Correlation between Shear Wave Velocity Assessed by Elastography and Stiffness given by Tensile Test in the Ovine Patellar Tendon
Kayser F1*, Bori E2, Armillotta N2, Innocenti B3, Hontoir F4, Vandeweerd JM5
1Centre Hospitalier Universitaire, Université Catholique de Louvain, NAMUR, site Godinne, Yvoir, Belgium
2Bio-Electro and Mechanical System, Université Libre de Bruxelles, Ecole Polytechnique de Bruxelles, Belgium
3Department of Bio-Electro and Mechanical System, Université Libre de Bruxelles, Ecole Polytechnique de Bruxelles, Belgium
4Namur Research Institute for Life Sciences, Integrated Veterinary Research Unit, Department of Veterinary Medicine, University of Namur, Belgium
5Namur Research Institute for Life Sciences, Integrated Veterinary Research Unit, Department of Veterinary Medicine, University of Namur, Belgium
*Corresponding author: Françoise Kayser, Centre Hospitalier Universitaire, Université Catholique de Louvain, NAMUR, site Godinne, Yvoir, Belgium.
Received: 08 February 2022; Accepted: 15 February 2022; Published: 18 February 2022
Article Information
Citation:
Kayser F, Bori E, Armillotta N, Innocenti B, Hontoir F, Vandeweerd JM. Correlation between Shear Wave Velocity Assessed by Elastography and Stiffness given by Tensile Test in the Ovine Patellar Tendon. Archives of Clinical and Biomedical Research 6 (2022): 248-259.
View / Download Pdf Share at FacebookAbstract
Background: Tendon injuries are very common in man, healing process often slow and incomplete, and available treatment options unsatisfactory. Mechanical properties such as stiffness can reflect the progress of tissue healing over time. Unfortunately, these measures cannot be recorded in vivo due to the invasive nature of the available conventional biomechanical testing methods. SWE can be used in vivo to investigate mechanical properties of tendons. Before clinical acceptance, SWE must be compared to tensile test values. The aim of our ex vivo study was to investigate whether SWE performed in vivo was correlated to stiffness assessed by an ex vivo uniaxial tensile test considered as gold standard.
Material and methods: Twelve patellar tendons in six healthy ewes underwent conventional ultrasound, color Doppler ultrasound and SWE in vivo followed by uniaxial testing performed ex vivo.
Results: The mean stiffness value of the 12 tendons was 45.69 N/mm (SD+/- 14.56). The mean shear wave velocity was 6.27 m/s (SD+/-0.6). There was a not statistically significant positive correlation between shear wave velocity and age (r=0.137, p=0.79), and a significant positive correlation between stiffness and SWV (r=0.87; p=0.02).
Conclusion: SWE can be a surrogate to biomechanical properties of the tendon with the advantage that it can be performed in vivo. It could be a useful imaging tool in the context of disease progression and healing follow-up. The SWV values obtained in our study could be considered as baseline values for further research studies on patellar tendons in the ovine model.
