Combinatorial Application of Hyaluronic Acid and Curcumin-Albumin Conjugate for Cartilage Repair in TNF- α Induced Inflammation in Rabbit Knee Joint
Deepa Sathee1, Sachin J Shenoy2, Arya Anil4, Sabareeswaran A3*, Lissy Kalliyana Krishnan1
1Division of Thrombosis Research, Biomedical Technology Wing, Sree Chitra Tirunal Institute for Medical Sciences and Technology, Thiruvananthapuram, Kerala, India
2Division of In-vivo models and testing, Biomedical Technology Wing, Sree Chitra Tirunal Institute for Medical Sciences and Technology, Thiruvananthapuram, Kerala, India,
3Division of Experimental Pathology, Biomedical Technology Wing, Sree Chitra Tirunal Institute for Medical Sciences and Technology, Thiruvananthapuram, Kerala, India,
4Division of Laboratory Animal Science, Biomedical Technology Wing, Sree Chitra Tirunal Institute for Medical Sciences and Technology, Thiruvananthapuram, Kerala, India
*Corresponding author: Dr. Sabareeswaran A, Division of Experimental Pathology, Biomedical Technology Wing, Sree Chitra Tirunal Institute for Medical Sciences and Technology, Thiruvananthapuram, Kerala, India
Received: 31 May 2021; Accepted: 10 June 2021; Published: 28 June 2021
Article Information
Citation:
Deepa Sathee, Sachin J Shenoy, Arya Anil, Sabareeswaran A, Lissy Kalliyana Krishnan. Combinatorial Application of Hyaluronic Acid and Curcumin-Albumin Conjugate for Cartilage Repair in TNF- α Induced Inflammation in Rabbit Knee Joint. Archives of Clinical and Biomedical Research 5 (2021): 519-537.
View / Download Pdf Share at FacebookAbstract
Osteoarthritis has emerged as a consequential disorder resulting from changing lifestyles, especially in the aged population. It is one of the most devastating degenerative joint diseases caused due to inflammation, wear and tear of articular cartilage leading to irreversible damage and physical trauma. Several intra-articular formulations are experimented with for restoring damaged cartilage. Many of them failed because of minimal effectiveness in establishing long-term therapeutic potential. We explored the cartilage regeneration potential of Hyaluronic acid (HA) on combining with dimethoxy curcumin-human serum albumin (DMCHSA) conjugate upon intra-articular administration. HA is known to possess immense lubrication property and is a well-recognized visco-supplement. The DMCHSA has the potential to suppress the action of inflammatory markers. So, a combinatorial approach anticipates an ideal therapeutic strategy to overcome the demerits of existing interventions.
Intra-articular injection of Tumor Necrosis Factor-α (TNF-α), repeatedly at 7-day intervals disrupted the cartilage morphology and produced an inflammatory knee joint model to study the therapeutic potential of DMCHSA-HA combination. Into separate inflamed knee-joint cartilage HA, DMCHSA and DMCHSA-HA were administered periodically to highlight the advantage of mixing the latter with the former.
Histopathology and gene expression analysis assessed the restoration potential of the treatment. We observed remarkable restoration of degenerated cartilage upon treatment with the DMCHSA-HA combination. The columnar arrangement of cells, regulated deposition of ECM components such as glycosaminoglycans (GAGs) & collagen, and synchronized expressions of inflammatory marker molecules suggested restoration of the treated defects.
The treatments with DMCHSA, HA, or HSA alone seemed inferior to DMCHSA-HA combination therapy. The study confirme
Keywords
<p>Hyaluronic acid; Dimethoxycurcumin; Osteoarthritis; Cartilage restoration; Inflammation</p>
Article Details
Abbreviations
COX-2 - Cyclooxygenase -2; DAB – 3,3'-Diaminobenzidine; DMC - Dimethoxy curcumin; DMCHSA - Dimethoxy curcumin- human serum albumin; DMSO - Dimethyl Sulphoxide; ECM - Extracellular matrix; EDTA - Ethylene Diamine Tetra Acetic acid; GAGs – Glycosaminoglycans; H&E - Haematoxylin and Eosin;HA - Hyaluronic acid; HRP - Horseradish peroxidize; HSA - Human serum albumin; IL-8 - Interleukin-8; MMP-13 - Matrix-metalloproteinase-13; NaOH - Sodium hydroxide; NBF - Neutral buffered formalin; NF-kB - Nuclear factor-kB; NSAIDs - Non-steroidal anti-inflammatory drugs; OA – Osteoarthritis; OARSI - Osteoarthritis Research Society International; TNF-α - Tumor necrosis factor-α
1. Introduction
Osteoarthritis (OA) is a disease characterized by cartilage degeneration which affects mainly the joints. It is a leading cause of disability in those above 50 years old [1]. The causative factors for the development of osteoarthritis are numerous; however, trauma due to physical injuries and inflammation at the articulating surfaces accounts for the main contributory factors [2]. The disease accompanies joint stiffness as well as pain affecting mobility. The intra-articular injections of corticosteroids, analgesics/anti-inflammatory drugs, polymerized collagen, anti-cytokine drugs, or hyaluronic acid are different treatment modalities in practice for OA [3]. Such treatments reduce disease severity; however, hyaluronic acid (HA) seemed more effective and safe for long-term usage [3]. It is a natural proteoglycan and is seen abundant in the extracellular matrix (ECM) of various organs and tissues. It is composed of non-sulfated glycosaminoglycans (GAGs) [4]. Due to its high lubrication ability, it functions as a visco-supplement in the degenerated knee cartilage.
