Emerging Mitochondria-Associated Molecular Target Therapies for Acute Myeloid Leukemia
Hirotaka Nakamura, Yosuke Minami*, SungGi Chi, Satoshi Uchiyama, Akihito Nagata, Nobuhiko Yamauchi
Department of Hematology, National Cancer Center Hospital East, Kashiwa, Japan
*Corresponding author: Yosuke Minami, Department of Hematology, National Cancer Center Hospital East 6-5-1 Kashiwanoha, Kashiwa, 277-8577, Japan
Received: 24 May 2021; Accepted: 1 June 2021; Published: 10 June 2021
Article Information
Citation:
Hirotaka Nakamura, Yosuke Minami, SungGi Chi, Satoshi Uchiyama, Akihito Nagata, Nobuhiko Yamauchi. Emerging Mitochondria-Associated Molecular Target Therapies for Acute Myeloid Leukemia. Archives of Clinical and Biomedical Research 5 (2021): 419-424.
View / Download Pdf Share at FacebookAbstract
The era of precision medicine for acute myeloid leukemia (AML) has arrived, and it is extremely important to detect actionable mutations relevant to treatment-related decision-making. However, the percentage of actionable mutations found in AML is approximately 50% at present, and therapeutic development is also needed for AML patients without such mutations. Nevertheless, recently approved drugs for AML treatment are less toxic than conventional intensive chemotherapy and can be combined with low-intensity treatments. Such combination therapies can improve prognosis, especially for elderly AML patients, who account for more than half of all AML cases. Thus, the treatment strategy for leukemia is changing drastically and showing rapid progress. In this review, we present novel mitochondria-associated molecular target therapies for AML, such as the use of BCL-2 and IDH inhibitors.
Keywords
<p>Acute myeloid leukemia (AML); Isocitrate dehydrogenases (IDH); B-cell leukemia/lymphoma 2 (BCL-2); Venetoclax; Enasidenib</p>
Article Details
1. Introduction
Acute myeloid leukemia (AML) is a genetically heterogeneous malignancy of hematopoietic stem cells. Conventionally, the prognosis of AML is determined based on chromosomal abnormalities and fusion genes. Intensive chemotherapy, such as 7 days of cytarabine + 3 days of anthracycline (7+3) or high-dose cytarabine, is a standard treatment for younger AML patients, and hematopoietic stem cell transplantation is considered based on the prognosis of AML. However, more than half newly-diagnosed AML patients are elderly [1] and ineligible for intensive chemotherapy. Such patients (older than 65 years of age) exhibit poor survival rates (5-year survival estimates <10%) [2, 3], and there is a large unmet need for their treatment.
Recently, whole-exome sequencing for AML has been performed using a next-generation sequencer in several studies [4, 5]. A few gene mutations were identified in each AML patient [4, 5]. Among them, FLT3 (28%), NPM1 (27%), DNMT3A (26%), and IDH1/2 (20%) mutations were observed in 20% to 30% of the cases, but the frequency of more than 10 other types of mutations was less than 10% [5]. Some of these low-frequency mutations are actionable mutations, which are defined as genetic aberrations in the DNA and would be expected to elicit a response to an approved targeted treatment that is available off-label or in clinical trials [6]. Since 2017, four new drugs targeting gene mutations (midostaurin, gilteritinib, ivosidenib, and enasidenib) have been approved by the US Food and Drug Administration (FDA) for AML treatment. The era of precision medicine for AML has arrived, and it is extremely important to detect actionable mutations relevant to treatment-related decision-making.
However, the percentage of actionable mutations found in AML is approximately 50% at present [5], and therapeutic development is also needed for AML patients without such mutations. The FDA has approved four drugs (venetoclax, CPX-351, mylotarg, and glasdegib) in addition to agents targeting actionable mutations. These recently approved drugs are less toxic than conventional intensive chemotherapy and can be combined with a low-intensity treatment such as low-dose cytarabine or azacitidine. Such combination therapies are expected to improve prognosis, especially for elderly AML patients, who account for more than half of all AML cases. Thus, the treatment strategies for leukemia are drastically changing due to the rapid development of new drugs. In this review, we present novel mitochondria-associated molecular target therapies for AML, such as the use of BCL-2 and IDH inhibitors.
