- Berwanger O, Nicolau J C, Carvalho A C, Jiang L, Goodman S G, Nicholls S J, et al. Ticagrelor vs Clopidogrel After Fibrinolytic Therapy in Patients With ST-Elevation Myocardial Infarction: A Randomized Clinical Trial. JAMA Cardiol 3 (2018): 391-399.
- Alexopoulos D, Theodoropoulos K C, Stavrou E F, Xanthopoulou I, Kassimis G, Tsigkas G, et al. Prasugrel versus high dose clopidogrel to overcome early high on clopidogrel platelet reactivity in patients with ST elevation myocardial infarction. Cardiovasc Drugs Ther 26 (2012): 393-400.
- Zhang H, van Olden C, Sweeney D, Martin-Rendon E. Blood vessel repair and regeneration in the ischaemic heart. Open Heart 1 (2014): e000016.
- Hiramori K. Major causes of death from acute myocardial infarction in a coronary care unit. Jpn Circ J 51 (1987): 1041-1047.
- Kim H K, Kim H B, Lee J M, Kim S S, Bae I H, Park D S, et al. Influence of Local Myocardial Infarction on Endothelial Function, Neointimal Progression, and Inflammation in Target and Non-Target Vascular Territories in a Porcine Model of Acute Myocardial Infarction. J Korean Med Sci 34 (2019): e145.
- Renault M A, Losordo D W. Therapeutic myocardial angiogenesis. Microvasc Res 74 (2007): 159-171.
- Yau J W, Teoh H, Verma S. Endothelial cell control of thrombosis. BMC Cardiovasc Disord, 15 (2015): 130.
- Muhlhauser J, Merrill M J, Pili R, Maeda H, Bacic M, Bewig B, et al. VEGF165 expressed by a replication-deficient recombinant adenovirus vector induces angiogenesis in vivo. Circ Res 77 (1995): 1077-1086.
- Schwarz E R, Speakman M T, Patterson M, Hale S S, Isner J M, Kedes L H, et al. Evaluation of the effects of intramyocardial injection of DNA expressing vascular endothelial growth factor (VEGF) in a myocardial infarction model in the rat--angiogenesis and angioma formation. J Am Coll Cardiol 35 (2000): 1323-1330.
- Vera Janavel G, Crottogini A, Cabeza Meckert P, Cuniberti L, Mele A, Papouchado M, et al. Plasmid-mediated VEGF gene transfer induces cardiomyogenesis and reduces myocardial infarct size in sheep. Gene Ther 13 (2006): 1133-1142.
- Hao X, Silva E A, Mansson-Broberg A, Grinnemo K H, Siddiqui A J, Dellgren G, et al. Angiogenic effects of sequential release of VEGF-A165 and PDGF-BB with alginate hydrogels after myocardial infarction. Cardiovasc Res 75 (2007): 178-185.
- Tao Z, Chen B, Tan X, Zhao Y, Wang L, Zhu T, et al. Coexpression of VEGF and angiopoietin-1 promotes angiogenesis and cardiomyocyte proliferation reduces apoptosis in porcine myocardial infarction (MI) heart. Proc Natl Acad Sci U S A 108 (2011): 2064-2069.
- Wu G, Rana J S, Wykrzykowska J, Du Z, Ke Q, Kang P, et al. Exercise-induced expression of VEGF and salvation of myocardium in the early stage of myocardial infarction. Am J Physiol Heart Circ Physiol 296 (2009): H389-395.
- Hedman M, Hartikainen J, Syvanne M, Stjernvall J, Hedman A, Kivela A, et al. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: phase II results of the Kuopio Angiogenesis Trial (KAT). Circulation 107 (2003): 2677-2683.
- Henry T D, Annex B H, McKendall G R, Azrin M A, Lopez J J, Giordano F J, et al. The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation 107 (2003): 1359-1365.
