Clinical Utility of EsoGuard® on Samples Collected with EsoCheck® as a Triage to Endoscopy for Identification of Barrett’s Esophagus – Interim Data from the CLUE Study
Dan Lister1, Andy Fine2, Shail Maheshwari3, Paul S. Bradley4, Victoria T. Lee5*, Brian J. deGuzman5, Suman Verma5, Lishan Aklog5
1Arkansas Heartburn Treatment Center, Heber Springs, AR, USA
2Colorado Primary Health Care, Littleton, CO, USA
3Center for Digestive Disease, Shenandoah, TX, USA
4Savii Health, Savannah, GA, USA
5Lucid Diagnostics Inc, New York, NY, USA
*Corresponding author: Victoria T. Lee, Lucid Diagnostics Inc, New York, NY
Received: 15 November 2023; Accepted: 21 November 2023; Published: 06 December 2023.
Article Information
Citation: Dan Lister, Andy Fine, Shail Maheshwari, Paul S. Bradley, Victoria T. Lee, Brian J. deGuzman, Suman Verma, Lishan Aklog. Clinical Utility of EsoGuard® on Samples Collected with EsoCheck® as a Triage to Endoscopy for Identification of Barrett’s Esophagus – Interim Data from the CLUE Study. Archives of Clinical and Biomedical Research. 7 (2023): 626-634.
View / Download Pdf Share at FacebookAbstract
Background: Barrett’s Esophagus (BE) is the only known precursor for esophageal adenocarcinoma (EAC). Recommendations are to screen patients with multiple risk factors; however, few eligible patients undergo evaluation. EsoGuard® (EG) is a commercially available biomarker test, which when used to analyze esophageal cells collected non-endoscopically with EsoCheck® (EC), may serve as an easily accessible and well-tolerated qualitative diagnostic tool. This study evaluates real-world clinical utility of EG as a triage to upper endoscopy (UE) for diagnosis of BE.
Methods: First data snapshot from the multi-center, observational Clinical Utility of EsoGuard (CLUE) trial. 275 subjects enrolled between February 23 - July 28, 2023. Patient demographics, risk factors, EG results, and next steps in management were collected. Clinical Utility was evaluated based on the impact of EG test results on physician’s decision to refer/not refer patients for UE evaluation
Results: Average age was 61.9 years, with similar distribution of males and females. 89.7% had chronic gastroesophageal reflux disease (GERD), and 73.8% had GERD plus three additional BE risk factors. EG positivity was 29.3% (68/232); 229 subjects had both EG results and a physician decision on UE referral. Positive agreement between EG(+) results and referral for UE was 100%; negative agreement between EG(-) results and non-referral was 99.3%. Overall concordance between EG results and UE referral was 98.8%.
Conclusions: The first snapshot of the CLUE study demonstrates physicians ordering EG/EC in the commercial setting are reliably utilizing it as a triage to UE for evaluation of patients at high risk of BE/EAC.
Keywords
<p>Barrett’s Esophagus; Esophageal Adenocarcinoma; EsoGuard; EsoCheck; Clinical Utility; Triage; Screening</p>
Article Details
1. Introduction
Barrett’s Esophagus (BE) is a metaplastic condition of the lower esophagus and the only known precursor for esophageal adenocarcinoma (EAC), a malignancy which has had increasing incidence in Western populations over the last 40 years [1]. Experts identify the hallmark of BE as the presence of intestinal metaplasia i.e., replacement of normal squamous epithelium with specialized columnar epithelium with intestinal-type goblet cells [2]. Screening for BE and surveillance of those diagnosed with disease is supported by multiple societal guidelines because contrary to the lethality of EAC, BE can be successfully treated using several endoscopic eradication therapies which achieve complete disease eradication in over 80-90% of patients [3-5]. Even with EAC, there is substantial improvement in survival if identified in the earliest stages, although this is infrequent as most patients present with dysphagia, by which time the cancer is usually advanced [6] [7]. As such, the underlying goal of BE screening is to reduce EAC mortalities via diagnosis in the pre-neoplastic stage followed by either surveillance (non-dysplastic BE) or treatment (dysplastic BE) to effectively halt disease progression.