Anakinra as Rescue Therapy to Treat Patients with Severe COVID-19 Refractory to Tocilizumab
Jaume Alijotas-Reig1*, Enrique Esteve-Valverde2*, Domingo Ruiz-Hidalgo3, Gabriel López-Sánchez4, Jaume Trapé-Pujol5, Francesc Miró-Mur6
1Senior consultant. Systemic Autoimmune Diseases Unit. Department of Internal Medicine-1,Vall d’Hebron University Hospital, Spain. Department of Medicine, Universitat Autònoma, Barcelona. Spain
2Department of Internal Medicine, Althaia Healthcare University Network of Manresa, Manresa, Barcelona, Spain
3Department of Internal Medicine, Althaia Healthcare University Network of Manresa, Manresa, Barcelona, Spain University Central de Catalunya, Barcelona, Spain
4Department of Internal Medicine, Althaia Healthcare University Network of Manresa, Manresa, Barcelona, Spain
5Head, Laboratory Department, Althaia Healthcare University Network of Manresa, Manresa, Barcelona, Spain
6Senior research, Systemic Autoimmune Research Unit, Vall d’Hebron Research Institute, Barcelona, Spain
*Corresponding Author’s: Jaume Alijotas-Reig, Senior consultant. Systemic Autoimmune Diseases Unit. Department of Internal Medicine-1,Vall d’Hebron University Hospital, Spain. Department of Medicine, Universitat Autònoma, Barcelona. Spain
Received: 17 November 2021; Accepted: 29 November 2021; Published: 10 December 2021
Article Information
Citation:
Jaume Alijotas-Reig, Enrique Esteve-Valverde, Domingo Ruiz-Hidalgo, Gabriel López-Sánchez, Jaume Trapé-Pujol, Francesc Miró-Mur. Anakinra as Rescue Therapy to Treat Patients with Severe COVID-19 Refractory to Tocilizumab. Archives of Clinical and Biomedical Research 5 (2021): 959-970.
View / Download Pdf Share at FacebookAbstract
Objective To evaluate the role played by anakinra in the treatment of patients with severe COVID-19 who fails to “accepted” standard of care and tocilizumab.
Methods We conducted a retrospective cohort study assessed in Althaia Health Network University and Vall d’Hebrón University Hospital, in Barcelona, Spain. We included patients with confirmed RT-PCR for SARS-CoV-2, moderate-to-severe acute respiratory distress syndrome [PaO2:FiO2] (PAFI) ≤200 mmHg, and hyperinflammation. All of them were primarily managed with non-invasive ventilation outside of the ICU and received standard of care with hydroxy chloroquine, lopinavir/ritonavir, azithromycin, and enoxaparin supplemented with tocilizumab. These patients received an additional administration of 100 mg subcutaneous anakinra twice a day (low-dose) or every 6-8 hours (medium-dose). Mechanical ventilation-free survival, ICU admission, respiratory function changes (PAFI), inflammatory markers, and survival were analysed.
Results Clinical and respiratory improvements were already present at 48 hours post-anakinra. PAFI increased 105.2% at 48 hours over anakinra administration and 166.9% at day 7. Respiratory function improvement was significant at day 7 (p<0.0001) with 10/12 (83%) responders having a PAFI ≥ 200 mm Hg. Despite of this improvement, inflammatory markers lasted to decrease. Two patients required ICU and temporary mechanical ventilation. No patients died and no adverse effects related to anakinra appeared. Short-time follow-up showed no relapses.
Conclusion In severe ARDS related to SARS-CoV-2 refractory to standard of care plus tocilizumab, low-to-moderate doses of anakinra as a rescue therapy showed effectiveness and safety, avoiding mechanical ventilation and deaths.