- Wang B, Cheheltani R, Rosano J, Crabbe D L, Kiani M F. Targeted delivery of VEGF to treat myocardial infarction. Adv Exp Med Biol 765 (2013): 307-314.
- Favaloro L, Diez M, Mendiz O, Janavel G V, Valdivieso L, Ratto R, et al. High-dose plasmid-mediated VEGF gene transfer is safe in patients with severe ischemic heart disease (Genesis-I). A phase I, open-label, two-year follow-up trial. Catheter Cardiovasc Interv 82 (2013): 899-906.
- Schumacher B, Pecher P, von Specht B U, Stegmann T. Induction of neoangiogenesis in ischemic myocardium by human growth factors: first clinical results of a new treatment of coronary heart disease. Circulation 97 (1998): 645-650.
- Laham R J, Rezaee M, Post M, Novicki D, Sellke F W, Pearlman J D, et al. Intrapericardial delivery of fibroblast growth factor-2 induces neovascularization in a porcine model of chronic myocardial ischemia. J Pharmacol Exp Ther 292 (2000): 795-802.
- Uchida Y, Yanagisawa-Miwa A, Nakamura F, Yamada K, Tomaru T, Kimura K, et al. Angiogenic therapy of acute myocardial infarction by intrapericardial injection of basic fibroblast growth factor and heparin sulfate: an experimental study. Am Heart J 130 (1995): 1182-1188.
- Jiang Z S, Padua R R, Ju H, Doble B W, Jin Y, Hao J, et al. Acute protection of ischemic heart by FGF-2: involvement of FGF-2 receptors and protein kinase C. Am J Physiol Heart Circ Physiol, 282(2002): H1071-H1080.
- Rubanyi G M. The design and preclinical testing of Ad5FGF-4 to treat chronic myocardial ischaemia. European Heart Journal Supplements 6 (2004): E12-E17.
- Grines C, Rubanyi G M, Kleiman N S, Marrott P, Watkins M W. Angiogenic gene therapy with adenovirus 5 fibroblast growth factor-4 (Ad5FGF-4): a new option for the treatment of coronary artery disease. Am J Cardiol 92 (2003): 24N-31N.
- Laham R J, Sellke F W, Edelman E R, Pearlman J D, Ware J A, Brown D L, et al. Local perivascular delivery of basic fibroblast growth factor in patients undergoing coronary bypass surgery: results of a phase I randomized, double-blind, placebo-controlled trial. Circulation 100 (1999): 1865-1871.
- Simons M, Annex B H, Laham R J, Kleiman N, Henry T, Dauerman H, et al. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: double-blind, randomized, controlled clinical trial. Circulation 105 (2002): 788-793.
- Grines C L, Watkins M W, Mahmarian J J, Iskandrian A E, Rade J J, Marrott P, et al. A randomized, double-blind, placebo-controlled trial of Ad5FGF-4 gene therapy and its effect on myocardial perfusion in patients with stable angina. J Am Coll Cardiol 42 (2003): 1339-1347.
- Cochain C, Channon K M, Silvestre J S. Angiogenesis in the infarcted myocardium. Antioxid Redox Signal 18 (2013): 1100-1113.
- Wollert K C, Meyer G P, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet 364 (2004): 141-148.
- Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet 367 (2006): 113-121.
- Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med 355 (2006): 1199-1209.
- Surder D, Mank R, Lo Cicero V, Moccetti T, Rufibach K, Soncin S, et al. Intracoronary injection of bone marrow-derived mononuclear cells early or late after acute myocardial infarction: effects on global left ventricular function. Circulation 127 (2013): 1968-1979.
- Traverse J H, Henry T D, Pepine C J, Willerson J T, Zhao D X, Ellis S G, et al. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the TIME randomized trial. JAMA 308 (2012): 2380-2389.
- Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 25 (2007): 2739-2749.
- Hare J M, Fishman J E, Gerstenblith G, DiFede Velazquez D L, Zambrano J P, Suncion V Y, et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA 308 (2012): 2369-2379.