[4] Diagnosis of BE is most frequently established when patients with refractory or severe gastroesophageal reflux disease (GERD) symptoms are found to have ≥1cm of “salmon colored mucosa” during upper endoscopy (UE) with presence of goblet cells on biopsy, but this approach to screening has several limitations. First, up to 44% of the population in Western countries have GERD [8], and when evaluating the incidence of other common risk factors (i.e., male sex, age >50 years, white race, etc.) it may not be realistic to perform screening endoscopy on everyone who meets criteria for elevated disease risk. Additionally, many patients with GERD utilize acid suppressive medications (recommended as part of disease management in published guidelines and from expert panels) [9] [10], and experience reasonable to good symptom control and may not seek or be referred for endoscopic evaluation, therefore BE in this population would continue to be missed. Unfortunately, while symptom control and reduced incidence of erosive esophagitis and peptic strictures are a benefit of acid suppressive medications, evidence suggests they do not reduce the risk of developing dysplasia or EAC in patients with BE [11, 12]. Clearly, better strategies for more widespread and earlier disease detection must be sought. One option is a two-step approach: first would be an easily accessible and non-invasive triage test to identify patients with high probability of disease, followed by a more invasive confirmatory test which also allows disease staging (i.e., UE with biopsies). EsoGuard® (EG) is a commercially available biomarker assay that when performed on esophageal mucosal cells sampled using the non-endoscopic, balloon based EsoCheck® (EC) device (EG/EC), offers a minimally invasive alternative to UE for initial qualitative detection of BE. This is an accepted strategy recognized by both the American College of Gastroenterology (ACG) and American Gastroenterological Association (AGA) [4, 13]. The goal of the ongoing, multicenter, prospective CLUE study is to capture real-world data from the commercial use of EG and evaluate the impact of test results on health care provider’s decision-making. CLUE focuses on patients with multiple risk factors that meet either ACG or (at minimum) AGA recommendations for BE screening and are at elevated risk for disease compared to the general population. The analysis presented here is for the first 275 subjects enrolled and for whom clinical utility data are available.
2. Methods
Prospective, multi-center, observational study to evaluate the utility of EG in the diagnosis of BE (CLinical Utility Study of EsoGuard® on Samples Collected with EsoCheck® as a Triage Test for Endoscopy to Identify Barrett’s Esophagus – CLUE; NCT06030180). Patient demographics, risk factors, EG results, and provider management decisions were recorded and analyzed for clinical utility assessment. Because the intended use of EG is as a triage to UE (with or without biopsies) the ‘gold standard’ confirmatory test, the key management decision captured in this study is the ordering physician’s decision whether to refer a patient for UE based on his/her EG result. Investigators are physicians who adopted EG/EC technology into their standard practice and were not deviating from usual care as part of study conduct. The first site was initiated 23-February-2023, and enrollment will continue until 500 evaluable subjects have been reached. We present the interim data collected through 28-July-2023 from the first 275 subjects enrolled across four study sites; interim data review is part of the approved study protocol. The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the WCG Institutional Review Board (IRB tracking number 20222402). All participating individuals signed informed consent prior to EC and collection of any study information.
2.1 EsoGuard® and EsoCheck® (EG/EC)
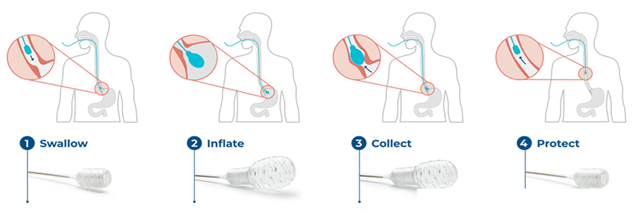
EG is a laboratory developed test (LDT) performed in a Clinical Laboratory Improvement Amendment (CLIA) certified and College of American Pathologists (CAP) accredited Central Lab that utilizes a set of genetic assays and algorithms which examine the presence of cytosine methylation at 31 different genomic locations on the vimentin (VIM) and Cyclin-A1 (CCNA1) genes. EG has been clinically validated in a developmental study published in 2018 and shown to have >90% sensitivity and specificity in detection of BE or EAC.[14] EG results are reported in a binary fashion (positive or negative) indicating presence or absence of methylation changes to suggest diagnosis of disease along the full BE progression spectrum, up to and including EAC. Quantity Not Sufficient (QNS) may be reported if the cell sample has insufficient DNA for EG analysis. Contaminated or otherwise unevaluable samples are reported as such, and the patient has the option to re-test. EC is an FDA 510K cleared, non-endoscopic device designed for the circumferential, targeted collection and retrieval of surface cells from the esophagus that can then be analyzed with diagnostic tests like EG. The unique, balloon-capsule technology allows for easy swallowing, non-traumatic cell sampling, and protection from specimen dilution during retrieval of the device through the upper esophagus and oropharynx (Figure 1). Cell collection is easy to perform in any office setting without sedation or specialized equipment, well-tolerated, and usually takes less than 5 minutes.