Often, instead of using HA alone, it is being used in a modified form wherein it is either chemically restructured or applied in combination with other substances such as drugs and cells for better and promising outcomes. The use of HA-based hydrogels provides a suitable environment for the cells embedded in the matrix to survive and synthesize important molecules crucial for tissue regeneration [5]. The Osteoarthritis Research Society International (OARSI) for arthritic treatment recommends about 64% success [4]. However, clinicians have failed to observe any remarkable change concerning the regulation of inflammatory response, despite intra-articular delivery.
Other commonly used treatments are non-steroidal anti-inflammatory drugs (NSAIDs) but after long- term usage, they show adverse effects on the gastrointestinal system, renal system, etc., [2]. Curcumin is known to possess numerous medicinal properties. Its anti-inflammatory potential could add to the cartilage protection property of HA [6]. One of the chemical forms of curcumin is dimethoxy curcumin (DMC) which exhibits potent anti-inflammatory actions [7]. However, the poor aqueous solubility of curcumin is a limitation that reduces its bioavailability. The solubility and bioavailability are increased by conjugating curcumin with albumin [8]. The anti-inflammatory effect of this molecule in human serum albumin (HSA) bound form was demonstrated in in vitro experiments using endothelial cells and chondrocytes as model cells in culture (Indian patent application in prosecution; No. 202041020471 of 15.05.2020) [9]. So, delivery of HA in combination with the anti-inflammatory drug DMCHSA can serve dual purposes. The current study focused on exploring the cartilage protective role of DMCHSA-HA in TNF-α induced inflammation in rabbit knee joint by histopathological assessment and gene expression analysis.
2. Materials and Methods
2.1 Preparation of DMCHSA conjugate
The DMCHSA conjugation employed the previously published protocol [9]. Briefly, the conjugate was prepared by adding 5mM CM (Sigma, USA) from stock solution (0.5M in Dimethyl Sulphoxide (DMSO- Merck)) with 200mg/ml of human serum albumin (HSA) (Intas Pharmaceutical Ltd India) followed by incubation for two hours. Passing the solution through a gel filtration column packed with Sephadex G- 50-300 beads (Sigma, USA) removed the unreacted curcuminoids. Based on the molar ratio (A280:A420), spectrophotometry (Hewlett Packard Diode Array 8543, Germany) identified fractions of concentrated DMCHSA, which were pooled, filtered using 0.22μm syringe filter (Merck, Millipore), and lyophilized (Edwards Modulyo, Edwards, UK) in 1ml aliquots. The DMC from replicate aliquots of conjugate were extracted into a 9:1 DMSO-water mixture and quantified based on a standard curve plotted using serially diluted CM in DMSO in the range of 1-12 μM. The Albumin in the conjugate was estimated using Lowry's protein assay. For further studies stored the lyophilized aliquots at 4°C.
2.2 Hyaluronic acid isolation
HA purification from the human umbilical cord employed the published procedure. Briefly, around 200- 250 g of umbilical cord tissue was washed free of bloodstains. Minced the tissue into small pieces, washed in 1L acetone two times, followed by a thorough wash with 3-4 changes of distilled water (D/W) overnight, kept at 4°C. Homogenized the tissue to fine particles and lowered the pH to 2.0 for pepsin digestion for 24h at 37 °C. Then the pH was raised to 7.4 for trypsin digestion for 24h at 37 °C. After digesting, centrifuged the mixture, and the pH of the supernatant adjusted to 2.0. Adding about 3 vol of 95% ethanol (~ 3L) precipitated the proteins and HA. After centrifuging, re-suspended the HA precipitate in ~100 ml double-distilled water and dialyzed (14,000 MW cut off) against 5L deionized water for 24 h. Transferred the solution into a beaker (~ 200 ml) and added a mixture containing 900 ml chloroform: 340 ml n-amyl alcohol: 200 ml double distilled water: 60 g sodium acetate: 32 ml glacial acetic acid. The mixture was shaken vigorously in a mechanical shaker for 10 min. After vigorous mechanical shaking for 10 min, collected the aqueous phase containing the hyaluronic acid. The repeat of the procedure eliminated all protein precipitate that appeared at the interface. Added two volumes of 95% ethanol (~ 400 ml) to the aqueous phase containing the hyaluronic acid. Centrifuged the solution for 30 min at 20,000 rpm at 4°C, dissolved the precipitate in double-distilled water (~ 200 ml), and dialyzed against deionized water for 24 -48 h with at least six changes of water. Syringe filtered (0.22µm) the dialyzed solution in a sterile laminar airflow bench and dispensed into small sterile glass vials. Deep froze and lyophilized until HA was dry. Measured the viscosity of 1mg ml-1 solution at 37 oC using Microviscometer - Lovis 2000M (Anton Paar); Used capillary diameter - 1.59mm.