Keywords
<p>Patellar tendon; Sheep; Shear wave elastography; Shear wave velocity; Stiffness; Uniaxial tensile test</p>
Article Details
Abbreviations:
CDUS: Color Doppler-Ultrasound; MRI: Magnetic resonance imaging; ROI: Region of interest; SE: Strain elastography; SWE: Shear wave elastography; SWV: Shear wave velocity; US: Ultrasound
1. Introduction
Tendon injuries and chronic tendinopathies occur commonly in man and are usually related to musculoskeletal pain and disability, significant morbidity and abnormal joint movement. The patellar tendon, besides the Achilles tendon and rotator cuff tendons, is often subject to tendinopathies [1]. To date, tendons are mainly evaluated clinically, by conventional Ultrasound (US), Color Doppler-ultrasound (CDUS) and Magnetic Resonance Imaging (MRI). Morphological changes of tendons such as echogenicity, homogeneity or thickness are well described by US [2]. Neovascularization in chronic tendinopathies can be documented by CDUS [3]. Inconveniently neither US and CDUS nor MRI inform on the mechanical properties of tendons. Tendon mechanical properties such as stiffness and elasticity are useful to quantify the progress of tissue healing over time. Stiffness is the ratio between the force applied to the tendon and its change in length. Elasticity, or Young’s modulus, is the ratio between the stress (ratio between the force applied and the cross-sectional area) and the strain (the ratio between the change in length and the initial length). Unfortunately, these measures cannot be recorded in vivo due to the invasive nature of the available conventional biomechanical testing methods. Ultrasound elastography, a more recent non-invasive technique, allows the investigation of those mechanical properties by applying a force either manually (Strain Elastography, SE) or mechanically (Shear Wave Elastography, SWE). SWE enables quantitative analysis of the tendon mechanical properties by measuring the shear elastic modulus and the Shear Wave Velocity (SWV) related to tissue elasticity. Unlike SE, SWE does not require a manual intervention by the ultrasonographer to produce strain, hence limiting operator biases [4-7]. To date sonoelastography has been widely used in diagnosis and follow-up of several diseases such as breast neoplasm [8, 9], liver fibrosis [10] and thyroid neoplasm [11]. Patellar tendon [12], Achilles tendon [12] and rotator cuff tendon [2] have also been explored by SWE [13]. It is reported to be an accessible, non-invasive, easy- to-use, fast, cost-effective, reproducible and reliable tool in clinical and research work providing quantitative data, with a high level of inter-operator agreement [4, 14]and minimal operator training. However, there is still a lack in standardization of elastography and there is no consensus about its application in tendon pathology. Animal models are required to transfer new technologies from research to clinical application [15]. The humanlike size of joints such as the knee (stifle) represents an advantage of the sheep compared to small animal models [16]. Sheep could be used to elucidate the clinical relevance and potential applications of SWE. The patellar tendon is a superficial easily accessible structure in living animals. The mechanical characteristics of the patellar tendon, such as elasticity and stiffness, are of capital importance and constitute major outcome measures in research studies.
Ultrasonographic, CT and MRI anatomy of the stifle have been described [17-19]. To date, the patellar tendon SWE properties have not been described in sheep, and there is therefore a need to document them in a population of research animals. Moreover, before clinical acceptance, the validation of SWE requires correlation to tensile test values. It is of paramount importance to establish the SWV values in normal ovine patellar tendons before using SWE in injured tendons. The aim of our ex vivo study was to investigate whether SWV in healthy ovine patellar tendons, determined by SWE tested in vivo, was correlated to stiffness assessed by an ex vivo uniaxial tensile test considered as gold standard.
2. Material and Methods
2.1. Animals
Six ewes (Ile de France; n= 6), from the Ovine Research Center of the University of Namur, were used. Their age ranged from 2 to 8 years. Previously to testing, the animals were kept free of movement in an outside paddock. Palpation of their stifle and locomotion were normal. No swelling, pain or lameness was observed. The experimental protocol 19006 VA was approved by the local ethical committee for animal welfare.
2.2. Ultrasound examination
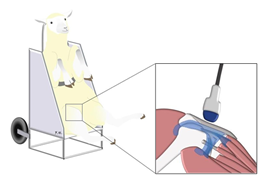
Animals were hold in a sitting position, in a chair specifically designed for sheep studies (Figure 1). Before ultrasound assessment, both left and right stifles were clipped, cleaned with water and soap, and shaved. The ultrasound examinations were performed with a high- resolution US scan (RS 85 Ultrasound System, Samsung Medison Co., Ltd Seoul Korea). A generous amount of coupling gel was used to improve transmission of US waves. The ultrasound transducer was hold manually for all examinations. All investigations were made at room temperature, with the tibia and femur forming an angle of approximately 95°. The examinations were performed by a radiologist with more than 20 years of experience in musculoskeletal ultrasound. All the data were acquired on the same day.
2.3. 2D Ultrasound
US examination included the evaluation of the entire patellar tendon, the patellar and tibial tuberosity attachments, the adjacent cranial soft tissues and the patellar fat pad using a L3- 12A and LA4-18B linear-array transducer. Tendon echogenicity and homogeneity were assessed in transverse and longitudinal planes. Abnormalities were searched and recorded if present.