2.2 Study Population
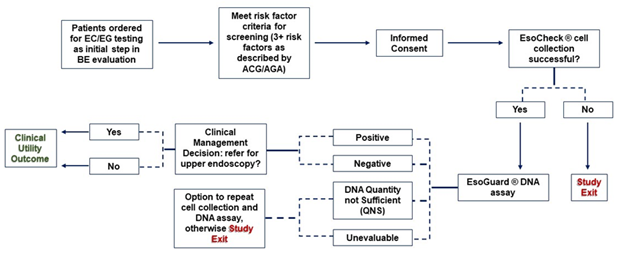
Eligible study participants are those whom a) their provider has made the independent clinical assessment of increased BE/EAC risk compared to the general population and b) determined medical necessity to test for BE using EG/EC. “Increased risk” is defined within the study as patients with ≥3 established risk factors, as described by the AGA in their 2022 clinical practice updates [13]. Established risk factors include male sex, White (Caucasian, non-Hispanic) race, chronic GERD, history of tobacco smoking, obesity, age >50 years old, and/or family history of BE or EAC in a first degree relative [4, 13]. Figure 2 provides a study schematic demonstrating the flow of the patient journey and data collected during the study.
2.3 Statistical Analysis
Subjects unable to successfully swallow the EC device could not contribute cellular DNA for EG analysis; these subjects are included in the summary of enrollment demographics and risk factors, but do not contribute to the clinical utility outcomes. Similarly, subjects with QNS or cell samples deemed unevaluable on EG were included in overall data analysis but did not contribute to the primary clinical utility outcome. The primary clinical utility outcome of this study is provider decision impact. This is measured by the agreement between positive or negative EG results and the provider decision to refer or not refer the patient for further endoscopic workup, respectively; overall concordance between the EG result and provider decision for UE referral is calculated. Continuous variables are summarized using the number of observations (n), mean, standard deviation (SD), median, minimum, and maximum, along with total number of patients contributing values. Categorical variables are described by frequency of counts and percentages. The total numbers of applicable subjects (N) are used as the denominator for percent calculations unless stated otherwise within a table footnote. Binomial exact two-sided 95% confidence interval are calculated wherever relevant.
3. Results
At the time of data snapshot, four clinical sites - each with a single, primary participating physician - had enrolled patients into CLUE. Two of four participating physicians are primary care providers/internists, one a foregut surgeon, and the fourth a gastroenterologist. The participating foregut surgeon is also an endoscopist. A total of 279 subjects signed informed consent for study participation, however four (4) were noted after consent to not appropriately meet inclusion criteria and were withdrawn early, resulting in 275 subjects contributing data for analysis.
3.1 Subject Characteristics and Risk Factors
Subject baseline characteristics and BE risk factors are summarized in Table 1. Four newly enrolled subjects were pending entry of any demographic information at the time of snapshot. Several other individuals had only partial data entry. Mean age was 61.9 years (SD 12.6 years), with a relatively equitable distribution among male vs. female sex. Most subjects (76.0%) were of White race (i.e., Caucasian non-Hispanic), and nearly 90% had a history of chronic GERD. Among the GERD cohort, average duration of symptoms was over 14 years; most affected subjects endorsed using acid suppressive medications with good symptom response. Other BE risk factors were also well-represented, although positive family history of BE/EAC in a first degree relative was expectedly infrequent (<3%). Most of the study population met ACG guideline criteria for BE screening, at 73.8%.