2.3 Inflammatory model
The study employed New Zealand white rabbits with an approximate weight of 2 to 3 kg in the age group of 8 to 12 months to develop inflammation of the knee joint. Before starting, Institutional Animal Ethics Committee (IAEC) approved the study (B-form No. SCT/IAEC-240/AUGUST/2017/94). To develop an inflammation model, injected TNF-α (500ng/ml) into the knee joint at the level of the middle patellar ligament to induce inflammation of the articular cartilage. At 5µM, the DMCHSA was a non-toxic but anti-inflammatory molecule in chondrocyte culture (IP No. 202041020471 of 15.05.2020). Used sterile normal saline as the medium of administration in joints in vivo.
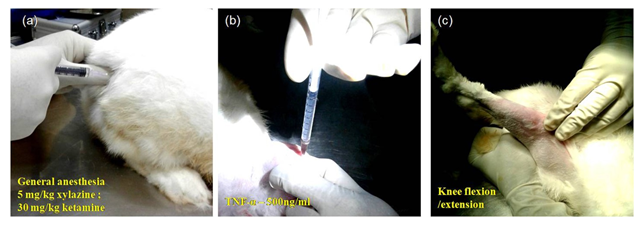
Figure 1: Images depicting the process of development of an inflammatory model in the rabbit knee. (a) Administering general anesthesia (b) administration of TNF-α into the synovial cavity (c) flexion and extension movement of knee joint post-administration. Repeated the procedure at 7-day intervals for administering a repeat dose of TNF-α and different treatments.
2.4 Experimental strategy
The study randomly allotted animals to five different groups. Noted animal weights (in Kg) before induction of anesthesia (5mg/Kg xylazine and 30mg/kg Ketamine) on all days. All five groups received TNF-α injections in the knee joint for four consecutive weeks at an interval of 7 days. All intra-articular injections (TNF-α, DMCHSA-HA, DMCHSA, HA, and HSA) used 1 ml sterile saline as the medium of administration. The sham control (group I) received TNF-α alone without any treatment. In all the animals, after injection of the inflammatory inducer, subjected joints to flexion and extension movement along with gentle massaging to get the injected solution evenly spread throughout the knee joint. The injection of TNF-α into the knee joint on the first day initiated induction of inflammation. Three more consecutive doses ending on the 21st day enhanced/continued the inflammatory effect. Treatment with drug molecules started along with the third dose of TNF-α on day 14, to ensure that the treatment started at the inflamed state. Group II received DMCHSA (5µM) and HA (4mg/ml) mixture in 1ml sterile saline. Group III received DMCHSA (5µM) in 1 ml sterile saline. Group IV received HA (4mg) in 1 ml sterile saline, and Group V received pharmacopeia grade HSA (0.2mg) in 1 ml. On day 35 of starting the experiment, i.e., seven days after the final treatment day, euthanized the animals and collected joints for further analysis. The summary of the experimental design is in the table given below.
|
Study groups |
Induction of inflammation |
Days on which TNF-α injections were given |
Treatments |
Days on which treatment was given |
Termination |
|
Group I |
TNF-α – 500ng/ml (Sham control) |
Day 0, 7, 14, 21 |
- |
- |
Day 35 |
|
Group II |
TNF-α – 500ng/ml |
Day 0, 7, 14, 21 |
CMHSA (5µM)+ HA (4mg) |
Day 14, 21, 28 |
Day 35 |
|
Group III |
TNF-α – 500ng/ml |
Day 0, 7, 14, 21 |
CMHSA (5µM) |
Day 14, 21, 28 |
Day 35 |
|
Group IV |
TNF-α – 500ng/ml |
Day 0, 7, 14, 21 |
HA (4mg) |
Day 14, 21, 28 |
Day 35 |
|
Group V |
TNF-α – 500ng/ml |
Day 0, 7, 14, 21 |
HSA (0.2mg) |
Day 14, 21, 28 |
Day 35 |
Table 1: Experimental design.
2.5 Assessments to establish restoration of defective tissues
Upon termination, collected a sample of cartilaginous tissue for qRT-PCR analysis of molecular markers of inflammation. Immersed the remaining part of each joint tissue in 10% neutral buffered formalin (NBF) (Merck) and subjected to decalcification using standard procedure.
2.5.1 qRTPCR analysis for inflammatory marker expressions: Used the cartilaginous tissue sample collected in Trizol for RNA isolation, followed by cDNA synthesis, and carried out qRT-PCR as per standard protocols [9]. The analyzed inflammatory markers include nuclear factor-kB (NF-kB), interleukin-8 (IL-8), matrix-metalloproteinase-13 (MMP-13), cyclooxygenase - 2 (COX-2).
|
Genes |
Amplicon size |
Primer Sequence |
|
GAPDH |
88 |
FP – TTTAACTCTGGCAAAGTG RP - TGGAATCATACTGGAACAT |
|
NF-kB |
123 |
FP – CAAGAAGTCCACAAACAC RP – ACCGATATGTCCTCTTTC |
|
IL-8 |
133 |
FP – GCTAAGAATACTGGAATTGT RP – TAGGATGTTGGCTGATAC |
|
MMP-13 |
84 |
FP – CAGTAACGAGGATGATGA RP - GGATTCAGAGGATGGTAG |
|
COX-2 |
145 |
FP – AACATCGTCAATAGCATTC RP - AGTAGGAGAGGTTAGAGAA |
Table 2: List of rabbit specific primes.