Keywords
<p>Anakinra; COVID-19; Corticosteroids; Hiperinflammation; Interleukin-1; Interleukin-6; SARS-.CoV-2; Tocilizumab.</p>
Article Details
1. Introduction
Severe acute respiratory syndrome related to Coronavirus-2 (SARS-CoV-2) infection causes the coronavirus disease 2019 (COVID-19). Clinical manifestations range from asymptomatic or mild forms, through flu syndrome to systemic inflammatory response with acute respiratory distress syndrome (ARDS). In areas where the COVID-19 pandemic is overwhelming, the number of patients with severe COVID-19 and ARDS has exceeded long the capacity of intensive care units (ICU). Since the declaration of a pandemic by the World Health Organization (WHO) in March 2020, clinicians have started a race to find an effective treatment to prevent and cure the development of the most serious forms of the disease. Current management of COVID-19 is supportive, and respiratory failure from ARDS is the main cause of death [1,2]. Mortality in patients with COVID-19 and ARDS who are admitted to the intensive care unit (ICU) is high, ranging from 26% to 78% [1-3]. Interestingly, mortality is increased in patients with pronounced systemic/ pulmonary inflammation and symptoms driven by IL-1 are the most common in COVID-19 patients. The current pharmacologic empiric management of these severe forms included biologic drugs, among them, interleukin (IL)-1 and IL-6 blockers [4,5]. In the current practice, IL-6 blocker tocilizumab (TCZ) is primarily used in addition with the “accepted” standard of care that includes: antiviral plus hydroxychloroquine plus prophylactic LMWH and sometimes methylprednisolone/dexamethasone [6] Other immune suppressive are also empirically been used, such as, intravenous immune globulins (IVIG), cyclosporine A (CyA), JAK/STAT inhibitors or plasma hiper immune [4]. Even so, some patients fail to this schema leading them to mechanical ventilation and eventually to death.
Anakinra is an interleukin IL-1 receptor antagonist that blocks activity of the pro inflammatory cytokines IL-1α and IL-1β and is used to treat auto inflammatory/rheumatic disorders at a daily dose of 100 mg subcutaneously [7]. Interestingly, a subgroup of patients with COVID-19 shows hyper inflammatory syndrome that resemble the cytokine storm related to macrophage activation syndrome, with release of IL-1, IL-6, IL-18, and interferon γ [3,4]. Cytokine blocking agents, including high-dose anakinra, are effective treatments for these disorders [8]. Preliminary series of patients with severe COVID-19 treated with low-to-high dose anakinra have yielded promising results [8-12]. In addition, anakinra has a remarkable safety when compared with other IL blockers particularly because of its short half-life, which allows prompt discontinuation, minimizing the risk of infection being, therefore, suitable for use in critically ill patients [5]. We aimed to assess clinical response at 48 hours, at 7 days and outcomes at discharge in 12 consecutive patients with severe COVID-19, ARDS, and hyper inflammatory syndrome outside the ICU who received anakinra in addition to non-invasive ventilation and who had been non–responders or refractory to “standard of care” plus tocilizumab.
2. Material and Methods
2.1 Patients and study design
From mid-March until end of May, patients with severe COVID-19 who required in-hospital management who failed to standard of care plus TCZ, were retrospectively assessed in two university hospitals, Althaia Network and Vall d’Hebron University Hospital, in Barcelona, Spain. COVID-19 was diagnosed by quantitative RT-PCR and either chest radiography or CT. Moderate to severe ARDS-related to SARS-CoV-2 was defined as acute-onset respiratory failure with bilateral infiltrates on chest radiography or CT, hypoxemia (ratio of the partial pressure of oxygen in arterial blood to the fractional concentration of oxygen in inspired air [PaO2:FiO2] ≤200 mm Hg with a positive end-expiratory pressure [PEEP] of at least 5 cm H2O), and no evidence of left atrial hypertension. Hyperinflammatory syndrome was defined as ferritin ≥ 800 ng/ml or C-reactive protein (CRP) ≥ 100 mg/L or D-dimer ≥ 1000 µg/L or IL-6 ≥ 40 pg/ml or some of them. At that time, the accepted “standard of care” included: azithromycin plus hydroxychloroquine plus lopinavir/ ritonavir plus prophylactic low-molecular weight heparin and low-to-moderate-dose methylprednisolone.