2.4. Color doppler ultrasound
Potential neovascularization, that could be a sign of tendon disorder, was assessed by CDUS, using the LA4-18B linear-array transducer, in transverse and longitudinal planes. The entire patellar tendon and the adjacent soft tissues were explored.
2.5. Shear wave elastography
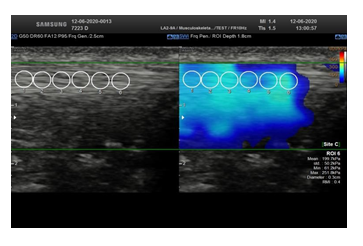
2D ultrasound was used to locate the patellar tendon and align the transducer longitudinally with the tendon fibers. When a correct image of the patellar tendon, without artefacts, was obtained, the shear wave mode was activated to obtain a color-coded elastogram. Through a dual display, the fiber-transducer alignment was constantly verified. The size of the rectangular acquisition box was defined previously to the data acquisition, to maximize the amount of tissue analyzed, avoiding the tendon extremities. The diameter of the circular ROI (Region of Interest) was held constant at 0.3 cm throughout the measurements in all the limbs. The ROI was set manually and was centered on the targeted patellar tendon. SWE was assessed in a longitudinal plane, parallel to the fiber orientation, with light pressure on the skin, basically in the mid portion of the tendon, avoiding the tendon extremities, using a LA2-9A linear-array transducer. The transducer was kept motionless during 8-12 seconds to acquire the color-coded elastogram. When the color in the color- coded elastogram was uniform, the image was frozen enabling an off-line analysis through the captured images (Figure 2). Three images were captured, with 6 measurements on each. For each PT, the SWV was assessed, expressed in m/s, as well as the shear elastic modulus expressed in kPa. In our study, we considered the SWV for correlations.
Figure 2: Dual display (left) showing a 2D ultrasound image used to locate the patellar tendon and align the tendon fibers longitudinally with the transducer. When a correct image of the patellar tendon was obtained (right), the shear wave mode was activated to obtain a color-coded elastogram. Three images were captured, with 6 measurements on each.
2.6. Gross anatomy
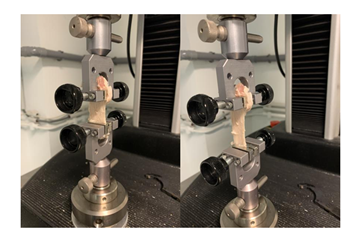
Animals were sacrificed by intravenous administration of pentobarbital (150 mg/kg). Both hind limbs were transected at the level of the mid-femur, immediately after death. The patellar tendon and its attachments on the patella and the tibial tuberosity were carefully dissected. The patella and tibial tuberosity were sawed at 1.5 cm from their tendon attachment in order to not affect the tendon insertion areas that are fundamental for transferring loads. Each harvested specimen consisting of an intact “patella-patellar tendon-tibial tuberosity” unit was individually wrapped in moistened gauze (with 0.9 % w/v NaCl), sealed in plastic bags, individually identified by a number and stored at -20°C until processed for biomechanical tests.
2.7. Biomechanical tests
All specimens were submitted to biomechanical tests on a same day. In six matched pairs of limbs (n=12), a uniaxial tensile test was performed on patellar tendons following a procedure described previously [20]. The frozen “patella-patellar tendon- proximal tibial” units were thawed to room temperature, cleaned thoroughly with 0.9 % w/v of NaCl solution and placed on a metal frame. The osseous parts of the specimen were cut with pliers to fit the clamp size (Figure 3). The camera was set to 100 fps. Force outputs were obtained via a dedicated 1 kN load cell (Lloyd Instruments Ltd). A series of 10 preconditioning stress-relaxation procedures were performed to obtain a good alignment of fibers prior to tensile testing since tendons had been previously manipulated and then stored at -20°C. As suggested in the ASTM D638 standard [21], the tensile test was performed with a crosshead speed of 5 mm/min. The axial force was recorded and paired with the relative displacement during the tests. The tensile test was performed until failure. The resulting force-displacement curve was analyzed. The linear region of the curve, corresponding to the elastic region, i.e., the elongation of the helical structure of collagen [22], was determined thanks to a dedicated software: a linear fitting process was used to determine the boundaries of the linear region and this latter was studied to obtain the relative stiffness of the tissue (obtained from the slope of the curve in the selected region). Tendon stiffness, expressed in N/mm, is defined as the ratio between the force applied on the tendon and the change of the tendon length.