2.5.2 Haematoxylin and Eosin (H and E) staining and light microscopy: To decalcify the bony tissue, placed the joints in nylon bags and incubated them in a solution comprising 12.5% Ethylene Diamine Tetra Acetic acid (EDTA) (Merck) (pH- 7) and sodium hydroxide (NaOH) (Merck). For effective and faster decalcification, stirred the solution continuously using a tabletop magnetic stirrer (York Scientific industries Pvt. Ltd, India). A needle prick test at regular intervals monitored decalcification. Grossed the decalcified tissue and made 2-3 mm thick longitudinal sections of cartilage and subchondral regions of the tissue. After achieving complete decalcification, processed the tissues using the standard steps of (i) fixation in 10% NBF; (ii) dehydration in series of alcohol (70, 80, and 100% - 2 changes); (iii) clearing in xylene (Merck) (2 changes) and, (iv) permeating paraffin using an automated tissue processor (Leica ASP 300, Germany). Semi-automated equipment (Leica EG1150H, Germany) prepared blocks of tissues embedded in paraffin. A rotary microtome (RM2550, Leica, Germany) cut sections (5μm) for immune-histochemical and other specific staining protocols. The standard H and E staining identified cellular arrangement in the cartilage. Briefly, keeping in a hot air oven at 60ºC for one hour, de-paraffinized the tissue sections. Cleared the de-paraffinized tissue sections in xylene (2 changes) and rehydrated in descending grades of alcohol (100, 95, 70%), 5min each with two changes in 100%. Then the rehydrated slides were placed in Hematoxylin (Sigma, USA) for 20min, washed in running tap water for 10min. Later, immersed tissue section in Eosin (Sigma, USA) for 2min and washed in running tap water for 10min. Subsequently, used an autostainer (Leica Autostainer XL, Germany), dehydrated tissue sections in ascending series of alcohol (70, 95, 100%), mounted the slides, and placed coverslip over the tissue section using an automated cover slipper (Leica CV5030, Germany). A trinocular light microscope (Nikon E 600, Nikon, Japan) analyzed and micrographed H and E-stained sections. The image processing at different magnifications used a digital camera (Nikon DS-Ri1, Japan) attached to the trinocular light microscope.
2.5.3 Immunohistochemistry (MMP-13): The procedure to retrieve antigens in the tissue sections employed heating in the pressure cooker in 10 mM citrate buffer for 20min. Quenched the endogenous peroxidases by incubating the tissue sections in 3% hydrogen peroxide for 20min. For blocking the nonspecific antibody binding, used 5% FBS. Stained the tissue slides with MMP-13 (1:1000) antibodies (Abcam) by incubating with the respective antibodies overnight at 4ºC. Washed the tissue sections in PBS, incubated for 2h at RT in dilute secondary antibodies (horseradish peroxidize (HRP)- conjugated anti-rabbit immunoglobulin- Abcam, UK), washed with PBS, incubated with 3,3'-diaminobenzidine (DAB), and finally washed with PBS for developing the antigen. Cleared the tissue sections, hydrated, nuclear stained with hematoxylin, viewed under a microscope, and micrographed as mentioned above (Leica DM IRB, Germany).
2.5.4 Alcian blue staining: Staining of the tissue sections with Alcian blue detected the GAGs. Rehydrated the de-paraffinized tissue slides and stained them with hematoxylin. Prepared the stain by dissolving 1g alcian blue (Himedia) in a 3% acetic acid (Merck) solution. Washed the slides and incubated with alcian blue for 30min. Washed the stained sections in running tap water. Then dehydrated the tissue sections in alcohol, cleared in xylene, mounted, and allowed to dry overnight. A light microscope (Leica DM IRB, Germany) acquired microphotographs at different magnifications.
2.5.5 Picrosirius red staining: Picrosirius red staining determined the presence of collagen in the tissue sections. Prepared the staining solution by dissolving 0.5g Sirius red (Sigma, USA) in a saturated solution of picric acid (Merck) in water. Briefly, rehydrated the de-paraffinized sections and stained for nuclei using hematoxylin. After washing, stained the slides by incubating them with Picrosirius red stain for one hour. Washed in two changes of acidified water and dehydrated in ascending series of alcohol with two changes in 100% alcohol and two changes in xylene. Mounted the stained sections using the mounting media and allowed them to dry overnight. A light microscope (Leica DM IRB, Germany) acquired microphotographs at different magnifications.
2.6 Statistics
The study carried out all qualitative and quantitative experiments at least in triplicates (n=3). All quantitative data are mean ± SD (Standard deviation). The analysis determined statistical significance among the individual group using a two-tailed t-test. P < 0.05 is considered statistically significant for all experiments and denoted as P < 0.05 (*); P < 0.01 (**); P < 0.001 (***) in illustrations.