Table 1: Subject Baseline Characteristics and BE/EAC Risk Factors
|
Characteristics |
Overall |
|
(N = 275) |
|
|
Age (Yrs) |
|
|
Mean ± SD |
61.9±12.6 (271) |
|
Median (Q1, Q3) |
64.0 (55.0,70.0) |
|
(Min, Max) |
(23.0,90.0) |
|
Sex |
|
|
Female |
46.1% (125/271) |
|
Male |
53.9% (146/271) |
|
Race |
|
|
Caucasian Non-Hispanic |
76.0% (206/271) |
|
Caucasian – Hispanic |
4.1% (11/271) |
|
Black or African American |
18.5% (50/271) |
|
American Indian or Alaskan Native |
0.7% (2/271) |
|
Asian, Native Hawaiian or Other Pacific Islander |
1.1% (3/271) |
|
Height (in) |
|
|
Mean ± SD |
67.6±4.0 (271) |
|
Median (Q1, Q3) |
68.0 (65.0,71.0) |
|
(Min, Max) |
(59.0,77.0) |
|
Weight (lbs) |
|
|
Mean ± SD |
205.8±46.2 (271) |
|
Median (Q1, Q3) |
201.0 (176.0,230.0) |
|
(Min, Max) |
(104.0,390.0) |
|
Calculated BMI (kg/m2) |
|
|
Mean ± SD |
31.6±6.3 (271) |
|
Median (Q1, Q3) |
31.0 (26.8,35.2) |
|
(Min, Max) |
(17.3,53.7) |
|
Obese (calculated BMI ≥30 kg/m2) |
56.8% (154/271) |
|
Smoking History |
|
|
Current |
18.3% (48/263) |
|
Former |
34.6% (91/263) |
|
Never-Smoker |
47.1% (124/263) |
|
Family history of BE or EAC |
2.6% (7/270) |
|
Chronic Gastroesophageal reflux disease (GERD) |
89.7% (243/271) |
|
Number of years of Gastroesophageal reflux disease (GERD) |
|
|
Mean ± SD |
14.1±11.6 (228) |
|
Median (Q1, Q3) |
10.0 (5.0,20.0) |
|
(Min, Max) |
(0.1,72.0) |
|
Is the subject taking, or has the subject taken acid-suppressing medications for management of GERD (e.g., H2 blockers, PPIs, etc.?) |
|
|
No |
18.5% (50/270) |
|
Yes |
81.5% (220/270) |
|
Are/were GERD symptoms controlled with the acid suppressing medications? |
|
|
No |
18.4% (40/217) |
|
Yes |
81.6% (177/217) |
|
3 or more established BE/EAC risk factors |
81.8%§ (225/275) |
|
(missing in components are assumed = NO) |
|
|
GERD + 3 or more additional risk factors |
73.8% (200/271) |
|
(i.e., cohort meeting ACG criteria for BE screening) |
Established BE/EAC risk factors are presented in bolded text
∮ This deviation from 100% (despite study inclusion criteria) is due to missing components/incomplete data entry being treated as NO for calculation of risk factors
3.2 EsoCheck Cell Collection
EC cell collection was performed in accordance with the device’s instructions for use (IFU, available upon request from https://www.luciddx.com/esocheck). EC cell collection information was documented for 272 subjects, among which 96.3% successfully completed the process (Table 2). Subjects unable to tolerate cell collection (3.7%, 10/272) were exited from the study early. Median cell collection time was 4 min; 119/267 subjects (44.5%) completed the cell collection in 3min or less and the fastest cell collections occurred in under one minute (rounded up to the nearest minute). A maximum collection time of 30min was seen in one individual who required several attempts to swallow the EC device. All subjects utilized small sips of water to facilitate device swallowing, and mean length of sampled esophagus was 6cm, both of which are appropriate per the device IFU.
Table 2: EsoCheck Cell Collection Characteristics
|
Characteristics |
Overall |
|
(N = 272) |
|
|
Was the EsoCheck cell collection successfully completed? |
|
|
No |
3.7% (10/272) |
|
Yes |
96.3% (262/272) |
|
Cell Collection Duration (min)§ |
|
|
Mean ± SD |
6.9±5.9 (267) |
|
Median (Q1, Q3) |
4.0 (2.0,12.0) |
|
(Min, Max) |
(1.0, 30.0) |
|
Length of sampled esophagus (cm) |
|
|
Mean ± SD |
6.0±1.2 (249) |
|
Median (Q1, Q3) |
6.0 (5.0,7.0) |
|
(Min, Max) |
(0.0,10.0) |
|
Were sips of water taken during swallowing of balloon capsule? |
|
|
Yes |
100.0% (266/266) |
|
Approximate volume of water consumed during the cell collection |
|
|
<100mL |
96.2% (256/266) |
|
>100mL |
3.8% (10/266) |
|
Was the lower esophageal sphincter (LES) able to be felt during the first or subsequent cell collection attempts? |
|
|
No |
5.6% (3/54) |
|
Yes |
94.4% (51/54) |
*For subjects who required more than one collection attempt, only the latest-most attempt was included in the count
∮ Rounded to the nearest minute when documented in the data capture system
3.3 EsoGuard Results and Clinical Utility Evaluation
Of the 272 subjects with EC cell collection information, 242 received EG results by the time of data snapshot, although only 232 were documented in the study database. Among those, 229 also had a documented management decision from their ordering physician regarding referral for UE (Table 3A). Just under 30% of the EG results returned positive (29.3%, 68/232) and 65.5% (152/232) returned negative. Eight subjects (3.4%) had insufficient DNA quantity in their cell samples for EG analysis (QNS), and four (1.7%) cell samples were unevaluable due to other factors (e.g., contamination). Just over 30% of subjects (70/229) were referred to UE following their EG results; the remainder were not. According to the investigators, the reason for over 95% of their UE referral decisions was because of a positive (29.7%) or negative (65.9%) EG result.