3. Statistical Analysis
Kolmogorov-Smirnov and Shapiro-Wilk tests were used to examine the normality of data (stiffness). Due to the normality of data, parametric tests were used. The Paired T test was used to compare observations that were not independent (difference in stiffness between left and right hindlimbs). Since there was no difference, one limb was randomly chosen within each pair and data obtained from that limb (SWV, tendon stiffness) was used for analysis. Correlations between mechanically measured SWV and tendon stiffness and age were assessed by Pearson correlation coefficient. Data were collected in Microsoft Excel and were analysed using Graph Pad Prism 8. A p-value less than 0.05 was considered to indicate a statistically significant difference.
4. Results
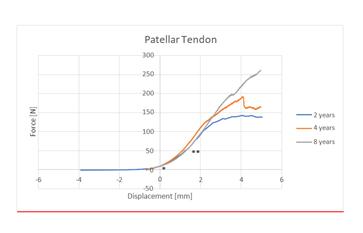
On ultrasound all tendons presented a homogeneous fibrillar structure. We noted no focal or global abnormal thickening. We found no abnormalities in the patellar and tibial tuberosity attachments. The cranial soft tissues adjacent to the patellar tendon and the patellar fat pad were homogeneous in all cases. No neovascularization was observed by CDUS nor in the patellar tendon neither in the adjacent soft tissues. The mean stiffness value of the 12 tendons was 45.69 N/mm (SD+/- 14.56). The tendon stiffness increased until failure. Since there was no difference in patellar tendon stiffness between left and right hindlimbs, one limb was randomly chosen within each pair and data obtained from that limb (Tendon stiffness) was used for analysis. Our tests confirmed that the patellar tendon had a typical non-linear behavior (Figure 4).
On SWE, the mean SWV value within the ROI was 6.27 m/s (SD+/-0.6).
We found a statistically not significant positive correlation between SWV and age (r=0.137, p=0.79).
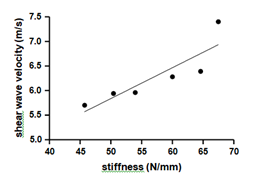
Our data showed a significant positive correlation (r=0.87; p=0.02) between stiffness and SWV (Figure 5).
Correlation between shear wave velocity and stiffness
5. Discussion
In this study the mean stiffness of patellar tendons was 45.69 N/mm (SD+/- 14.56). As previous studies were conducted in other animal models and reported only Young’s modulus, our data constitute, to the best of our knowledge, the first reference values for patellar tendon stiffness in sheep. With soft tissue structures, as they are quite irregular, the estimation of the cross-sectional area during the test may be quite difficult and could induce some measure errors in the determination of the stress (ratio between the force applied and the cross-sectional area) and therefore of the Elastic Modulus. For that reason, we decided to measure the stiffness as our technique is accurate in evaluating force and displacement. Moreover, stiffness is a structural property, i.e., a property of the tendon as a whole, while elasticity is a material property (related to the material that constitutes a structure), meaning that it could change locally and not be homogeneous along the tendon length. We found a statistically not significant positive correlation between SWV and age (r=0.137, p=0.79). This may be explained by progressive stiffening of connective tissues with ageing as shown in a study performed in canine patellar tendons [23]. Similarly, a former study showed altered passive biomechanical properties of the muscle-tendon unit in normal ageing rats, with increased stiffness and decreased relaxation response in the Achilles tendons of middle-aged animals [24]. The association between ageing and increased tendon stiffness may have different explanations. Collagen crosslinks, resulting from the formation of advanced glycation end products, limit fiber-fiber and fibril- fibril sliding, reduce the viscoelasticity of the tendon and increase the tendon stiffness [25]. Concomitantly fascicle sliding has been described to decrease with ageing [26]. The reduced gliding properties of fascicular sheets may be explained by decreased proteoglycan 4 and elastin mRNA expression in tendons [27].