When clinical and respiratory function data did not improve or worsen, patients were put on TCZ 600 mg intravenous (iv) in single or double doses 12 hours apart. Patient number 1 was also put on IVIG and patient 5 on remdesivir. If patients showed no improvement in the ventilatory values, a rescue therapy with anakinra (Kineret®, Swedish Orphan Biovitrum, Stockholm, Sweden) was started.
Patients younger than 18 years-old, those already admitted to the ICU for mechanical ventilation, with evidence of bacterial infection before anakinra, individuals with concomitant administration of other cytokine blockers or JAK-inhibitors, and people who had been enrolled concomitantly in clinical trials were excluded. Time of response, mechanical ventilation-free survival, survival, changes in ferritin, D-dimer, C-reactive protein, lymphocyte count and clinical status was registered at 24 hours (not depicted), 48 hours, and 7 days after anakinra treatment. Clinical condition, pulmonary function at discharge, re-admissions and mortality were also evaluated.
2.2 Treatment with anakinra
Anakinra 100 mg twice subcutaneously (compassionate use) was added. Two cases (number 11 and 12) received 3-day course of 100 mg q6 hours with further reduction doses. Treatment was maintained empirically 7 days until achieve sustained clinical benefit, defined as a reduction of inflammatory markers and, particularly, a sustained respiratory improvement for at least 2 days. In patients achieving sustained clinical benefit, discontinuation of anakinra was followed empirically by decreasing dose each three days until cessation which was deemed useful to avoid possible inflammatory relapses. Lack of clinical improvement at 7 days, the apparition of bacteraemia/sepsis or side-effects were considered causes to cease anakinra treatment.
2.3 Main Outcomes
Primary outcome was to evaluate the clinical response, particularly the pulmonary function and the mortality rate. Arbitrarily, we defined PAFI ≥ 200 mmHg at day 7 post anakinra as marker of response. Secondly, to evaluate the number of cases that needed MV and ICU admissions. Owing to their clinical status and the emergency of the situation, patients were orally informed and all of them gave their consent orally. This information was reordered in an electronic medical history.
2.4 Ethics
This study has been carried out in accordance with The Declaration of Helsinki for experiments involving humans. Furthermore, ethical approval for this study - Project No PR (AG)204/2020 - was provided by the Ethical Committee of Vall d’Hebron University Hospital, Barcelona, Spain met in regular session on March, 2020.
2.5 Statistics
Data were analyzed with GraphPad Prism version 6 (San Diego, Cal, USA) and statistics performed with the embedded software. Comparisons among treatment progression were done with non-parametric one-way ANOVA (Dunn’s multiple comparisons).
3. Results
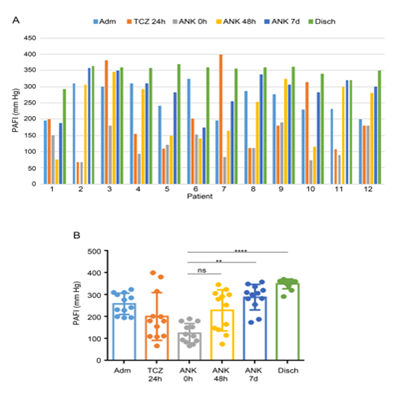
Fifteen patients received subcutaneous (sc) anakinra after standard treatment plus tocilizumab and plus non-invasive ventilation failed. Three out of 15 cases were excluded because they only received anakinra for 2 days. Finally, 12 cases were included for analyses. Main epidemiological data, clinical and analytical results at patient admission are depicted in Table 1. Briefly, the mean age was 60 (range: 20-80), with male predominance (8/4). At admission, all showed tachypnea and PAFI were abnormal in 8/12 (66.67%) cases. Ferritin, CRP, LDH, D-dimer and IL-6 levels were elevated in all cases. All but three (75%) showed absolute and marked lymphocytopenia (Table 1). Main clinical and analytical findings after TCZ and anakinra administration are shown in Tables 1 and 2. Before anakinra, fifty percent of cases had PAFI ≤ 100 mm Hg ant the remainder ≤ 150 mm Hg. Interestingly, clinical and respiratory improvement began at 48 hours post anakinra in 10/12 (83%) cases. In the responders at 48-hours, PAFI increases a mean of 133.80 mm Hg (range: 28-239) and in percentage a mean of 132.01% (range: 23.33-356.72). While considering all patients, PAFI percentage increased 105.18% (range: -50.00-356.72).
