Huaier Compensates Impaired Signal Transfer Inter/Intra Neurons in Central and Peripheral Nervous Systems
Manami Tanaka1*, Tomoo Tanaka1, Fei Teng2, Hong Lin2, Ning Li3, Zhu Luo3, Sotaro Sadahito4, Toshiyuki Suzuki5, Ding Wei6 and Zhengxin Lu7
1Bradeion Institute of Medical Sciences, Co. Ltd., Kanagawa 259-1145, Japan
2BGI-Shenzhen, Building NO.7, BGI Park, Shenzhen 518083, China
3BGI-Japan, Kobe 650-0047, Japan
4Department of Surgery, Kameda-Morinosato Hospital, Kanagawa 243-0122, Japan
5Department of Surgery, Oiso Hospital, Tokai University School of Medicine, Kanagawa 259-0198, Japan
6Japan Kampo NewMedicine, Co. Ltd., Tokyo 103-0025, Japan
7QiDong Gaitianli Medicines Co. Ltd., Jiangsu Province, China
*Corresponding author: Manami Tanaka, Bradeion Institute of Medical Sciences, Co., Ltd., Japan
Received: 30 May 2021; Accepted: 17 June 2021; Published: 25 June 2021
Article Information
Citation:
Manami Tanaka, Tomoo Tanaka, Fei Teng, Hong Lin, Ning Li, Zhu Luo, Sotaro Sadahito, Toshiyuki Suzuki, Ding Wei and Zhengxin Lu. Huaier Compensates Impaired Signal Transfer Inter/Intra Neurons in Central and Peripheral Nervous Systems. Archives of Clinical and Biomedical Research 5 (2021): 484-518.
View / Download Pdf Share at FacebookAbstract
Huaier (Trametes robiniophila murr) provides significant efficacy not only on cancer, but also various physiological disorders caused by disrupted transcriptional control on multiple signaling pathways. In the process of MEGA-DATA analysis of human transcriptomes, we observed impaired signal transfer in the central and peripheral nervous systems. Here we demonstrate the details of molecular systems to compensate impaired neural transmission, especially by mutated transcripts correlated with neurodegenerative alterations. These significant rescue of signal transfer inter/intra neurons was observed in the patients with the hereditary mutated EGFR, and receptor tyrosine kinases such as c-MET, HER2/neu (erbB2), Htt, Parkin, APP, SOD1, ALS2, and many oncogenes and tumour suppressor genes. The similar compensation has been also reported in the patients with Huaier and conventional chemotherapy using platinum (II) complex. Huaier treatment prevented those patients from pathogenesis, but influenced to cause mild depression. The epigenetic potential seems to influence the pathogenicity in these hereditary mutations, and that typically observed in the defects in DNA mismatch repair systems. With KEGG pathway characterization, Huaier showed significant effects on the retrieval of normal function inter/intra neurons. Although we have observed only one case successfully recovered from Parkinson’s disease, the further roles of each representative molecules have not yet defined, and no changes identified in the mechanism of mutations in translation and transcription processes. The present study demonstrated detailed molecules and signaling pathways involved in the onset of neurodegenerative diseases, and the significant effects of Huaier to retain and rescue the impaired neurotransmission and signal transfer. Neurodegenerative damages are inevitably caused by chemical administration, and also by ageing process. However, it is emphasized
Keywords
<p style="text-align:justify">Huaier (<em>Trametes robiniophila murr</em>); cancer therapy; neurodegenerative diseases (Alzheimer’s’ disease, Parkinson’s diseases, Huntington’s diseases, and amyotrophic lateral sclerosis); neural transmitters and signal transfer pathways; EGFR, epidermal growth factor receptor (ErbB-1, HER1 in humans); c-MET, tyrosine-protein kinase Met or hepatocyte growth factor receptor (HGFR); <em>HER2/neu</em>; receptor tyrosine-protein kinase erbB-2, (former CD340); SLC6A4 solute carrier family 6 member 4 (Htt; Huntington’s disease); APP (glycoprotein amyloid-beta precursor protein); SOD1(superoxide dismutase gene); ALS2 (Alsin GTPase underlying in hereditary amyotrophic lateral sclerosis); platinum (II) complex (FORLFIRINOX)</p>
Article Details
Introduction
The successful treatment and prevention of neurodegenerative diseases are the topics in medical science [1, 2]. There are abundant failed promises, and the solution has not been obtained yet.