Table 3A: Summary of EsoGuard Results and Physician Decisions on Endoscopy Referral
|
Characteristics |
Overall |
|
(N = 272*) |
|
|
Was the EsoGuard assay completed on the collected cell sample? |
|
|
No** |
4.1% (10/242) |
|
Yes |
95.9% (232/242) |
|
EsoGuard assay result: |
|
|
NEGATIVE |
65.5% (152/232) |
|
POSITIVE |
29.3% (68/232) |
|
QUANTITY NOT SUFFICIENT (QNS) |
3.4% (8/232) |
|
UNEVALUABLE |
1.7% (4/232) |
|
Was the subject referred for upper endoscopy? |
|
|
No |
69.4% (159/229) |
|
Yes |
30.6% (70/229) |
|
Provide the reason for referring or not referring the patient for an endoscopy: |
|
|
Due to NEGATIVE EsoGuard Result |
65.9% (151/229) |
|
Due to POSITIVE EsoGuard Result |
29.7% (68/229) |
|
OTHER |
4.4% (10/229) |
|
Other, please specify: |
|
|
Endoscopy required for evaluation of reflux surgery§ |
10.0% (1/10) |
|
Patient refused endoscopy? |
10.0% (1/10) |
|
QNS or unevaluable EsoGuard result – pending repeat test; no endoscopy referral until further results available |
50.0% (5/10) |
|
Unevaluable EsoGuard result – subject referred for endoscopy rather than repeat test, given his/her risk factors |
10.0% (1/10) |
|
QNS EsoGuard result – subject not warranted for endoscopy without a positive result |
20.0% (2/10) |
*All subjects who completed EsoCheck cell collection are included in this count, even if EsoGuard results have not yet been processed; average time from cell collection to results is 7-14 days; some results may also have been received by the ordering provider but not yet entered in the study database
**Cell samples shipped to the Central Lab for analysis but for which EsoGuard results are still pending were reported here as “not completed” by some sites
∮ Subject had a negative EG result and was scheduled for upper endoscopy for non-screening purposes
3.4 Subject had a positive EG result and was referred for endoscopy, but refused scheduling of the procedure
EG results and their relationship to UE referral were evaluated by subject risk cohort (those either meeting ACG screening criteria or not) and presented in Table 3B. Three subjects with non-binary EG results (two QNS and one unevaluable) were pending endoscopy referral decisions. Two EG(+) subjects and one EG(-) subject with endoscopy referral decisions were missing risk factor and/or demographic information and therefore could not be classified into either the ACG vs. non-ACG cohorts; these subjects were excluded from counts within those cohorts but still contributed to analysis of the full study cohort. All (100%) of subjects with EG(+) results were referred for confirmatory UE. This was consistent across both risk cohorts. Only one subject with EG(-) result was referred for UE, and all others were not. One subject with an unevaluable result was referred directly to UE rather than repeating EG/EC.
Table 3B: EsoGuard Results and Endoscopy Referral Decisions by Risk Cohort
QNS = DNA quantity not sufficient for EsoGuard analysis
-Two EG positive and one EG negative subject with endoscopy referral decisions did not have complete risk factor information and therefore were excluded from the counts for ACG vs. non-ACG cohorts, however contributed to counts for the full study cohort
Three subjects with reported EG results were pending UE referral information i.e., difference between n = 232 subjects with EG results (all results, including QNS and unevaluable) and n = 229 with endoscopy referral decisions
*Two subjects pending referral information
**One subject pending referral information
The primary clinical utility outcome of provider decision impact analyzed only the subjects with binary EG results and a documented physician decision on UE referral, of which there were 220 (Table 4). This primary outcome was analyzed on a study level and on a per-site level. The overall concordance between EG results and UE referral pattern was 98.9% (study level); all sites except one had 100% concordance.