3. Results
3.1 In vivo inflammation induction using TNF-α
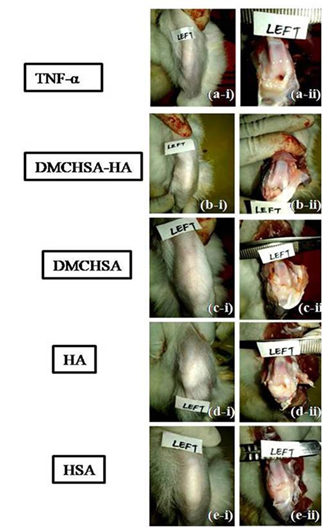
Upon administration of TNF-α, there were no visible symptoms of inflammation. On examining after 24h, the surface temperature on the knee was higher than the other parts of the body. A light swelling on the injected knee joint signified mild inflammation. Touching on the knees seemed painful; the animals withdrew their legs backward, indicating an inflammatory response in the TNF-α injected joints. The histopathological assessment of H&E stained tissues showed disruption of the columnar state to scattered cell arrangement (Figure 2a). Fibrillations were evident (Figure 2b) on the cartilage surface. Also, the analysis revealed lymphocyte infiltration and diapedesis in the TNF-α injected knee joints (Figure 2c). Upon sacrificing the animals on the study termination day (35th day of initial day of TNF-α injection), there was no visible edema on the experimental knee sites in any of the groups (Figure 3). The measured viscosity of HA was Dynamic Viscosity = 60.32 mPa/s; Kinetic Viscosity = 54.316 mm2/s, qualifying it as a good visco-supplement.
Figure 2: Effect of TNF-α injection on tissue integrity. H&E stained sections of the knee joint (a) TNF-α (500 ng/ml) injected knee joint with scattered cells; (b) Disrupted superficial layer with delamination and fissures in cartilage; (c) infiltration of polymorphonuclear cells, lymphocytes, and engorged capillaries with red blood cells.
3.2 Inflammatory marker expression analysis
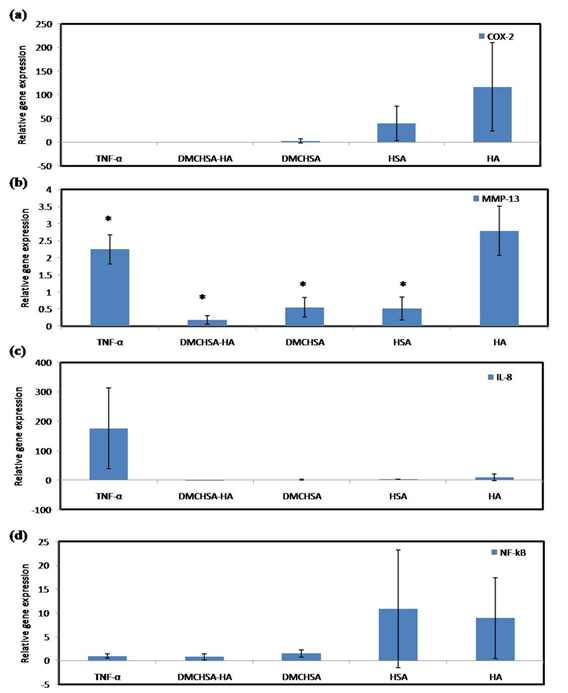
Treatment with TNF-α, up-regulated MMP-13, and IL-8 in the knee joint cartilage tissue. Induction of inflammation with four consecutive injections of TNF-α in 7 d intervals, both MMP-13 and IL-8 expressions significantly increased than those in normal tissue. Except in tissues treated with HA, all others such as DMCHSA, HSA, and DMCHSA-HA treated tissues seemed to restore MMP-13 expression and were comparable to normal tissues. With all treatments, including HA alone, the tissues restored IL-8 expression similar to those in normal tissues. The COX-2 and NF-kB expressions in HA-treated and HSA-treated tissues increased but not when treatments were with DMCHSA and DMCHSA-HA. The results indicate that the HA or HSA alone caused inflammation. However, treatment with DMCHSA-HA has restored the inflammatory response indicated by COX-2 and NF-kB expressions.
3.3 Histopathological assessment
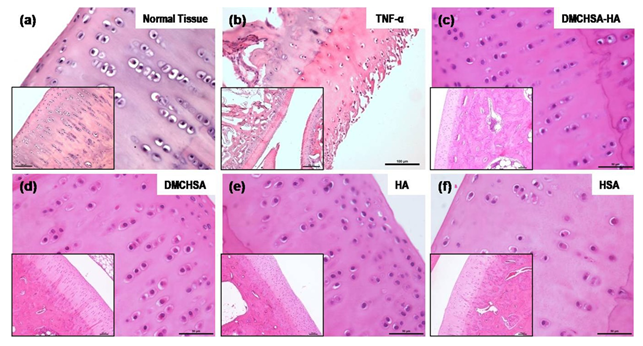
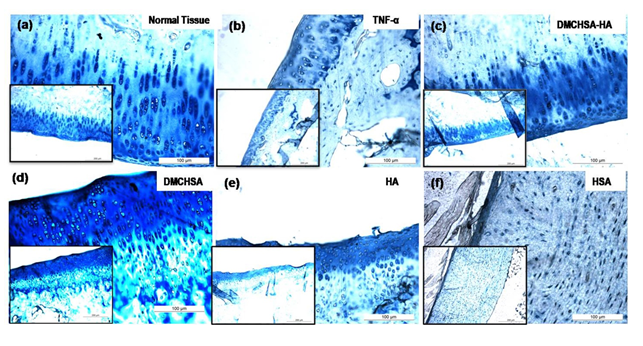
3.3.1 H and E evaluation: The H and E stained images of knee joint sections show the proper alignment of the chondrocytes in their typical columnar arrangement similar to that in normal tissue (Figure 5a). Whereas in the TNF-α induced tissue, delamination of superficial cartilage layer, fissures running down the cartilage layer and the disrupted typical columnar arrangement of cells, loss of chondrocytes, and acquisition of a scattered pattern confirmed inflammatory changes (Figure 5b). The inflamed joint tissues, treated with DMCHSA-HA (Figure 5c) and DMCHSA (Figure 5d), showed restoration in terms of the columnar arrangement of cells similar to normal tissue. The scattered, small clusters of few chondrocytes seen in HSA-treated tissue (Figure 5f) suggested a slightly better effect in HA-treated tissue (Figure 5e).