At the beginning of our investigation on molecular basis of anti-cancer efficacy of Huaier, we used transgenic Drosophila flies with overexpressing non-phosphorylatable Yorkie (Yki:V5S168A) as a cancer model [3]. This model was later revealed to be useful to screen the candidate compound for amyotrophic lateral sclerosis (ALS), however, nearly 5 years’ research resulted in vain. Recently, it was broad-cast announced that the one of medicine for Alzheimer’s disease might delay the onset for several months, but also emphasize that they could not identify no possible candidate drugs, even by the screening of thousands and thousands compounds in vitro. Simultaneously with our genome scope project from 2018 [4-10], we always anticipated to have a clue for the treatment of ALS, and other neurodegenerative diseases such as Huntington’s disease, Alzheimer’s disease, and also Parkinson’s disease.
In contrast, currently cancer becomes controllable disease, with a combination of modern technology of surgical dissection, targeting radiology, immunotherapy, and effective conventional chemotherapy with supplemental Huaier treatment [6, 8, 9]. Huaier was defined as effective for cancer prevention with a small dose (3g per day) [4], and also regulates iPS/ES production and normal differentiation for the regeneration of damaged tissues [8, 9].
The demand for a solution to major neurodegenerative diseases then remains unsolved, not to say a solution to prevention of stress accumulation by ageing. It is very strange to find that a final goal resembles very much to rejuvenescence or immortality, just the same objective ordered by Qin Shi Huang, the first Emperor in Qin dynasty, at B.C. 247−210 [11-14]. Surprisingly, the outcome of the ancient expedition was Huaier, which contributed health maintenance, but no contribution to immortality.
In our genome scope project, we happened to identify the remarkable genetic alterations in meningioma patient [7]. This patient had a long history of many sufferings irrelevant each other, such as migraine headache, symptoms from hormonal discoordination, mild depression, continuous skin rashes (occasionally appeared in different places), symptoms from accumulated stresses such as hepatic dysfunction, sleep disorders. The memory loss was often observed, which leads her to a lack of conformity to social expectations. Until present time, Huaier treatment (20g per day for 2 years) resulted in 1) swift recovery of the injury by surgical operation, and 2) the prevention of the recurrence, as frequently observed in similar cases in Japan.
The identified mutations were identified among all the members of the epidermal growth factor receptor (EGFR; ErbB-1; HER1 in humans), among ErbB family of receptors, a subfamily of four closely related receptor tyrosine kinases: EGFR (ErbB-1), HER2/neu (ErbB-2), Her 3 (ErbB-3) and Her 4 (ErbB-4) [7, 15-20]. Huaier influenced a broad spectrum of impaired systems including; cell growth and differentiation; proliferation; survival; mortality; angiogenesis; for the control of carcer progression and tumorigenesis, together with apoptosis; chemoresistance; cytokine-cytokine receptor interaction; for cancer treatment, mismatch repair; nucleotide excision repair systems; for prevention of carcinogenesis and stem cell control, together with neural transmitter and signal transfer intra/inter neurons [9].
Surprisingly, by the screening of KEGG characterization analysis [21], we identified severe disturbance in signal transfer network in neurons, represented by the mutations in HTT (Huntington’s; SLC6A4 solute carrier family 6 member 4 [22]); 5HTT; OCD1; SERT; 5-HTT; SERT1; hSERT; 5-HTTLPR which encodes an integral membrane protein that transports the neurotransmitter serotonin from synaptic spaces into presynaptic neurons.
Similar down-regulation in neural transmission and signal transfer was observed in the patients treated with platinum (II) complex (FORFIRINOX, FOLFOX6, and Cisplatin) [6, 23-25]. The molecular basis for the severe adverse events by those platinum (II) complex therapy was begun with the total destruction of RNA synthesis, followed by massive down-regulation of transcripts in every signaling pathway, especially in signal transfer in central and peripheral nervous systems within 90 days. Huaier successfully recoverd the impaired function by 60g per day administration for 6 months, followed by 20g per day for an year [6].
The present study, together with the former paper [6], demonstrates the related molecules and siganaling pathways for compensation of impaired signal transfer inter/intra neurons in the central and peripheral nervous systmes. Huaier administration contributed to the rescue of impaired cell communication systems by influencoing a wide variety of signal transferring pathways. The treatment focused on specified target molecules should have a certain limitation to influence all these alterations, some with up-rebulation, and the others with down-regulation at a time. There are scarce medicine or candidate compounds even for mutated EGFR and receptor tyrosine kinases. The present study thus provides a clue for the demand for successful treatment and prevention of neurodegenerative diseases by Huaier.
Materials and Methods
Project Design and patients’ profile
The present study specifically focussed on Huaier effects to the neurological systems. Patients’ characterization and information have been introduced previously [4].
Huaier compounds were provided by the manufacturer for this purpose with a strict control on transfer to Japan, good condition for maintenance, and provision to the patient volunteers, just as the same as the previous reports.