The two non-responders (patients number 1 and 6) were also discharged after long-hospital stay. Infectious complications appeared in patient 1 with bacteriuria and bacteremia. At 48 hours post anakinra, CRP, D-dimer, ferritin levels and lymphocyte count improved only in 30-40% of cases. At day 7, clinical status and PAFI showed improvement in all patients but two 10/12 (83%) accomplished the criteria of respiratory improvement (PAFI ≥ 200 mm Hg; Table 1; Figure 1A). Overall, at this time a significant increase of PAFI [288.6 ± 58.14 mm Hg (mean ± SD), p<0.0001] was observed over PAFI at anakinra administration [124.5 ± 44.42 mm Hg] (Figure 1B). At discharge, improvement or normalization of inflammatory parameters and respiratory function were attained in all treated cases. Patient 1 and 6 were considered as anakinra non-responders because PAFI were < 200 mmHg at day 7. Patients number 1 and 7 needed mechanical ventilation (MV). The latter case was put on MV but could be extubated at day 3 post anakinra. The reminder patients did not require MV. No deaths occurred in this series (Supplementary Table S1). Patients remained in the hospital for an average of 20.8 days (range: 14-35) and were discharged with subsequent follow-up in outpatient clinic. After 4-8 weeks, no patients needed to be readmitted.
Legend/Footnote: PAFI evolution during patient hospitalization. (A) Patients from 1 to 12 are showed individually and each bar represent PAFI assessment at a specific time during treatment. (B) Comparisons of PAFI among treatments over time. Each dot represents a patient. Non-parametric one-way ANOVA with Dunn’s multiple comparisons (ns, non-significant; ** p<0.01; **** p<0.0001). Legend: Adm, admission; TCZ 24h, 24 hours after tocilizumab administration; ANK 0h, at the moment of anakinra administration; ANK 48h, 48 hours after anakinra administration; ANK 7d, 7 days after anakinra administration; Disch, discharge.
Table 1: Mean epidemiological features. Clinical and analytical results of patients at admission and after tocilizumab and anakinra administration
*Optiflow
Admin: administration; ANK: Anakinra; AoI: Aortic Insufficiency; BR: Breath rate; CKD: Chronic Kidney Disease; CRP: C-Reactive protein; DDO: Days since disease onset; DLP: Dyslipidemia; DM2NID: Non-insulin dependent diabetes mellitus II; HBP: High Blood Pressure; IL-6; Interleukin 6; Lympho: Total lymphocytes; PAFI: PaO2/FiO2; TCZ: Tocilizumab; U: Unknown.
4. Discussion
In this retrospective case-series of 12 patients with poor response to accepted standard of care plus TCZ for severe COVID-19, a 7-day course of low-to-moderate subcutaneous anakinra showed already clinical benefit at 48 hours in 10 of 12 cases with maintenance at 7 days and until discharge. This fact allowed us to avoid intubation in all but one responders. Treatment was well tolerated, and discontinuation was not followed by relapses. Our study is the first reporting benefits in non-intubated patients with severe COVID-19 and ARDS treated with anakinra who previously failed to IL-6 blockade with TCZ treatment.