In the current study, a mean value of SWV of 6.27 m/s (SD+/-0.6) was found and there was a significant positive correlation (r=0.87; p=0.02) between stiffness and SWV. A former study performed ex vivo in human Achilles tendon by Haen et al. demonstrated a statistical correlation between shear modulus given by SWE and elastic modulus given by tensile test [28]. Feng et al. showed a significant correlation between shear wave modulus and stiffness index obtained from a hand-held Myoton PRO device in healthy human Achilles tendon [29]. Several ex vivo studies performed in swine [5, 30], New Zealand white rabbits [31] and bovines [32] showed a linear correlation between SWV measured by SWE and Young’s modulus given by biomechanical tests. Most studies have correlated ex vivo measurement of SWE to ex vivo biomechanical testing of dissected tendons [5, 28-32]. The originality of our study was to investigate, in vivo, the patellar tendon biomechanical properties and to correlate them directly to the ex vivo biomechanical testing of dissected bone-tendon-bone units, creating a baseline database for further research use in testing therapeutics for tendon healing.
By connecting bone to bone, the patellar tendon is different from other tendons. A greater portion of the tendon is in proximity to bony insertion sites that may result in a tendon with a greater range of stiffness values. Therefore, the tendon may be more susceptible to subtle changes in the imaging area that occur between testing sessions or trials as pointed out by Peltz et al. [6]. In the present study, the ultrasonographer was aware of ultrasonography anatomy of the ovine stifle and dedicated time to adequately position the transducer at mid-tendon, to avoid bias in measurement of the tendon SWE. However, our study has some limitations. First, though the position of the animal in the chair and the position of the limbs and instruments were held constant, since we performed the SWE in living non-sedated animals knee joint movement control was not always optimal and eventually muscle contracture may have changed slightly the flexion angle of the stifle and therefore the SWV values. Former studies reported variations of SWE measurements with knee flexion. Shear elastic modulus [33] and shear wave velocity [34] were shown to be higher in the flexed knee. It is important to consider the conditions of measurement techniques well before any study in order to allow comparison of the results [35]. In our study we controlled the testing position as much as possible to avoid this influence, but minimal limb movement cannot be excluded. In upcoming studies, a custom-designed mold with straps to keep the limb in a fixed position could be developed. Unfortunately, all sheep do not support being constrained and sedation might be required, what could induce another bias.
Second, SWE was performed in vivo, in a relative static condition, opposed to the force- displacement test conducted ex vivo, in a non-static manner, with progressive force application. But that was precisely the objective of this study to compare non-invasive in vivo techniques to post-mortem ex vivo methods. We showed that SWV obtained in clinical condition i.e., in living animals, is correlated to SWV values obtained ex vivo. Similarly, Haen et al. reported that shear wave modulus in clinical-like condition was significantly correlated to shear modulus in harvested, not yet elongated Achilles tendon [28]. Third, although bone insertions were preserved for optimal tendon fixation during tensile test, a minimal slipping in the clamps may have occurred. Fourth, our study included a small number of limbs. The known wide range of normal patellar tendon shear modulus [7, 36, 37], and hence shear wave velocities, may require a larger sample to be tested in order to obtain more reliable reference values. To date, due to the subjective nature of the available common clinical evaluation methods, the success of conservative, non-conservative and rehabilitative treatments remains still controversial. Assessing healing as outcomes is important. The ovine patellar tendon could be used as a research model by creating injury either by surgery or by collagenase technique. SWE would allow a quantitative manner to track tendon stiffness changing in the healing process and assess the efficacy of novel therapies. The improved tendon mechanical properties, as measured by SWE, may be considered as an objective evaluation in tendon recovery.
Another future challenge for clinicians and researchers is the early detection of the disease that would allow effective strategies to attenuate the disease process before its chronic development. We can speculate that SWE may detect early changes in biomechanical properties related to tendinopathy, even before any changing may be diagnosed on US or CDUS. Currently, the effects of those novel management strategies on shortening or improving the healing process are based on some animal studies, as New Zealand white rabbits [38], but none have been conducted in sheep. Even if our study showed that age does not seem to have a major impact on biomechanical results, the most appropriate sheep population for those future studies would be young adults since there are indications in the scientific literature that degradation of the biomechanical properties can occur with age [25,39].