The present study was strictly conducted according to the guidelines of the Declaration of Helsinki and the principles of good clinical practice [4-10]. Written informed consent was obtained from the patients. This clinical research was applied according to the Consolidated Standards of Clinical Research Trials guidelines and was applied to the Japanese Medical Association on 9th February 2018, and approved on 5th March, 2018 (ID: JMA-IIA00335). The project has been strictly conducted with a monthly review by the ethics committee consisted by the experts on Medicine, Nursing, Laws, Pharmaceutics and Business Community (first committee held on 9th February, 2018).
We used Huaier compounds as complementary therapy, without any chemotherapy and radiotherapy which disrupt the molecular systems. Only surgical operation was allowed if applicable, even in the period of during Huaier therapy. We thus planned and initiated an open-style, before-after controlled study, using peripheral blood as sampling materials to understand the almost all molecular events in each Huaier taking patient. The sampling materials were total blood, the same as reported previously [4-10]. To compare with the other sampling, RNA extraction using nuclear cell components in peripheral blood rapidly reflects the biophysiological changes, and that more sensitive to monitor the course of any treatment than any other samples such as dissected organs.
Total RNA and small RNA analysis
RNA extraction, miRNA library construction, and Total RNA- and small non-coding RNA-sequencing on the MGISEQ-2000 and BGISEQ-500 platform [26, 27] were processed in BGI, Shenzhen, China, as descrived previously [4-10]. The subseuent bioinformatics work was also processed in BGI, Shenzhen, China. The detailed protocols were provided and demonstrated at BGI website: http://www.bgitechsolutions.com/.
The identified DEGs (differentially expressed genes) and DESs (differentially expressed small RNAs) were analyzed between samples and do clustering analysis and functional annotations.
With quantitative analysis of DEGs, we performed Gene Ontology (GO) classification by three categories of molecular biological function, cellular component and biological process, with a consideration of time course of Huaier administration. We also analysed every signal transduction pathway by KEGG pathway classification (https://www.genome.jp/kegg/) [21]. Furthermore, we applied the enrichment analysis of DEG in KEGG database.
The obtained novel transcripts and small nuclear non-coding RNA have been deposited to The NCBI GEO (GSE157086), and continuously up-loaded with newly identified sequences throughout the project period [4-10].
Results
Once case of recovery observed in the patient diagnosed as Parkinson’s disease
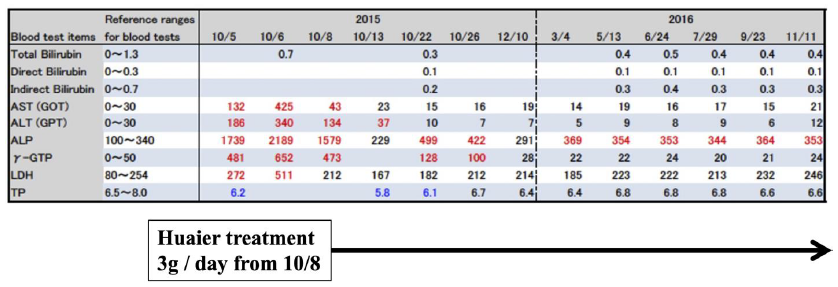
The successful recovery of one case diagnosed by Parkinson’s disease was shown in Fig. 1. One patient of 87 years’ old female also had a familial liver dysfunction. She was administered several drugs by as many independent clinics. As shown in Fig. 1, striking improvement was observed within one night after 3g of Huaier administration, and finally, succeeded to rescue liver function within normal level in the end. Of course, the symptoms and disorders were all disappeared. We could not identify the molecular mechanisms underneath of the Huaier effect in this patient in 2015, since it was before the beginning of our genome scope project.
The result seemed to be dependent not only from Huaier treatment, but also from the withdrawal of the other chemical administration. The patient maintained a good condition up to 2021.