Figure 4: Graphical representation of qRTPCR data in terms of relative gene expression demonstrating the anti-inflammatory action of different treatments on inflammatory marker regulation upon injection with TNF-α (500ng) in knee joints; (a) MMP-13, (b) COX-2, (c) IL-8, (d) NF-kB. Values are represented as Mean ±SD; n=3.P < 0.05 (*); P < 0.01 (**); P < 0.001 (***). Fold change is quantified relative to GAPDH expression using the 2-∆∆Ct method, upon normalization with normal tissues.
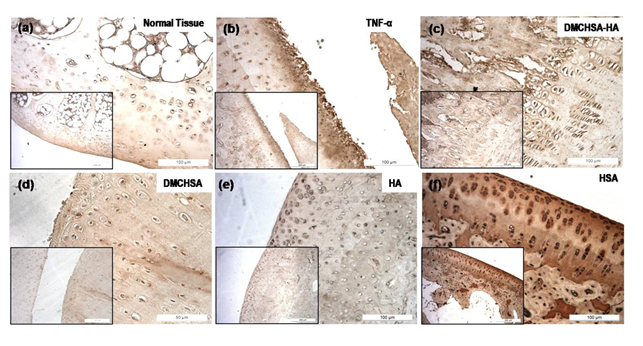
3.3.2 MMP-13 by immunohistochemistry: Cartilage sections immune stained with MMP-13 antibodies showed a negligible level of MMP-13 in the case of HA-treated tissue (Figure 6e) and even lower to that in DMCHSA-treated tissue (Figure 6d).
On the other hand, the HSA-treated tissues (Figure 6f) showed the highest MMP-13, which was even higher than that in TNF-α administered tissues (Figure 6b) which are not comparable to that in normal tissue (Figure 6a). In the DMCHSA-HA treated tissues (Figure 6c), MMP-13 appeared similar to that in normal tissue indicating restoration of the tissue property.
3.3.3 GAGs deposition: Deposition of GAGs in the ECM of cartilage sections stained by Alcian blue showed the lowest GAG deposition in HSA-treated tissues (Figure 7f) in concurrence with high MMP-13 protein deposition. In the case of DMCHSA-HA treated tissues (Figure 7c), the GAG deposition was comparable to that in normal tissue, suggesting restoration to native tissue architecture upon treatment of inflammation-induced tissues with the DMCHSA-HA combination. Neither HA-treated (Figure 7e) nor HSA-treated tissues (Figure 7f) showed GAG deposits similar to the normal tissue. Therefore, the injected HA has degraded by 35 days; stained GAG seems to be the newly deposited molecules.
Figure 7: Representative images of cartilage tissue sections stained with Alcian blue depicting the presence of GAGs, processed from animals subjected to different treatment conditions: (a) Normal tissue; (b) TNF-α injected; (c) DMCHSA-HA treated; (d) DMCHSA treated; (e) HA treated; (f) HSA treated.
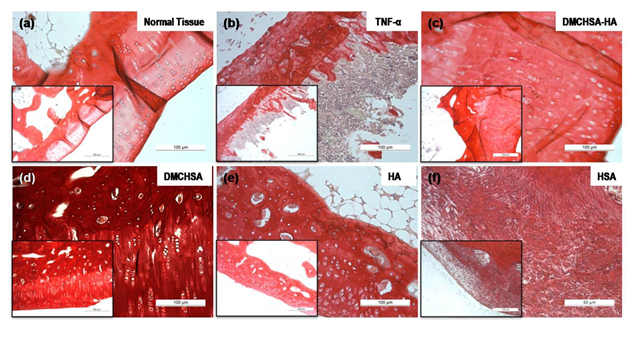
3.3.4 Collagen deposition: The HSA-treated tissues (Figure 8f) showed a scant and irregular deposit of collagen deposition. The absence of cell alignment correlates well with the high MMP-13 in HSA-treated tissues. Collagen deposit was high in DMCHSA-treated tissues, which may be due to the low amount of MMP-13 in these tissues. The collagen content was well controlled and similar to those in normal tissue upon treatment of inflammation-induced tissue with the DMCHSA-HA combination. Similarly, in TNF-α injected followed by HSA- treatment, collagen content in tissues was lower than that in normal tissue and correlated to the high MMP-13 expressions in these tissues.
Figure 8: Representative images of cartilage tissue sections stained with picrosirius red depicting the presence of collagen, processed from animals subjected to different treatment conditions. (a)Normal tissue; (b) TNF-α injected; (c) DMCHSA-HA treated; (d) DMCHSA treated; (e) HA treated; (f) HSA treated.
4. Discussion
Many among the elderly population above the age of 50 suffer from movement disabilities caused due to pain and stiffness in joints, termed osteoarthritis. Several factors worsen the disease condition from mild to moderate or severe cartilage damages resulting in irreversible cartilage loss in some cases. One of the biochemical changes observed during osteoarthritis is caused by TNF-α inducing inflammation in the cartilage [10]. A similar process occurred in the in vivo model of knee joint inflammation developed in the current study. For studying the pathological changes occurring in cartilage during inflammation, the rabbit is a suitable model due to the ease with which surgical procedures can be carried out [11].