6. Conclusion
In conclusion, by assessing tendon properties, SWE may be a promising technique to improve tendon disease diagnosis and to assess progress, tendon healing and efficacy of treatments or interventions. Shear wave modulus values obtained in our study could be considered as preliminary reference values in ovine patellar tendon for further research studies.
Acknowledgments
We acknowledge V. Simon for his help. This study did benefit from a grant of the Fondation Mont Godinne and was supported by the University of Namur (UNamur), NARILIS (Namur Research Institute for Life Science) and Benetec (providing the SAMSUNG ultrasound device).
Competing interests
None of the authors of this paper has a financial or personal relationship with people or organizations that could inappropriately influence or bias the content of the paper.
- Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendinopathy. Clinics in Sports Medicine 22 (2003): 675-692.
- Davies SG, Baudouin CJ, King JB, et al. Ultrasound, computed tomography and magnetic resonance imaging in patellar tendinitis. Clinical Radiology 43 (1991): 52-56.
- Weinberg EP, Adams MJ, Hollenberg GM. Color Doppler sonography of patellar tendinosis. American Journal of Roentgenology 171 (1998):743-744.
- Hsiao MY, Chen YC, Lin CY, et al. Reduced patellar tendon elasticity with aging: in vivo assessment by Shear Wave Elastography. Ultrasound in Medicine & Biology 41 (2015): 2899-2905.
- Zhang ZJ, Fu SN. Shear elastic modulus on patellar tendon captured from Supersonic Shear Imaging: correlation with tangent traction modulus computed from Material Testing System and test-retest reliability. PLoS One 8 (2013): 68216.
- Peltz CD, Haladik JA, Divine G, et al. Shearwave elastography: repeatability for measurement of tendon stiffness. Skeletal Radiology 42 (2013):1151-1156.
- Kot BCW, Zhang ZJ, Lee AWC, et al. Elastic modulus of muscle and tendon with shear wave ultrasound elastography: variations with different technical settings. PLoS One 7 (2012): 44348.
- Song EJ, Sohn Y-M, Seo M. Tumor stiffness measured by quantitative and qualitative shear wave elastography of breast cancer. The British Journal of Radiology 91 (2018): 20170830.
- Pesce K, Binder F, Chico MJ, et al. Diagnostic performance of shear wave elastography in discriminating malignant and benign breast lesions: Our experience with QelaXtoTM software. Journal of Ultrasound 23 (2020): 575-583.
- Trebicka J, Gu W, de Ledinghen V, et al. Two-dimensional shear wave elastography predicts survival in advanced chronic liver disease. GUT Journal gutjnl 2020 (2021): 323419.
- Tuan PA, Duc NM, An M, et al. The Role of Shear Wave Elastography in the Discrimination Between Malignant and Benign Thyroid Nodules. Acta Informatica Medica 28 (2020): 248-253.
- Coombes BK, Tucker K, Vicenzino B, et al. Achilles and patellar tendinopathy display opposite changes in elastic properties: a shear wave elastography study. Scandinavian Journal of Medicine & Science in Sports 28 (2018): 1201- 1208.
- Dirrichs T, Quack V, Gatz M, et al. Shear Wave Elastography (SWE) for the evaluation of patients with Tendinopathies. Academic Radiology 23 (2016): 1204- 1213.
- Payne C, Watt P, Cercignani M, et al. Reproducibility of shear wave elastography measures of the Achilles tendon. Skeletal Radiology 47 (2018): 779-784.
- Cook JL, Hung CT, Kuroki K, et al. Animal models of cartilage repair. Bone & Joint Research 3 (2014): 89-94.
- Little CB, Smith MM. Animal models of osteoarthritis. Current Rheumatology Reviews 4 (2008): 175-182.
- Kayser F, Hontoir F, Clegg P, et al. Ultrasound anatomy of the normal stifle in the sheep. Anatomia, Histologia, Embryologia 48 (2019): 87-96.
- Vandeweerd JM, Kirschvink N, Muylkens B, et al. A study of the anatomy and injection techniques of the ovine stifle by positive contrast arthrography, computed tomography arthrography and gross anatomical dissection. The Veterinary Journal 193 (2012): 426-432.