Huaier effects on multiple signal transfer intra/inter neurons in the central and peripheral nervous systems
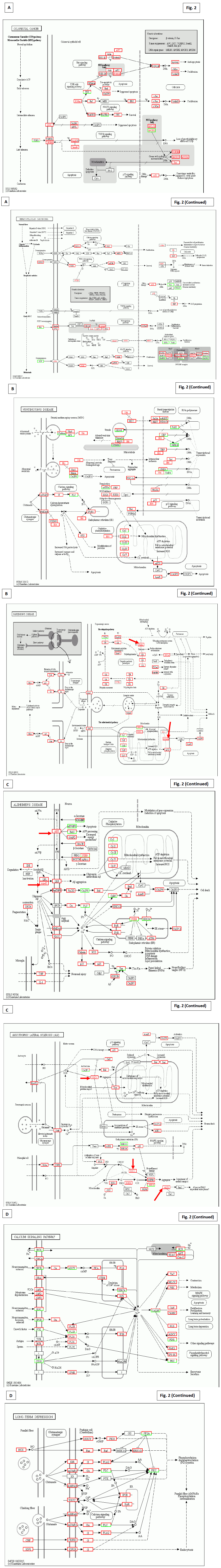
KEGG characterization results of the patient used in the present study (the patient No. 14 in the original paper [4]) were demonstrated to indicate multiple mutations and their compensation in Figs. 2-4, observed after 3 months’ Huaier administration, with a comparison to the successful results to retain normal condition after 2 years’ of Huaier treatment. The mutation detected in oncogenes and tumour suppressor genes, and consequent alterations in major signalling pathways were noted in each panel, as used in the previous reports (the patient No. 8 in the original paper [4]).
In Fig. 2A, panels explained the Huaier effects on basic diseases and disorders. The patient was 61 years’ old female, and had various symptoms irrelevant each other, such as migraine headache, symptoms from hormonal discoordination, mild depression, continuous skin rashes (occasionally appeared in different places), symptoms from accumulated stresses such as hepatic dysfunction, sleep disorders. The memory loss was occasionally observed, which leads her to a slight lack of conformity to social expectations (usually within normal limits). In walk-in clinic, multiple cysts formation in liver and kidney, and multiple polyps in colon were detected. As the appearance of multiple cysts in liver was all of a sudden, we, including the patient and her family, immediately decided to have Huaier therapy, together with endoscopic dissection of colorectal polyps and treatment on latent Helicovacter pylori infection in stomach. The cure of rashes and other disorders were disappeared within 3 months, and no cancer progression and other neurological and psychological disorders were not identified ever after 3 years now. Mutated oncogenes and suppressor genes were listed in the gray box. Special attention should be paid to the mutation in the DNA mismach repair genes hMLH 1, 2, 3 and 6.
Fig. 2B, C, and D clealy indicates the mutated molecules, their lineage to multiple signaling pathways correlated with neurotransmittion, and consequent influence to total signaling cascades. Mutated molecules were indicated by red letter, and the up-regulation by highlighted by red box, whereas downregulation by green box. Extreme up-regulation of signaling molecules were clealy demonstated in the panels. These were the results after 3 months after Huaier administraion 20g/day, and these effects were observed from the first 30 days of Huaier treatment.
Fig. 2B and C indicate the rescue of mutated molecules in the representative neurodegenerative diseases. It was of particular concern to identify the mutations in APP (glycoprotein amyloid-beta precursor protein for Alzheimer’s disease), ApoE (apolipoprotein E), SOD1 (superoxide dismutase gene), and ALS2 (Alsin GTPase underlying in hereditary amyotrophic lateral sclerosis for amyotrophic lateral sclerosis) indicated by red arrows.
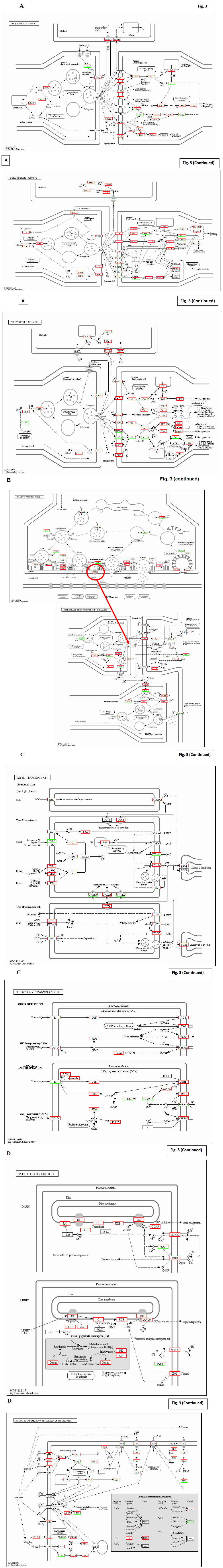
Fig. 2D shows detailed functional rescue in closely related pathway and in psychological system. In addition, Fig. 2A and B demonstrate further influences to neurotransmission, siganal transfer intra neurons, in the central nervous system. As for sensory nervous systems, Fig. 3A and B show the taste, hearing, and visual sense systems.