2. Drug Targeting Isocitrate Dehydrogenase Mutation
Isocitrate dehydrogenases (IDHs) are enzymes that catalyze the oxidative decarboxylation of isocitrate to alpha-ketoglutarate (α-KG) [7]. IDHs are divided into three types. IDH1 is expressed in the cytoplasm and IDH2/3 in the mitochondria. Mutant IDH acquires a new function and produces an oncometabolite called R-2-hydroxyglutarate (2-HG) from α-KG. This conversion reduces normal α-KG, and α-KG-dependent ten-eleven translocation-2 (TET2) function deteriorates. As a result, histone demethylation is not performed correctly, and disorders of cell differentiation occur. 2-HG contributes to cancer by inhibiting various enzymes such as TET and histone demethylase. IDH1 or IDH2 mutations occur in 15% to 20% of AML patients and are more prevalent in patients with a normal karyotype [5, 8]. Enasidenib inhibits both R140Q- and R172K-mutated IDH2 [9]. In a phase I/II trial, 100 mg/d enasidenib showed an ORR of 38.8% with a cCR of 29.0% for 214 patients with R/R IDH2 mutant AML [10]. The median OS for all 214 R/R AML patients who received 100 mg/d enasidenib was 8.8 months (95% CI, 7.7-9.6 months). Enasidenib was well tolerated in this study. Special side effects such as IDH differentiation syndrome with fever, dyspnoea due to lung infiltrates, pleural effusion, and leukocytosis occurred in 6.4% of the participating patients [10]. The FDA approved enasidenib for the treatment of R/R AML with IDH2 mutations in 2017. Enasidenib combined with intensive chemotherapy achieved a cCR (CRi or CRp) of 72% in an open-label, multicenter, phase I study including 89 patients recently diagnosed with AML with an IDH2 mutation [11]. Currently, a phase III trial evaluating the clinical benefit of enasidenib combined with induction, consolidation, and maintenance therapy for patients with newly diagnosed IDH2-mutated AML is ongoing (NCT03839771).
Ivosidenib is a selective IDH1 mutation inhibitor. Ivosidenib showed an ORR of 41% (CR 22%, CRi 8%) as a single agent in a phase I dose-escalation and dose-expansion study including 258 R/R AML patients with IDH1 mutation [12]. The median OS of the primary efficacy population was 8.8 months (95% CI, 6.7-10.2 months). In this study, IDH differentiation syndrome occurred in 3.9% of patients administered an ivosidenib dose of 500 mg daily. Based on the results of this study, ivosidenib was approved by the FDA on May 2, 2019 for the treatment of recently diagnosed AML with IDH1 mutation in patients who are at least 75 years old or are unfit for intensive chemotherapy. In the frontline setting, ivosidenib (500 mg daily) in combination with intensive chemotherapy showed a clinical efficacy (cCR) of 80% in a phase I trial including 60 AML patients recently diagnosed with an IDH1 mutation [11]. Ivosidenib in combinational therapy (intensive or low-intensity chemotherapy) is currently being studied in randomized phase III trials investigating previously untreated AML patients with an IDH1 mutation (NCT03839771 and NCT03173248).
3. BCL-2 Family Proteins and BCL-2 Inhibitor Venetoclax
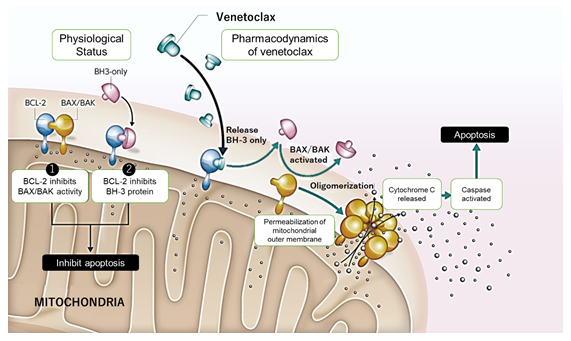
B-cell leukemia/lymphoma 2 (BCL-2), which is one of the BCL-2 family proteins that also include BCL-XL and MCL-1, promotes cell survival. BCL-2 regulates the mitochondrial apoptotic pathway and plays an important role in the chemoresistance and survival of AML blasts [13-15]. Venetoclax is a potent, selective inhibitor of BCL-2 but not BCL-XL or MCL-1 (figure 1) [16, 17]. In a phase II trial, venetoclax showed an ORR of 19% (6/32, CR 6%, CRi 13%) as a single agent for patients with r/r AML or unfit for intensive chemotherapy [18]. An international phase Ib/II study evaluated the safety and efficacy of venetoclax in combination with low-dose cytarabine (LDAC) for elderly patients with previously untreated AML and ineligible for intensive chemotherapy [19]. In this study, the CR/CRi rate was 54% (CR 26% + CRi 28%, 95% CI, 42-65%) and the median OS was 10.1 months (95% CI, 5.7-14.2 months) for 82 AML patients who received 600 mg venetoclax. In contrast, a large, multicenter phase Ib dose-escalation and expansion study was conducted to evaluate the safety and efficacy of venetoclax in combination with azacitidine or decitabine for elderly patients with previously untreated AML and ineligible for intensive chemotherapy (n = 145) [20]. The CR/CRi (CR 37%, CRi 30%) rate was 67%, with an ORR of 68% (99/145), and the median OS for all the patients was 17.5 months (95% CI, 12.3 months to “not reached”) in this study.