The features like delamination of superficial layer, fibrillation observed in the cartilage surface, loss of chondrocytes, and scattered arrangement of cells in the cartilage matrix are characteristic features of damaged cartilage [12]. Besides, the infiltrating lymphocytes, diapedesis, and higher level of inflammatory markers confirmed the creation of the disease in the model for studying the therapeutic potential of different therapeutic molecules.
The anti-TNF-α drugs can reduce the inflammatory changes in damaged cartilage. Other currently available treatments include joint replacement surgeries, analgesics, anti-inflammatory drugs (NSAIDs), intra-articular injections of hydrogel-based systems, etc. These strategies have improved the recovery from disease conditions but consuming these drugs for long periods carries the risk of adverse side effects. Some treatment strategies that need multiple intra-articular injections into the knee joints may not be ideal due to the adverse effects upon long-term use.
Ideally, a novel intra-articular formulation should provide anti-inflammatory effects as well as lubrication in the joints. So a combination of HA and curcumin could be a good option. Different studies have proven that curcumin can reduce inflammation in biological systems [6, 9, 13]. A similar effect is evident in the current study in the form of suppression of inflammatory markers. A small clinical trial showed the advantage of curcumin in lessening the morbidities experienced during osteoarthritic conditions [14]. Curcumin reduced joint pains in osteoarthritic patients [15]. Of the different analogs of curcumin, DMC is efficiently bound to HSA, thereby exhibiting anti-inflammatory action [9]. Therefore, this study proposed that adding an appropriate lubricating agent such as HA could provide additional benefits to DMC.
Many clinicians recommend HA as an alternative therapy to reduce the pain at joints by providing a cushioning effect for the joints [16]. The HA preparations are commercially available in modified forms that improve effectiveness in therapeutic applications. Besides, HA contributes to the anti-inflammatory effect as an inhibitor molecule to many pain-sensitive receptors [17]. Contradictory to this hypothesis, no significant recovery of patients is confirmed in clinical trials [16]. Treatment with HA reported lower levels of inflammatory reactions at the joints. The beneficial effect of the molecule may get compromised by the injection procedure leading to the sustenance of inflammatory response [18]. Both advantages, as well as "no effects at all", have been reported in clinical trials. The proposed reason for such huge variations might be the experimental variables including the difference in the HA degrading enzymes in different individuals [3]. Efforts have accomplished cytomodulin/HA treatment for correcting damaged cartilage [19]. Few experimental data suggest that HA exerts its action via receptor-mediated pathways. CD44 expressed by chondrocyte surface is a possible target for HA and MMPs, both molecules competing for the same receptor might be the reason for suppressing MMPs [20].
The most prominent ECM component present in cartilage is collagen and is responsible for providing mechanical strength to the tissue [21]. The collagen present in the ECM of cartilage is also responsible for ensuring the proper structural features for executing its involvement in mechanical strength [22]. In this study, DMCHSA-HA treated tissues showed maximum collagen deposits. The control tissues of HSA treatment resulted in disorganized and poor collagen compared to all other tissues. This result correlates with the relative MMP-13 gene and protein expressions. The more the MMP-13 expressed, the higher collagen cleavage, and the lesser deposition is expected. One of the primary functions of MMP-13 is disrupting the ECM by cleaving collagen present in the matrix [23]. As the TNF-α caused up- regulation of MMP-13 expression, the cleavage of collagen is high. Damage to the chondrocyte and inflammatory changes reduce collagen deposition and distribution throughout the ECM. So, the collagen deposition in cartilage is dependent on the quantity of MMP-13 and the presence of healthy chondrocytes. Therefore, anti-inflammatory drugs for cartilage regeneration must specifically target the suppression of MMP-13 and must serve as MMP-13 inhibitors. Another important criterion to assess the functionality of cartilage is the presence of GAGs. These molecules play a role in hydrating the cells and the tissue, more so in an avascular environment [24]. The hydration of cartilage is necessary to provide flexibility during movement. Another function of GAGs is to control the activity of MMPs by binding to specific sites on the latter and inhibiting or activating it based on the stimuli [25]. In this study, GAGs are maximum in DMCHSA-HA treated tissues. Also, the high GAG was inversely proportional to the MMP-13.
5. Conclusion
This study established a cartilage inflammatory model upon intra-articular injection of TNF-α repeatedly in a specific interval. The combination of HA and DMCHSA injections in inflamed knee joints regulates cellular function restoring the native columnar arrangement of cells. The DMCHSA-HA injections reversed the expression of MMP-13. The MMP-13 regulated ECM content, including GAGs and collagen. Therefore, the anti-inflammatory action of the curcumin and the biological functions of HA worked synergistically and restored the architecture of the cartilage tissue defect.
Funding
The Technology development fund (TDF-6216) scheme of Sree Chitra Tirunal Institute for Medical Sciences and Technology (SCTIMST), Trivandrum, India, provided financial support for conducting the study.
Author Contributions
- Deepa S prepared DMCHSA-HA; and organized animal experiments and tissue analysis; compiled data from in vivo experiments, and drafted the manuscript.
- Sachin J Shenoy and Arya Anil executed all the animal experiments.