Table 4: Primary Clinical Utility Outcome – Provider Decision Impact
|
Analysis Set |
Subjects with Binary EG Result |
EG(+) subjects referred to UE |
EG(-) subjects not referred to UE |
Concordance between EG results and UE referral (95% CI) |
|
(95% CI) |
(95% CI) |
|||
|
Overall |
220 |
100.0% (94.7%, 100.0%) |
99.3% (96.4%, 100.0%) |
98.9% (96.9%, 100.0%) |
|
Site ID = 01 |
22 |
100.0% (29.2%, 100.0%) |
94.7% (74.0%, 99.9%) |
83.1% (51.1%, 100.0%) |
|
Site ID = 02 |
42 |
100.0% (75.3%, 100.0%) |
100.0% (88.1%, 100.0%) |
100.0% (100.0%, 100.0%) |
|
Site ID = 03 |
71 |
100.0% (81.5%, 100.0%) |
100.0% (93.3%, 100.0%) |
100.0% (100.0%, 100.0%) |
|
Site ID = 05 |
85 |
100.0% (89.7%, 100.0%) |
100.0% (93.0%, 100.0%) |
100.0% (100.0%, 100.0%) |
Discussion
Despite well-established criteria defining patients at increased risk for BE and multiple published societal guidelines for screening, a significant diagnostic gap remains; most patients who could benefit from screening are not being screened [15]. When different modalities for BE screening were reviewed and compared – including traditional UE, transnasal endoscopy, video capsule endoscopy, and minimally invasive sampling devices combined with analysis of cellular markers – it was apparent those with the highest diagnostic accuracy (i.e., endoscopy) were also associated with the lowest transportability, patient convenience, or acceptance [16]. This supports the concept of a two-step process for improved BE diagnosis: the first step being a well-tolerated, highly sensitive, non-invasive triage test which is accessible for the larger, at-risk population; the second step would be a confirmatory test (for triage ‘positive’ patients only) with high diagnostic accuracy but lower convenience – namely UE with or without biopsy. Patient triage via non-endoscopic testing strategies has accumulated interest, with the most widespread literature available for CytoSponge, a swallowable sponge on a string, paired with immunohistochemistry (trefoil factor/TTF3) [17]. Comparative modeling analyses have even shown that use of this diagnostic approach in primary care settings can be cost effective [18]. In China, balloon-based esophageal cell collection has been successful in supporting cytology screening for esophageal cancer [19]. Aside from being the only commercially available non-endoscopic esophageal cell collection device on the U.S market, advantages of EsoCheck compared to the other devices include the unique, balloon-capsule design, which allows targeted cell collection and specimen protection. Specifically for diagnosis of BE, a disease in which cellular changes originate and are localized to the distal esophagus, balloon inversion within the EC capsule after targeted collection in the distal esophagus avoids cellular dilution and contamination as the device is removed through the upper esophagus and oropharynx. Additionally, as seen in CLUE, the EC cell collection process is fast, with a median cell collection time of only four minutes (note - the mean duration was skewed by the presence of one extreme outlier), and very well tolerated with less than four percent of patients unable to swallow the device; no patients reported complaints or complications to their physicians following the visit. This contrasts with sponge-based cell collection devices that take a minimum of 7-10 minutes for the gel capsule to dissolve in the stomach, and run the risk of string detachment [17, 20]. The EsoGuard Esophageal DNA Test, which utilizes targeted next generation sequencing and validated algorithms to detect abnormal DNA methylation patterns, in turn has significant advantages over cytology. Unlike cytology, which requires highly trained experts to accurately classify cells, the EG assay is automated, easily scalable, and not subject to inter-observer variability. As seen in the CLUE data snapshot, binary EG test results were available in approximately 95% of patients, which remains well within standards of biomarker tests performed in CLIA-certified laboratories. Patients included within this CLUE interim analysis accurately represent the target BE testing population as described by GI society guidelines, namely patients with multiple risk factors - the majority of which have chronic GERD of long-standing duration. Over 80% of the chronic GERD patients in the study were on acid suppressive medications, 81.6% of which reported good symptom control and would therefore have been less likely to seek out or been referred for UE. The observed EG positivity rate of 29.3% may seem high compared to reported BE prevalence rates of 5-15% cited in the literature, however this number is reasonable in the context of the higher risk study population (majority of subjects with 4 or more established BE risk factors). Published BE prevalence rates are also likely an under-reporting of true disease prevalence due to historically low rates of screening, leading to under-diagnosis [21, 22]. Indeed, literature shows that less than 20% of patients in the U.S who are diagnosed with EAC have any preceding diagnosis of BE, and only 10% of high-risk individuals undergo endoscopic BE screening [23, 24]. The cause is likely multifactorial, including lack of any characteristic constellation of symptoms associated with BE, poor patient understanding of their own disease risk, and fears around the discomfort or inconvenience of UE [25]. Office-based, non-endoscopic testing with EG/EC can address these patient concerns by improving accessibility and minimizing invasiveness. Given the intended utility of EG as a high-sensitivity triage test, it is expected that the EG positivity rate should be higher than true disease prevalence, so as not to risk missing any patients with disease.