In severe COVID-19, activation of inflammasome NLRP3 participates in the release of high amounts of pro-inflammatory cytokines, particularly IL-1 and IL-18. Furthermore, the activation of NF-kB pathway favors the release of IL-6 and IL-8 [4,8]. Thus, it appears to be a rationale for using IL-1 and/or IL-6 blockers in the therapeutics of COVID-19. TCZ administered with the same protocol used in cytokine release syndrome has provided encouraging results [13]. However, cases with TCZ failure do exist, prompting the search for other cytokine blockers. In addition, since IL-1 can induce IL-6 release, it is possible that anakinra could be more effective blocking the cytokine storm. Anakinra is a recombinant, human interleukin-1 receptor antagonist. The possibility to be administered sc or iv route is an advantage, although the 95% bioavailability after a single sc bolus administration makes unnecessary the iv route [5,14]. Data on the doses able to block IL-1α/IL-1β in severe COVID-19 are unknown yet. Off-label doses of anakinra either 100 mg twice daily sc or up to 10 mg/kg/day iv have been used [9-12]. The length of anakinra treatment is also empirical. A time-course of 7-10 days has been the most used for the clinicians [9-12]. Noteworthy, in our series, only two patients were admitted in ICU and invasive ventilation was temporary needed. Importantly, there were no deaths. Although this series was not including controls, the expected mortality in similar cases not treated with anakinra was as high as 27-78% [1-3].
The accepted clinical risk factors for poor outcomes in severe COVID-19 were poorly represented in our series. Only 5 cases were older than 65. Overt hypertension and heart diseases were not disclosed at admission. These facts could explain in part the absence of mortality in these patients. However, we think anakinra could have played a major role to achieve a good outcome. PAFI has been the better objective marker for severity and for good recovery too. The increase of PAFI at 48 hours post-anakinra was present and really notable at day 7, with a clinical improvement and decreasing oxygen requirements. Similar results have been found by Cavalli et al [9], Dimopoulos et al [10], Aouba et al [11] and Portoli et al [12]. Nevertheless, in our study markers of hyperinflammation did not diminish in all cases as fast as expected. In these previously mentioned series, the mortality ranged from 0 to 40% although this latter result happened in cases with hemophagocytic syndrome related to COVID-19 [13]. Recently, Navarro-Millán et al [15] reported that sc administration of anakinra was able to avoid MV and reduce mortality in ARDS patients with COVID-19. Similar results have been reported by Kyriazopoulou et al [16] meta-analysis, using iv anakinra with or without associated corticosteroid use and Balkhair et al [17], both series with pneumonia-associated COVID-19 patients requiring oxygen supplementation. On contrary, the CORIMUNO-19 Collaborative Group stated anakinra did not improve outcomes in patients with mild-to-moderate COVID-19 pneumonia [18].
In our series, bacterial infection was only present in one case that resolved with antibiotics. Thus, a 7-day course of anakinra did not suppose an added risk factor for bacterial superinfection in these non-intubated patients. The short half-live of anakinra (4 to 6 hours) and relatively short length of treatment may suppose an advantage compared with other biologic drugs such as IL-6 or TNF inhibitors in terms of preventing bacterial infection [5,15]. However, this study has several limitations, particularly a small sample size and the absence of controls. The strong point is the selected cases that include only those patients refractory to corticosteroids and IL-6 blockade.
In conclusion, in severe ARDS related to SARS-CoV-2 refractory to standard of care plus tocilizumab, low-to-moderate dose of anakinra as a rescue therapy showed effectiveness and safety, avoiding ICU admissions, mechanical ventilation and deaths.
Acknowledgements
We thank Dr. Ferrán Martinez-Valle for helping us in the collection of some patients. We also thank Ms. Ariadna Anunciacion-Llunell for helping us in the drafting of the manuscript.
Disclosure of Interest
The authors stated that they do not have any commercial or any other type of interest that may have influenced the drawing up and the results of this paper.