- Sabareeswaran A performed the histopathological analysis of cartilage tissues, analyzed and interpreted the data compiled by each of them, and edited the manuscript.
- Lissy K Krishnan conceived the idea, designed animal experiments, and guided Deepa during the studies.
Declaration of Competing of Interest
The authors declare no competing interests (neither financial nor non-financial) in the publication of the data in this manuscript.
Acknowledgment
The Director SCTIMST and Head BMT Wing are acknowledged for providing valuable support and facilities. The authors are thankful to the Technology Development Fund (TDF-6216), for providing financial support. All members of the animal facility are acknowledged for their whole-hearted support. The data presented in this manuscript is part of an IP application filed in India and is in prosecution. The title is "Hyaluronic acid- curcumin- human serum albumin composition for restoring inflammation-induced damage of knee joint" with a Provisional Specification at the Indian Patent Office on 29.10.2020 under application reference no.202041047225.
References
- Haq I, Murphy E, Dacre J. Osteoarthritis. Postgraduate Medical Journal 79 (2003): 377-383.
- Mora JC, Przkora R, Cruz-Almeida Y. Knee osteoarthritis: pathophysiology and current treatment modalities. Journal of Pain Research 11 (2018): 2189.
- Bowman S, Awad ME, Hamrick MW, et al. Recent advances in hyaluronic acid-based therapy for osteoarthritis. Clin Transl Med 7 (2018).
- Masuko K, Murata M, Yudoh K, et al. Anti-inflammatory effects of hyaluronan in arthritis therapy: Not just for viscosity. Int J Gen Med 2 (2009): 77-81.
- Li H, et al. The Application of Hyaluronic Acid-Based Hydrogels in Bone and Cartilage Tissue Engineering. Advances in Materials Science and Engineering 20 (2019).
- Fadus MC, Lau C, Bikhchandani J, et al. Curcumin: An age-old anti-inflammatory and anti-neoplastic agent. Journal of Traditional and Complementary Medicine 7 (2017): 339- 346.
- Patwardhan RS, Checker R, Sharma D, et al. Dimethoxycurcumin, a metabolically stable analog of curcumin, exhibit anti-inflammatory activities in murine and human lymphocytes. Biochem Pharmacol 82 (2011): 642-657.
- Thomas C, Pillai LS, Krishnan L. Evaluation of Albuminated Curcumin as Soluble Drug Form to Control Growth of Cancer Cells and lt;i> in Vitro</i>. Journal of Cancer Therapy 05 (2014): 723-734.
- Sathee D, Krishnan LK. Aqueous Solubility and Bioavailability of Albumin Conjugated Curcumin in Endothelial Cells Allege Potential of the Formulation for Anti-Inflammation Therapy. Cardiology and Cardiovascular Medicine 5 (2021): 86-105.
- Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology 39 (2002): 237-246.
- Moran CJ, Ramesh A, Brama PAJ, et al. The benefits and limitations of animal models for translational research in cartilage repair. J Exp Orthop 3 (2016).
- Adams CS, Shapiro IM. The Fate of the Terminally Differentiated Chondrocyte: Evidence for Microenvironmental Regulation of Chondrocyte Apoptosis. Critical Reviews in Oral Biology and Medicine 13 (2002): 465-473.
- Aggarwal BB, Gupta SC, Sung B. Curcumin: an orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br J Pharmacol 169 (2013): 1672-1692.
- Goulart M, Partar D, Cunha L, et al. Ab0792 Curcumin in Osteoarthritis Treatment: The Present State of Evidence. Annals of the Rheumatic Diseases 78 (2019): 1867-1867.
- Perkins K, Sahy W, Beckett RD. Efficacy of Curcuma for Treatment of Osteoarthritis. J Evid Based Complementary Altern Med 22 (2017): 156-165.
- Medvedeva EV, et al. Repair of Damaged Articular Cartilage: Current Approaches and Future Directions. Int J Mol Sci 19 (2018).
- Nicholls MA, Fierlinger A, Niazi F, et al. The Disease-Modifying Effects of Hyaluronan in the Osteoarthritic Disease State. Clinical Medicine Insights. Arthritis and Musculoskeletal Disorders 10 (2017).
- Wen DY. Intra-articular Hyaluronic Acid Injections for Knee Osteoarthritis. AFP 62 (2000): 565.
- Park SH, et al. An injectable, click-crosslinked, cytomodulin-modified hyaluronic acid hydrogel for cartilage tissue engineering. NPG Asia Materials 11 (2019).
- Gupta RC, Lall R, Srivastava A, et al. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front. Vet. Sci 6 (2019).
- Eyre D. Articular cartilage and changes in Arthritis: Collagen of articular cartilage. Arthritis Res 4 (2002): 30-35.
- Sophia Fox AJ, Bedi A, Rodeo SA. The Basic Science of Articular Cartilage. Sports Health 1 (2009): 461-468.
- Howes JM, et al. The Recognition of Collagen and Triple-helical Toolkit Peptides by MMP-13. J Biol Chem 289 (2014): 24091-24101.
- Casale J, Crane JS. Biochemistry, Glycosaminoglycans in StatPearls, Treasure Island (FL): StatPearls Publishing (2020).
- Tocchi A, Parks WC. Functional interactions between matrix metalloproteinases and glycosaminoglycans. The FEBS Journal 280 (2013): 2332-2341.


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 