Based on these results, venetoclax was approved in combination with LDAC and HMAs (azacitidine or decitabine) by the FDA in 2018. Ongoing trials are evaluating the efficacy of venetoclax in combination with FLT3 inhibitors, intensive chemotherapy, or decitabine (NCT03625505, NCT03214562, and NCT03404193) in the relapsed and refractory settings. Several trials assessing the clinical benefit of venetoclax in low-intensity or intensive treatments in the front-line setting are ongoing (NCT02993523, NCT03069352, NCT03941964, and NCT03709758). For previously untreated patients who were ineligible for intensive chemotherapy, OS was longer, and the incidence of remission was higher among those who received azacitidine + venetoclax than among those who received azacitidine alone [21].
4. Conclusions and Future Perspectives
Many clinical trials evaluating the efficacy of promising investigational drugs for AML are ongoing, and more drugs will go to market than ever before. Several new agents can create overlapping treatment options, especially for elderly, unfit AML patients and R/R AML patients. Henceforth, the proper use of these new agents will be one of the issues in AML treatment. Physicians should select an optimal treatment depending on factors such as age, performance status, comorbidities, and genetic mutations. In particular, genome profiling analysis upon diagnosis will be needed to select an optimal first-line treatment.
Funding
This paper was supported by the National Cancer Research and Development expenses grant.
Conflicts of Interest
Y.M. received research funding from Ono and CMIC, and honoraria from Bristol-Myers Squibb, Novartis, Astellas, and Daiichi-Sankyo. The other authors declare no conflict of interest.
Author Contribution
H.N., S.C., and Y.M. wrote the first draft and all the authors revised the manuscript.
References
- Surveillance, Epidemiology, and End Results Program. 1988-2014 (SEER 13) (2019).
- Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood 107 (2006): 3481-3485.
- Vasu S, Kohlschmidt J, Mrózek K, et al. Ten-year outcome of patients with acute myeloid leukemia not treated with allogeneic transplantation in first complete remission. Blood Adv 2 (2018): 1645-1650.
- Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N. Engl. J. Med 374 (2016): 2209-2221.
- Genomic and Epigenomic Landscapes of Adult De Novo Acute Myeloid Leukemia. N. Engl. J. Med 368 (2013): 2059-2074.
- Carr TH, McEwen R, Dougherty B, et al. Defining actionable mutations for oncology therapeutic development. Nat. Rev. Cancer 16 (2016): 319-329.
- Cairns RA, Mak TW. Oncogenic Isocitrate Dehydrogenase Mutations: Mechanisms, Models, and Clinical Opportunities. Cancer Discov 3 (2013): 730-741.
- Mardis ER, Ding L, Dooling DJ, et al. Recurring Mutations Found by Sequencing an Acute Myeloid Leukemia Genome. N. Engl. J. Med 361 (2009): 1058-1066.
- Yen K, Travins J, Wang F, et al. AG-221, a first-in-Class Therapy Targeting Acute Myeloid Leukemia Harboring Oncogenic IDH2 Mutations. Cancer Discov 7 (2017): 478-493.
- Stein EM, DiNardo CD, Fathi AT, et al. Molecular remission and response patterns in patients with mutant-IDH2 acute myeloid leukemia treated with enasidenib. Blood 133 (2019): 676-687.
- Stein EM, DiNardo CD, Fathi AT, et al. Ivosidenib or Enasidenib Combined with Induction and Consolidation Chemotherapy in Patients with Newly Diagnosed AML with an IDH1 or IDH2 Mutation Is Safe, Effective, and Leads to MRD-Negative Complete Remissions. Blood 132 (2018): 560-560.
- DiNardo CD, Stein EM, de Botton S, et al. Durable Remissions with Ivosidenib in IDH1-Mutated Relapsed or Refractory AML. N. Engl. J. Med 378 (2018): 2386-2398.
- Pan R, Hogdal LJ, Benito JM, et al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov 4 (2014): 362-375.
- Vo T-T, Ryan J, Carrasco R, et al. Relative mitochondrial priming of myeloblasts and normal HSCs determines chemotherapeutic success in AML. Cell 151 (2012): 344-355.
- Konopleva M, Contractor R, Tsao T, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell 10 (2006): 375-388.
- Pan R, Ruvolo VR, Wei J, et al. Inhibition of Mcl-1 with the pan–Bcl-2 family inhibitor (–)BI97D6 overcomes ABT-737 resistance in acute myeloid leukemia. Blood 126 (2015): 363-372.
- Souers AJ, Leverson JD, Boghaert ER, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med 19 (2013): 202-208.
- Konopleva M, Pollyea DA, Potluri J, et al. Efficacy and Biological Correlates of Response in a Phase II Study of Venetoclax Monotherapy in Patients with Acute Myelogenous Leukemia. Cancer Discov 6 (2016): 1106-1117.
- Wei AH, Strickland SA, Hou J-Z, et al. Venetoclax Combined With Low-Dose Cytarabine for Previously Untreated Patients With Acute Myeloid Leukemia: Results From a Phase Ib/II Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol 37 (2019): 1277-1284.
- DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 133 (2019): 7-17.
- DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med 383 (2020): 617-629.


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 