The 98.8% concordance between EG results and UE referral demonstrate that CLUE physicians are consistently utilizing EG as a triage to endoscopy. 100% of EG(+) subjects were referred for further UE work-up, and 99.3% of EG(-) received no additional diagnostic evaluation. This is consistent with the physician’s own self-reporting, with >95% of their documented referral reasons being either a negative or positive EG result (Table 3A). This remained true even for the cohort of patients that specifically met ACG guideline criteria for screening. The ACG screening guidelines for BE are arguably some of the more stringent compared to those of other GI societies, given their requirement that all patients have chronic GERD (defined as five or more years of frequent symptoms) and at least three additional risk factors [26]. These patients could be clinically justified in proceeding straight to UE for BE screening, however in all except one individual with negative EG results (112/113, 99.1%), triage with EG was able to save them from the more burdensome, uncomfortable, and higher-risk diagnostic procedure. The singular subject with a negative EG results who was sent for UE did so for non-screening purposes; the UE was performed for pre-operative workup of planned anti-reflux surgery (Table 3A and 3B). This demonstrates provider confidence in negative EG results and the ability to rule out BE. Results of the clinical utility analysis are unsurprising, given that specialty Societies including the AGA and ACG have already recognized non-endoscopic cell collection paired with DNA biomarkers as an acceptable approach to initial BE screening [4, 13]. The focus of this manuscript was on real-world provider decision impact, and we recognize that absence of patient outcomes may be deemed a limitation. However, the intent of technologies like EG/EC is to facilitate early diagnosis through more widespread testing of high-risk individuals, and increased patient and provider awareness of BE. The intent is not to change standards of care following establishment of a diagnosis. There are clear guidelines for management of patients once diagnosed - including timing of surveillance and indications for ablative therapy - which are expected to improve immediate and long-term patient outcomes [4, 27, 28]. It is not within the scope of a triage test like EG or studies like CLUE to ensure patient or provider compliance with those guidelines. Another potential limitation of this study is the small number of enrolling sites (n=4) and investigators; the number of sites and physicians is planned to double over the remainder of the study. It is important to note that despite the small number of investigators, multi-disciplinary representation was still achieved, with three different specialty types. In short, the early experience in CLUE appears to support EG as an effective triage to UE, which can be used in both primary care and specialty settings to assist in physician decision-making. This approach could facilitate increased testing of patients at high risk for BE/EAC, while also focusing UE resources on those patients with the highest pre-procedure probability of disease.
Conclusions:
Review of data from the first snapshot of the CLUE study demonstrates that physicians who have adopted EG/EC into their clinical practices are reliably utilizing EG as a triage to UE for diagnostic evaluation of patients at high risk of BE/EAC. EG(+) individuals are consistently referred for confirmatory UE, whereas EG(-) subjects are being spared the more invasive test.
Conflict of Interests:
D.L, A.F, S.M, and P.S.B declare no conflicts of interest. V.T.L, B.J.D, and L.A are executive members of PAVmed Inc., of which Lucid Diagnostics Inc. is a subsidiary, and own stock and/or options in the parent company. S.V is an executive member of Lucid Diagnostics Inc. and owns stock and/or options in the company.
Acknowledgements and Funding:
The presented research was fully funded by Lucid Diagnostics Inc.
References:
- Thrift AP. Global burden and epidemiology of Barrett oesophagus and oesophageal cancer. Nat Rev Gastroenterol Hepatol 18 (2021): 432-443.
- Clermont M and GW Falk. Clinical Guidelines Update on the Diagnosis and Management of Barrett's Esophagus. Dig Dis Sci 63 (2018): 2122-2128.
- Shaheen NJ. Durability of radiofrequency ablation in Barrett's esophagus with dysplasia. Gastroenterology 141 (2011): 460-468.