- Hare J M, Traverse J H, Henry T D, Dib N, Strumpf R K, Schulman S P, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol 54 (2009): 2277-2286.
- Gao L R, Chen Y, Zhang N K, Yang X L, Liu H L, Wang Z G, et al. Intracoronary infusion of Wharton's jelly-derived mesenchymal stem cells in acute myocardial infarction: double-blind, randomized controlled trial. BMC Med 13 (2015): 162.
- Shin J Y, Hu W, Naramura M, Park C Y. High c-Kit expression identifies hematopoietic stem cells with impaired self-renewal and megakaryocytic bias. J Exp Med 211(2014): 217-231.
- Mansour S, Roy D C, Bouchard V, Stevens L M, Gobeil F, Rivard A, et al. One-Year Safety Analysis of the COMPARE-AMI Trial: Comparison of Intracoronary Injection of CD133 Bone Marrow Stem Cells to Placebo in Patients after Acute Myocardial Infarction and Left Ventricular Dysfunction. Bone Marrow Res 2011 (2011): 385124.
- Tendera M, Wojakowski W, Ruzyllo W, Chojnowska L, Kepka C, Tracz W, et al. Intracoronary infusion of bone marrow-derived selected CD34+CXCR4+ cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: results of randomized, multicentre Myocardial Regeneration by Intracoronary Infusion of Selected Population of Stem Cells in Acute Myocardial Infarction (REGENT) Trial. Eur Heart J 30 (2009): 1313-1321.
- Adams J W, Ramirez J, Ortuno D, Shi Y, Thomsen W, Richman J G, et al. Anti-thrombotic and vascular effects of AR246686, a novel 5-HT2A receptor antagonist. Eur J Pharmacol 586 (2008): 234-243.
- Brasil D, Temsah R M, Kumar K, Kumamoto H, Takeda N, Dhalla N S. Blockade of 5-HT(2A) receptors by sarpogrelate protects the heart against myocardial infarction in rats. J Cardiovasc Pharmacol Ther 7 (2002): 53-59.
- Przyklenk K, Frelinger A L, Linden, Whittaker P, Li Y, Barnard M R, et al. Targeted inhibition of the serotonin 5HT2A receptor improves coronary patency in an in vivo model of recurrent thrombosis. J Thromb Haemost 8 (2010): 331-340.
- Belcher P R, Drake-Holland A J, Hynd J, Noble M I. Dispersion of coronary artery thrombi by antagonism of platelet serotonin receptor in the dog. Cardiovasc Res 26 (1992): 292-296.
- Rajesh K G, Suzuki R, Maeda H, Murio Y, Sasaguri S. 5-HT2 receptor blocker sarpogrelate prevents downregulation of antiapoptotic protein Bcl-2 and protects the heart against ischemia-reperfusion injury. Life Sci 79 (2006): 1749-1755.
- Wilczewska A Z, Niemirowicz K, Markiewicz K H, Car H. Nanoparticles as drug delivery systems. Pharmacol Rep 64 (2012): 1020-1037.
- Bejarano J, Navarro-Marquez M, Morales-Zavala F, Morales J O, Garcia-Carvajal I, Araya-Fuentes E, et al. Nanoparticles for diagnosis and therapy of atherosclerosis and myocardial infarction: evolution toward prospective theranostic approaches. Theranostics 8 (2018): 4710-4732.
- Zhu K, Wu M, Lai H, Guo C, Li J, Wang Y, et al. Nanoparticle-enhanced generation of gene-transfected mesenchymal stem cells for in vivo cardiac repair. Biomaterials 74 (2016): 188-199.
- Vandergriff A C, Hensley T M, Henry E T, Shen D, Anthony S, Zhang J, et al. Magnetic targeting of cardiosphere-derived stem cells with ferumoxytol nanoparticles for treating rats with myocardial infarction. Biomaterials 35 (2014): 8528-8539.