Figure 2: The detailed analysis of the Huaier effects on each cancer by KEGG biological pathways in the patient with sudden appearance of multiple cysts in liver and kidney. Multiple polyps in colon were also found and endoscopically dissected. The results demonstated in the panels were the results by 3 month after Huaieer administration. Panel A; The pathway characterization according to the originally suspected diseases, hepatocellular carcinoma and colorectal cancer, by the clinical data. The name of mutated expressed genes was indicated by red color, and up-regulated genes were highlighted by red box, whereas down-regulated ones by green box. Mutated oncogenes and gene suppressor genes were specifically written in gray box in each panel. Panel B; KEGG characterization on molecular modifications in Huntington’s disease and Parkinson’s disease. Note the mutations in Htt (Huntington’s disease) and Parkin (Parkinson’s disease) indicated by red arrows. Panel C; KEGG characterization on molecular modifications in Alzheimer’s disease and amyotrophic lateral sclerosis. Note the mutations in APP (glycoprotein amyloid-beta precursor protein for Alzheimer’s disease), ApoE (apolipoprotein E), SOD1 (superoxide dismutase gene), and ALS2 (Alsin GTPase underlying in hereditary amyotrophic lateral sclerosis for amyotrophic lateral sclerosis) indicated by red arrows. Panel D; KEGG characterization of the representative pathways, closely correlated with neural transmission. The relating psychological influence of Huaier administration was also demonstrated. Note that no genetic alterations were detected, only quantitative changes (up-regulation by red box, down-regulation by green box).
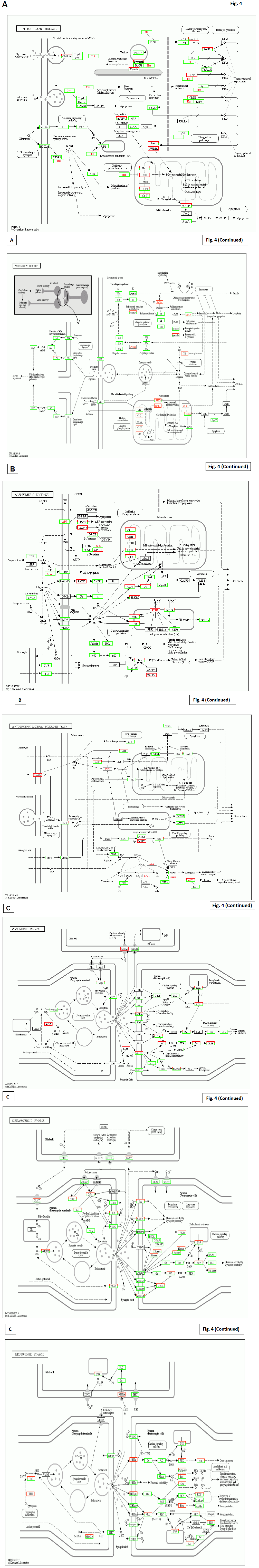
Fig. 4 is a compariton to the results from the other patient after 2 years’ Huaier treatment (20g/day), with similar symptoms with multiple cysts in liver and pancreas. This patient, 81 years’old male, has the same mutations shown in Figs. 1-3.
Figure 3: KEGG characterization of signal transfer intra/inter neurons. Panel A; From Up to bottom, the rescue of neurotransmission in; cholinergic synapse, Dopaminergic synapse, and serotonergic synapse. Quantitative regulation of signal transfer was highlighted in abundant molecules by red box. Panel B; Synaptic vesicle cycle were specifically focused. Panel C; The enhancement of signal transfer in taste transduction, olfactory transduction was demonstrated. Panel D; The enhancement of signal transfer in phototransduction and thermal recognition by inflammatory mediator was demonstrated.
The strong enhancement of neurotransmission was the typical observation after Huaier administration, and it took 2 years to be down-regulated into normal level. It was speculated to have some disorders or subjective symptoms at the beginning of Huaier treatment, but there were no complaint in daily life at all. The similar down-regulation was observed in the patient of pancreatic cancer with both lung metastasis, treated with conventional therapy using FOLFIRINOX [25] together with Huaier 60g/day as reported.
From the results shown in Figs. 2-4, the rescue of the impaired function by mutated molecules, with or without subjective symptoms, required to cope with multi-dementional biological systems with abundant quantity of molecules. It seems to indicate that Mono-targetted drugs or simple combination of possible compounds were not enough solution to the reality of the process of pathogenesity [3, 4, 9, 28].
Epigenetic insights into pathogenesis with mutated transcriptomes and compensation by Huaier
The post-transcriptional control, epigenetic processes seemd to matter the differences in pathogenecity among family members, from the observation of the altered levels of DNA repair enzymes such as MGMT, MLH1-3, and p53 [5, 7]. In addition, miRNA genes are also drastically altered which associated with CpG islands, that may be repressed by epigenetic methylation [3, 7].