- Shaheen NJ. Diagnosis and Management of Barrett's Esophagus: An Updated ACG Guideline. Am J Gastroenterol 117 (2022): 559-587.
- Qumseya B. ASGE guideline on screening and surveillance of Barrett's esophagus. Gastrointest Endosc 90 (2019): 335-359.
- Qiao Y. Surveillance in Patients with Barrett's Esophagus for Early Detection of Esophageal Adenocarcinoma: A Systematic Review and Meta-Analysis. Clin Transl Gastroenterol 6 (2015): 131.
- Hamouda A. Presentation and survival of operable esophageal cancer in patients 55 years of age and below. World J Surg 34 (2010): 744-749.
- El-Serag HB, S Sweet, CC Winchester, et al. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 63 (2014): 871-880.
- Katz PO. ACG Clinical Guideline for the Diagnosis and Management of Gastroesophageal Reflux Disease. Am J Gastroenterol 117 (2022): 27-56.
- Yadlapati R, CP Gyawali, and J.E. Pandolfino. AGA Clinical Practice Update on the Personalized Approach to the Evaluation and Management of GERD: Expert Review. Clin Gastroenterol Hepatol 20 (2022): 984-994.
- Hu Q. Proton Pump Inhibitors Do Not Reduce the Risk of Esophageal Adenocarcinoma in Patients with Barrett's Esophagus: A Systematic Review and Meta-Analysis. PLoS One 12 (2017): 0169691.
- Brusselaers N, L Engstrand, and J Lagergren. Maintenance proton pumps inhibition therapy and risk of oesophageal cancer. Cancer Epidemiol 53 (2018): 172-177.
- Muthusamy VR, S Wani, CP Gyawali, et al. AGA Clinical Practice Update on New Technology and Innovation for Surveillance and Screening in Barrett's Esophagus: Expert Review. Clin Gastroenterol Hepatol 20 (2022): 2696-2706.
- Moinova HR. Identifying DNA methylation biomarkers for non-endoscopic detection of Barrett's esophagus. Sci Transl Med 10 (2018): 5848.
- Amitabh Chak M. Update on Barrett Esophagus Diagnosis and Management 19 (2023).
- Spechler SJ, DA Katzka, and RC Fitzgerald. New Screening Techniques in Barrett's Esophagus: Great Ideas or Great Practice? Gastroenterology 154 (2018): 1594-1601.
- Fitzgerald Cytosponge-trefoil factor 3 versus usual care to identify Barrett's oesophagus in a primary care setting: a multicentre, pragmatic, randomised controlled trial. Lancet 396 (2020): 333-344.
- Heberle CR. Cost Effectiveness of Screening Patients With Gastroesophageal Reflux Disease for Barrett's Esophagus With a Minimally Invasive Cell Sampling Device. Clin Gastroenterol Hepatol 15 (2017): 1397-1404.
- Shen O. Cytologic screening for esophageal cancer: results from 12,877 subjects from a high-risk population in China. Int J Cancer 54 (1993): 185-188.
- Cytosponge Cell Collection Device Instructions for Use (IFU), Medtronic, Editor (2003).
- Runge TM, JA Abrams and NJ Shaheen. Epidemiology of Barrett's Esophagus and Esophageal Adenocarcinoma. Gastroenterol Clin North Am 44 (2015): 203-231.
- Qumseya BJ. Systematic review and meta-analysis of prevalence and risk factors for Barrett's esophagus. Gastrointest Endosc 90 (2019): 707-717.
- Tan MC. Systematic review with meta-analysis: prevalence of prior and concurrent Barrett's oesophagus in oesophageal adenocarcinoma patients. Aliment Pharmacol Ther 52 (2020): 20-36.
- Shaheen NJ, GW Falk, PG Iyer, et al. Gerson, ACG Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. Official journal of the American College of Gastroenterology | ACG 111 (2016): 30-50.
- Kolb JM. Patient Knowledge, Risk Perception, and Barriers to Barrett's Esophagus Screening. Am J Gastroenterol 118 (2023): 615-626.
- Komanduri, S. and D.A. Farina, A Practical Approach to Screening and Surveillance of Barrett’s Esophagus. Foregut 1 (2021): 25-31.
- Shaheen NJ. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med 360 (2009): 2277-2288.
- Shaheen NJ. Safety and efficacy of endoscopic spray cryotherapy for Barrett's esophagus with high-grade dysplasia. Gastrointest Endosc 71 (2010): 680-685.


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 