- Oh K S, Song J Y, Yoon S J, Park Y, Kim D, Yuk S H. Temperature-induced gel formation of core/shell nanoparticles for the regeneration of ischemic heart. J Control Release 146 (2010): 207-211.
- Oduk Y, Zhu W, Kannappan R, Zhao M, Borovjagin A V, Oparil S, et al. VEGF nanoparticles repair the heart after myocardial infarction. Am J Physiol Heart Circ Physiol 314 (2018): H278-H284.
- Fujii H, Sun Z, Li S H, Wu J, Fazel S, Weisel R D, et al. Ultrasound-targeted gene delivery induces angiogenesis after a myocardial infarction in mice. JACC Cardiovasc Imaging 2 (2009): 869-879.
- Chong A J, Pohlman T H, Hampton C R, Shimamoto A, Mackman N, Verrier E D. Tissue factor and thrombin mediate myocardial ischemia-reperfusion injury. Ann Thorac Surg 75 (2003): S649-S655.
- Erlich J H, Boyle E M, Labriola J, Kovacich J C, Santucci R A, Fearns C, et al. Inhibition of the tissue factor-thrombin pathway limits infarct size after myocardial ischemia-reperfusion injury by reducing inflammation. Am J Pathol 157 (2000): 1849-1862.
- Raivio P, Lassila R, Petaja J. Thrombin in myocardial ischemia-reperfusion during cardiac surgery. Ann Thorac Surg 88 (2009): 318-325.
- Raivio P, Kuitunen A, Suojaranta-Ylinen R, Lassila R, Petaja J. Thrombin generation during reperfusion after coronary artery bypass surgery associates with postoperative myocardial damage. J Thromb Haemost 4 (2006): 1523-1529.
- Fazal J, Chen J, Weinheimer C, Kovacs A, Pan H, Wickline S. P4656 Thrombin targeted nanoparticles limit ischemia reperfusion injury in acute myocardial infarction. European Heart Journal (2018).
- Pan H, Lindon A, Grabau R, Dominguez W, Wickline S. P3100 Anti-thrombin nanoparticles limit ischemia-reperfusion injury and no-reflow in myocardial infarction. European Heart Journal (2019).

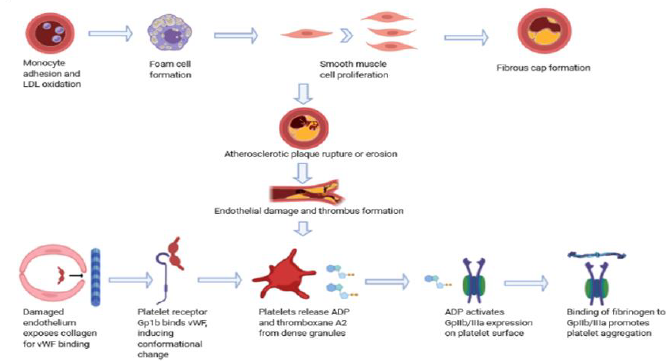
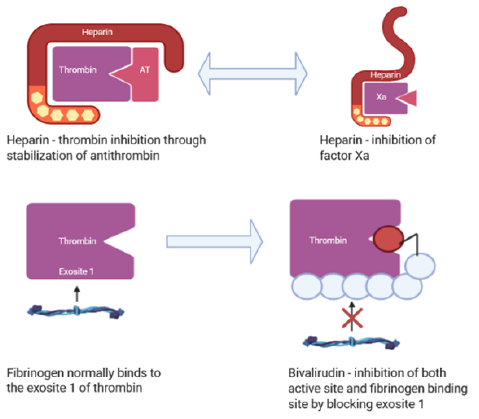

 Impact Factor: * 5.6
Impact Factor: * 5.6 Acceptance Rate: 74.36%
Acceptance Rate: 74.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 