At the same time, alteration of the expression level of WASP and SUMO (Small Ubiquitin-like Modifier) were detected to support the compensation of defected functions in signaling pathways such as; protein stability, nuclear-cytosolic transport, and transcriptional regulation [29-32]. The SUMO-1 modification of RanGAP1 (the first identified SUMO substrate) was also involved for compensation of Ubiquitin mediated proteolysis by trafficking from cytosol to nuclear pore complex.
DYRK1A (dual-specificity tyrosine-regulated kinase 1A), a kinase with multiple implications for embryonic development, especially in the nervous system, has been well known to link all these functions written above [33], and the results obtained from the present study indicated the similar potential of Huaier to link multiple signaling pathways. By the way, no alterations in DYRK1A were found in this patient and family.
Discussion
Thus, the present study provides detailed clue to the molecular events occurring to the normal Japanese population, latent possibility to cause neurodegenerative diseases and disorders and how to compensate the impaired functions by mutated molecules. It is a general understanding that, when signaling pathways interact with one another they form networks, they allow cellular responses to be coordinated, often by combinatorial signaling events [34-36]. At the molecular level, such responses include changes in the transcription or translation of genes, and epigenetic post-transcriptional and conformational changes in proteins. These molecular events are in total the basic mechanisms controlling cell growth, proliferation, metabolism and many other processes.
Ageing process resulted in disrupted transcriptional control on various signaling pathways, which perturbed the smooth transmission of the information to or inside brain. Consequently, it jeopardizes the decision in these individuals on the behavior at the time of encountering the environmental stress and personal relationship in a society. A repeat length polymorphism in the promoter of sodium: neurotransmitter symporter family gene has been shown to affect the onset of depression, however, no significant relationship has not been detected by MEGA-DATA analysis technology [26, 37] throughout our clinical research.
The mode of action of Huaier very much resembles to the reported functions of DYRK1A [33]. There might be more agents with the similar potential, but not yet introduced in public before. However, a strategy for successful prevention and treatment of neurodegenerative diseases, with multiple hereditary mutations, requires to rescue many disrupted transcriptional controls which resulted in the rescue of functions. In addition, Huaier effects improved slightly on memory loss, especially on a long-term memory as shown in Fig. 2D. The compensation for signal transfer seemed to rescue the neurotransmission to the brain [38-41].
More importantly, the mutated transcripts in APP (glycoprotein amyloid -beta precursor protein) [42], ApoE (apolipoprotein E) [43] , Parkin (465 -residue E3 ubiquitin ligase) [44], Htt (Huntington) [45], SOD1 (super oxide dismutase Cu-Zn) [46, 47] and ALS2 (Alsin) [48] were identified in the patients (Fig. 2 B and C). These mutations were reported for their crucial roles to decide the pathogenicity and onset time of the diseases. Those molecules and encoding genes were all defined as critical in the pathogenesis. It is surprising to identify mutations in all these not only in this patient analysed in the present study, but also many volunteer cancer patients in our clinical research. The patients themselves and their family member did not show the symptoms and disorders so far, which strongly suggested that the pathogenicity of these neurodegenerative diseases were not only hereditary factors but also epigenetic influences. In addition, it is notable to find their original birth places were South-Western counties in Japan.
Although the individual genomic potential to undergo those changes and modulations after Huaier administration is the key for the rescue of disrupted transcriptional control, Huaier has a significant potential to compensate for the impaired cell to cell communications [38, 39]. We emphasize that Huaier can not cure the system or process to mutate the gene expression of these molecules. Huaier does compensate the impaired functions.
Acknowledgements
The authors wish to thank cancer patient volunteers and many healthy volunteers kindly collaborated with the present study. We also wish to thank Prof. Dr. Tongbiao Zhao, Professor of Stem Cell and Immunology, Institute of Zoology, Chinese Academy of Sciences, China, for critical review and the comments on the project scheme; the reviewing committee for the medical ethics and safety monitoring of the project. The present study was grant-in-aid from QiDong Gaitianli Medicines Co., Ltd. And Japan Kampo NewMedicine, Co., Ltd.
Author contributions
T.T., M.T., designed the study from the clinical observation of the cancer patients with Huaier treatment (as a complementally therapy), and managed the sampling and clinical assessment of the patient volunteers, statistically analyzed the data, and drafted the manuscript. F.T., H.L., managed total RNA and small nuclear RNA sequencing and conducted systematic analysis of the data. S.S. and T. S., contributed clinical diagnosis and treatment of the patients, together with the assessment of QOL and the effects of Huaier administration, Z.L., D.W., contributed to the provision of Huaier granules and clinical evaluation of the data, especially focused on Immunological evaluation.
Conflict of interest
The authors have no competing interest to declare.