- Vandeweerd JM, Kirschvink N, Muylkens B, et al. Magnetic resonance imaging (MRI) anatomy of the ovine stifle. Veterinary Surgery 42 (2013): 551-558.
- Innocenti B, Larrieu JC, Lambert P, et al. Automatic characterization of soft tissues material properties during mechanical tests. Muscles, Ligaments and Tendons Journal 7 (2018): 529-537.
- ASTM D638. Standard Test Method for Tensile Properties of Plastics. ASTM Int 2014.
- Kirkendall DT, Garrett WE. Function and biomechanics of tendons. Scandinavian Journal of Medicine & Science in Sports 7 (1997): 62-66.
- Haut RC, Lancaster RL, DeCamp CE. Mechanical properties of the canine patellar tendon: some correlations with age and the content of collagen. Journal of Biomechanics 25 (1992): 163-173.
- Plate JF, Wiggins WF, Haubruck P, et al. Normal aging alters in vivo passive biomechanical response of the rat gastrocnemius-Achilles muscle-tendon unit. Journal of Biomechanics 46 (2013): 450-455.
- Gautieri A, Passini FS, Silvan U, et al. Advanced glycation end-products: Mechanics of aged collagen from molecule to tissue. Matrix Biology 59 (2017): 95-108.
- Thorpe CT, Udeze CP, Birch HL, et al. Capacity for sliding between tendon fascicles decreases with ageing in injury prone equine tendons: a possible mechanism for age- related tendinopathy? European Cells & Materials 25 (2013): 48-60.
- Kostrominova TY, Brooks SV. Age-related changes in structure and extracellular matrix protein expression levels in rat tendons. Age 35 (2013): 2203-2214.
- HaenT-X, Roux A, Soubeyrand M, et al. Shear waves elastography for assessing of human Achilles tendon’s biomechanical properties: an experimental study. Journal of the Mechanical Behavior of Biomedical Materials 69 (2017): 178-184.
- Feng YN, Li YP, Liu CL, et al. Assessing the elastic properties of skeletal muscle and tendon using shearwave ultrasound elastography and MyotonPRO. Scientific Reports 8 (2018): 17064.
- Hsiao YY, Yang TH, Chen PY, et al. Characterization of the extensor digitorum communis tendon using high-frequency ultrasound shear wave elastography. Medical Physics 47 (2020): 1609-1618.
- Martin JA, Biedrzycki AH, Lee KS, et al. In vivo measures of shear wave speed as a predictor of tendon elasticity and strength. Ultrasound in Medicine & Biology 41 (2015): 2722-2710.
- Rosskopf AB, Bachmann E, Snedeker JG, et al. Comparison of shear wave velocity measurements assessed with two different ultrasound systems in an ex-vivo tendon strain phantom. Skeletal Radiology 45 (2016): 1541-1551.
- Hardy A, Rodaix C, Vergari C, et al. Normal range of patellar tendon elasticity using the sharewave elastography technique: an in vivo study in normal volunteers. Surgical Technology International 31 (2017): 227-230.
- Kuervers EJ, Firminger CR, Edwards WB. Effect of knee angle and Quadriceps muscle force on Shear-Wave Elastography measurements at the Patellar Tendon. Ultrasound in Medicine & Biology 47 (2021): 2167-2175.
- Yu M, Wu J, Hou J, et al. Young's Modulus of Bilateral Infraspinatus Tendon Measured in Different Postures by Shear Wave Elastography Before and After Exercise. Journal of Orthopaedic Surgery 13 (2021): 1570–1578.
- Zhang ZJ, Ng GYF, Fu SN. Effects of habitual loading on patellar tendon mechanical and morphological properties in basketball and volleyball players. European Journal of Applied Physiology 115 (2015): 2263-2269.
- Mannarino P, Lima KMM, Fontenelle CRC, et al. Analysis of the correlation between knee extension torque and patellar tendon elastic property. Clinical Physiology and Functional Imaging 38 (2018): 378-383.
- Chiu YH, Chang KV, Chen IJ, et al. Utility of sonoelastography for the evaluation of rotator cuff tendon and pertinent disorders: a systematic review and meta-analysis. European Radiology 30 (2020): 6663-6672.


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 