Author’s Contribution
Jaume Alijotas-Reig (JAR) Enrique Esteve-Valverde (EEV) Domingo Ruiz-Hidalgo (DRI) Gabriel López-Sánchez (GLS) Jaume Trapé-Pujol (JTP), Francesc Miró-Mur (FMM)
Conception or design of the work: JAR; EEV; DRI; FMM
Data acquisition: JAR; EEV; GLS; JTP; FMM
Analysis and interpretation of data for the work: All authors
Drafting the work: JAR; EEV; FMM
Revising the work critically for important intellectual content: All authors
Final approval of the version to be published: All authors
Agreement to the work contents: All authors
Funding
This study has been supported in part, by a grant from the SEMCC (Sociedad Española de Medicina y Cirugía Cosmética –Spanish Society of Cosmetic Medicine&Surgery-).
Data Availability Statement
All data related to patients and their complementary tests and exams have been retrieved and are available from the medical records belonging to the Vall d’Hebron University Hospital, Barcelona, and Althaia Healthcare University Network of Manresa (Barcelona), both in Spain.
References
- Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelliet A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 323 (2020):1574-81
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395 (2020): 497-506.
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395 (2020): 1054–62.
- Alijotas-Reig J, Esteve-Valverde E, Belizna C, Selva-O'Callaghan A, Pardos-Gea J, Quintana A, et al. Immunomodulatory therapy for the management of severe COVID-19. Beyond the anti-viral therapy: A comprehensive review.. .Autoimmun Rev 19 (2020):102569.
- Muñoz-Jiménez A, Rubio-Romero E, Marenco de la Fuente JL. Proposal for the use of anakinra in acute respiratory distress secondary to Covid-19. Reumatol Clin 17 (2020): 309-12.
- World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected Interim guidance 13 March (2020).
- Fleischmann RM, Schechtman J, Bennett R, Handel ML, Burmester GR, et al. Anakinra, a recombinant human interleukin-1 receptor antagonist (r-metHuIL-1ra), in patients with rheumatoid arthritis: A large, international, multicenter, placebo-controlled trial. Arthritis Rheum 48 (2003): 927-34.
- Grom AA, Horne A, De Benedetti F. Macrophage activation syndrome in the era of biologic therapy. Nat Rev Rheumatol 22 (2016): 259-68.
- Cavalli G, De Luca G, Campochiaro C, Della-Torre E, Ripa M, Canetti D, et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol 2 (2020): e325-31.
- Dimopoulos de Mast Q, Markou N, Theodorakopoulou M, Komnos A, Mouktaroudi M, et al. Favorable Anakinra Responses in Severe Covid-19 Patients with Secondary Hemophagocytic Lymphohistiocytosis. Cell Host & Microbe 28 (2020):117-23.
- Aouba A, Baldolli A, Geffray L, Verdon R, Bergot E, Martin-Silva N, et al. Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: case series. Ann Rheum Dis 79 (2020): 1381-2.
- Pontali E, Volp S, Antonucci G, Castellaneta M, Buzzi D, Tricerri F, et al. Safety and efficacy of early high-dose IV anakinra in severe COVID-19 lung disease. J Allergy Clin Immunol 146 (2020): 213-5.
- Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, Panoskaltsis N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med 355 (2006): 1018-28.
- Kineret, INN-Anakinra. European Medicines Agency (2020).
- Navarro-Millan-I, Sattui-Cortes S, Siegel C, Kuntz-Crow M. Use of Anakinra to prevent mechanical ventilation in severe COVID-19: A case series. Arthritis Rheumatol 72 (2020): 1990-7.
- Kyriazopoulou E, Huet T, Cavalli G, Gori A, Kyprianou M, Pickkers P, et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol 2 (2020): e393–e400.
- Balkhair A, Al-Zakwani, M. Al Busaidi, A. Al-Khirbash, S. Al Mubaihsi, BaTaher, J A. et al. Anakinra in hospitalized patients with severe COVID-19 pneumonia requiring oxygen therapy: results of a prospective, open-label, interventional study. Int. J. Infect. Dis 103 (2021): 288–296.
- CORIMUNO-19 Collaborative Group. Effect of anakinra versus usual care in adults in hospital with COVID-19 and mild-to-moderate pneumonia (CORIMUNO-ANA-1): a randomised controlled trial. Lancet Respir. Med 9 (2021): 295–304.


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 