Author information
Readers are welcome to comment on the paper. Correspondence should be addressed to T.T. (ttanaka@bradeion.com) and M.T. (manami-tanaka@bradeion.com), and those researchers contributed equally to this work. Requests for Huaier extract and commercially-available granules should be addressed to D.W. (teii@newkampo.co.jp) and M.T. (manami-tanaka@bradeion.com).
References
- Rubinsztein DC. The roles of intracellular protein-protein-degradation pathways in neurodegeneration. Nature 443 (2006): 780-786.
- Bredesen DE, Rao RV, Mehlen P. Cell death in the nervous system. Nature 443 (2006): 796-802.
- Tanaka T, Suzuki T, Nakamura J, Kawamura Y, Sadahiro S, et al. Huaier regulates cell fate by the rescue of disrupted transcription control in the Hippo signaling pathway. Arch Clin Biomed Res 1 (2017): 179-199.
- Tanaka M, Tanaka T, Teng F, Lin H, Li N, et al. Huaier Induces Cancer Recovery by Rescuing Impaired Function of Transcription Control Based on the Individual Genomic Potential. Arch Clin Biomed Res 4 (2020): 817-855.
- Tanaka M, Tanaka T, Teng F, Lin H, Li N, et al. Anti-cancer effects of Huaier on prostate cancer; miRNA-mediated transcription control induced both inhibition of active progression and prevention of relapse. J Altern Compl Integr Med 7 (2021): 146-155.
- Tanaka M, Tanaka T, Teng F, Lin H, Li N, et al. Complete remission of the severe advanced stage cancer by miRNA-mediated transcriptional control of Bcl-xL with Huaier therapy compared to the conventional chemotherapy with platinum (II) complex. Arch Clin Biomed Res 5 (2021): 230-261.
- Tanaka M, Tanaka T, Teng F, Lin H, Li N, et al. Huaier inhibits cancer progression correlated with the mutated EGFR and other receptor tyrosine kinases (c-MET/erbB-2) by down-regulation of multiple signal transduction pathways. Arch Clin Biomed Res 5 (2021): 262-284.
- Tanaka M, Tanaka T, Teng F, Lin H, Li N, et al. Huaier inhibits cancer progression and induces tissue regeneration by transcriptional regulation of pluripotency of stem cells. J Altern Compl Integr Med 7 (2021): 162−172.
- Tanaka M, Tanaka T, Teng F, Lin H, Li N, et al. Huaier therapy for successful recovery of cancer and health maintenance: Steady progress and the end of failed promise. Arch Clin Biomed Res 5 (2021): 457-483.
- Tanaka T, Tanaka M, Teng F, Lin H, Li N, et al. Molecular basis of Huaier effects on immunomodulation, and natural selection of iPS cells with stable growth in vivo. [PowerPoint Slides]. J Pharm Res Dev. (2021): https://unisciencepub.com/ppts/
- Li L, et al. Progress on experimental research and clinical application of Trametes robiniophila. Bull Chin Cancer 16 (2007): 110-113.
- Sun Y, Sun T, Wang F, Zhang J, Li C, et al. A polysaccharide from the fungi of Huaier exhibits anti-tumor potential and immunomodulatory effects. Carbohydr Polym 92 (2013): 577-582.
- Song X, Li Y, Zhang H, Yang, Q. The anticancer effect of Huaier (Review). Oncol Rep 34 (2015): 12-21.
- Wang X, Wang N, Cheung F, Lao L, Li C, et al. Chinese medicines for prevention and treatment of human hepatocellular carcinoma: current progress on pharmacological actions and mechanisms. J Integr Med 13 (2015): 142-164.
- Herbst RS. Review of epidermal growth factor receptor biology. Int J Rad Oncol Biol Physics 59 (2004): 21-6.
- Bottaro DP, Rubin JS, Faletto DL, Chan AM, Kmiecik TE, et al. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science 251 (1991): 802-804.
- Cooper CS. The met oncogene: from detection by transfection to transmembrane receptor for hepatocyte growth factor. Oncogene 7 (1992): 3-7.
- Comoglio PM, Trusolino L, Boccaccio C. Known and novel roles of the MET oncogene in cancer: a coherent approach to targeted therapy. Nat Rev Cancer 18 (2018): 341-358.
- Zhang H, Berezov A, Wang Q, Zhang G, Drebin J, Murali R, Greene MI. ErbB receptors: from oncogenes to targeted cancer treatment. J Clin Invest 117 (2007): 2051-2058.
- Coussens L, Yang-Feng TL, Liao YC, Chen E, Gray A, et al. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science 230 (1985): 1132-1139.
- Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res 36 (2008): 480-484.
- Coleman JA, Gfreen EM, Gouaux E. X-ray structures and mechanism of the human serotonin transporter. Nature 532 (2016): 334-339.
- Fishel JL, Formento P, Ciccolini J, Rostagno P, Etienne MC, et al. Impact of the oxaplatin-5 fluorouracil-folinic acid combination on respective intracelluylar determinants of dfug acticity. Br J Cancer 86 (2002): 1162-1168.
- Michele Peyrone (1813-1883). Discoverer of Cisplatin, Platinum Metals Review 54 (2010): 250-256.
- Conroy T, Hammel P`, Hebber M, Ben Abdelghani M, Wei AC, et al. FOLFIRINOX or Gemcitabine as adjuvant therapy for pancreatic cancer. New Eng J Med 375 (2018): 2395-2406.
- Peng Z, Cheng Y, Tan BC, Kang L, Tian Z, et al. Comprehensive analysis of RNA-Seq data reveals extensive RNA editing in a human transcriptome. Nat Biotechnol 30 (2012): 253-260.
- Ramsköld D,Luo S,Wang Y, Li R, Deng Q, et al. Full-Length mRNA-Seq from single cell levels of RNA and individual circulating tumor cells. Nat Biotechnol 30 (2013): 777-782.
- Chongtham A, Agrawal N. Crucumin modulates cell death and it protective in Huntington’s disease model. Sci Rep 6 (2016): 18736.
- ClarkVE, Erson-Omay EG, Serin A, Yin J, Cotney J, et al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science 339 (2013): 1077-80.
- Hay RT. SUMO: a history of modification. Mol Cell 18 (2005): 1-12.
- Miki H, Sasaki T, Takai Y, Takenawa T. Induction of filopodium formation by a WASP-related actin-depolymerizing protein N-WASP. Nature 391 (1998): 93-96.
- Mahajan R, Delphin C, Guan T, Gerace L, Melchior F. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell 88 (1997): 97-107.
- Fernández-Martínez P, Zahonero C, Sánchez-Gómez P. DYRK1A: the double-edged kinase as a protagonist in cell growth and tumorinenesis. Mol Cell Oncol 30 (2015): e970048.
- Papin JA, Hunter T, Palsson BO, Subramaniam S. Reconstruction of cellular signalling networks and analysis of their properties. Nature Rev Mol Cell Biol 6 (2005): 99-111.
- Krauss G. in Biochemistry of Signal Transduction and Regulation. 2008; Wiley-VCH. p. 15. ISBN 978-3527313976.
- Mo JS, Park JW, Guan, KL. The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep 25 (2014): 642-65.
- Song Y, Li L, Ou Y, Gao Z, Li E, et al. Identification of genomic alterations in oesophageal squamous cell cancer. Nature 509 (2014): 91-95.
- Papin JA, Hunter T, Palsson BO, Subramaniam S. Reconstruction of cellular signalling networks and analysis of their properties. Nature Rev Mol Cell Biol 6 (2005): 99-111.
- Krauss G. in Biochemistry of Signal Transduction and Regulation. (2008) Wiley-VCH. p. 15. ISBN 978-3527313976.
- Bailey CH, Kandel ER, Si K. The persistence of long-term memory: a molecular approach to self-sustaining changes in learning-induced synaptic growth. Neuron 44 (2004): 49-57.
- Barco A, Bailey CH, Kandel ER. Common molecular mechanisms in explicit and implicit memory. J Neurochem 97 (2006): 1520-1533.
- Priller C, Bauer T, Mitteregger G, Krebs B, Kretzschmar HA, Herms J. Synapse formation and function is modulated by the amyloid precursor protein. J Neurosci 26 (2006): 7212-21.
- Puglielli L, Tanzi RE, Kovacs DM. "Alzheimer's disease: the cholesterol connection". Nat Neurosci 6 (2003): 345-51.
- Seirafi M, Kozlov G, Gehring K. Parkin structure and function. FEBS J 282 (2015): 2076-88.
- The Huntington's Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. (PDF). Cell 72 (1993): 971-83.
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362 (1993): 59-62.
- Cardoso RMF, Silva CHTP, Araujo APU, Tanaka T, Tanaka M, Garratt RC. Structure of the cytosolic Cu, Zn superoxide dismutase from Schistosoma mansoni. Biol Cryst Acta Cryst. D60 (2004): 1569-1578.
- Hadano S, Hand CK, Osuga H, Yanagisawa Y, Otomo A, et al. A gene encoding a putative GTPase regulator is mutated in familial amyotrophic lateral sclerosis 2. Nat Genet 29 (2001): 166-73.


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 