Aortic Arch Surgery Under Warm Conditions (Moderate to Mild Hypothermia)
Schachner Bruno*, Ratschiller Thomas, Benedikt Peter, Huber Florian, Mair Roland, Gottsberger Jessica, Zierer Andreas
Department of Cardiothoracic and Vascular Surgery, Kepler University Hospital, Medical Faculty, Johannes Kepler University Linz, Linz, Austria
*Corresponding author: Schachner Bruno, Department of Cardiothoracic and Vascular Surgery, Kepler University Hospital, Medical Faculty, Johannes Kepler University Linz, Linz, Austria
Received: 01 October 2020; Accepted: 08 October 2020; Published: 20 October 2020
Article Information
Citation: Schachner Bruno, Ratschiller Thomas, Benedikt Peter, Huber Florian, Mair Roland, Gottsberger Jessica, Zierer Andreas. Aortic Arch Surgery Under Warm Conditions (Moderate to Mild Hypothermia). Archives of Clinical and Biomedical Research 4 (2020): 551-560.
View / Download Pdf Share at FacebookKeywords
<p>Aortic Arch Surgery</p>
Article Details
1. History of Aortic Arch Surgery and Hypothermia
In 1950, Lewis et al first used the concept of hypothermic cardiac arrest in animal experiments. In 1952 they reported the first atrial septal defect closure under this concept [1]. The first reported cardiopulmonary bypass in human was performed in 1953 under the leadership of Gibbon. Shortly thereafter the first replacement of an ascending aorta was performed by DeBakey and Cooley in 1956 [2]. The first systemic case series of aortic arch surgery using hypothermic circulatory arrest was published in 1975 by Griepp et al[3]. The mean temperature measured in this case series was 14°C oesophageal and 18°C rectal. Three out of four patients survived the procedure.
2. Physiology and Anatomy
The mean adult brain weight is1400g. Approximately 15% of the cardiac output is used for cerebral perfusion. Under normothermic condition the oxygen consumption is 3ml/min/g brain mass [4]. During normal body temperature condition until deep hypothermia the brain has the ability to autocorrect the intracerebral blood pressure. Between 50 and 150mm/Hg mean arterial pressure the cerebral autoregulation generates a constant cerebral perfusion pressure. Intracerebral pressure should not be above 15mm/Hg [5]. This system is deactivated when the core temperature drops below 6-12° Celsius [4]. Most of patients with aortic pathologies have increased blood pressure which is one cause of the impairment of the autoregulation. In combination with cardiopulmonary bypass and hypothermia this system is almost deactivated. The blood supply of the brain is mainly provided by four arteries. The internal carotid arteries (left and right) and the vertebral arteries (left and right). The internal carotid arteries provide the blood flow for the anterior cerebral arteries and the middle cerebral arteries. The vertebral arteries are smaller and support the basilar arteries and posterior cerebral arteries. Together with the communication arteries they form the Circle of Willis. This is the basic structure of cerebral perfusion. If this structure is intact every feeding artery can provide the cerebral perfusion.
3. Hypothermic Circulatory Arrest
During a hypothermic circulatory arrest, it is obligatory to protect the brain, the spinal cord and the visceral organs from hypoxic damage. Especially neurons react sensitive to hypoxia. Before cerebral perfusion was used, keeping the procedure during cardiac arrest short, was the only way to protect the patient from neurologic damage. During hypothermia the metabolic rate in the body drops about 50% with temperature reduction of 10 degree of baseline [6]. The definition of moderate, deep or profound hypothermia is heterogenic within the literature [Table 1].
3.1 Brain
During circulatory arrest and normal body temperature the time until neurologic damage occurs is limited. Irreversible neurologic damage can already occur within 5 minutes or less, however this time can be extended up to 30-40 minutes in deep hypothermic circulatory arrest. At 28°C body temperature the metabolic rate drops at least 50%. In combination with selective perfusion the tolerance for systemic circulatory arrest is prolonged and perioperative neurologic deficits are reduced [7, 9, 10]. At the same time core temperatures below 28°C are associated with visceral organ damage due to inflammatory response [11].
3.2 Spinal cord
Until today the ischemic tolerance for the spinal cord during circulatory arrest is not well studied. In a porcine model the ischemic tolerance for the spinal cord was investigated. After cross clamping the aorta distal to the subclavian artery the animals were woken up. The ischemic tolerance time was 20 minutes at normal body temperature (37°C) and could be extended to 120 minutes during hypothermia (20°C). The metabolic rate reduction seems to be the same as for cerebral tissue. The ability for the spinal cord to tolerate ischemic periods longer than the brain seems to be a fundamental difference. The brain has a baseline tolerance of 5 minutes and the spinal cord of 20min [13]. In a metanalysis it was shown that during circulatory arrest under hypothermic conditions the paraplegia rate is 2.1% overall. In the subgroup with circulatory arrest over 60 minutes the rate for paraplegia was as high as 18,2% [14].
3.3 Visceral organs
For the visceral organs, the time without damage under circulatory arrest is much longer than for brain. Visceral organs may even profit from a higher body temperature during cardiac arrest. The inflammatory response and severe hepatorenal dysfunction can be lowered in mild to moderate hypothermia. In an animal trial with 24 pigs damage for visceral organs depending on the core temperature was evaluated. Normothermic, moderate and deep hypothermic arrest were compared. The inflammatory response and the histological damage in organ tissue were the lowest in the moderate hypothermic group [11]. Nevertheless, lower body perfusion should be re-established as soon as possible. In very complex aortic arch procedures with an extended timeframe an placement of an balloon tipped catheter connected to the arterial cardiopulmonary bypass line down the descending aorta should be considered. The safe time limit of selective antegrade cerebral perfusion during moderate hypothermia circulatory arrest is yet to be defined. Nevertheless, there is growing evidence in the literature that lower body perfusion should restart again after not much longer than 60 minutes [15, 16].Tarola et al. investigated a whole-body perfusion concept. In this study selective ACP in moderate hypothermia and whole-body perfusion were compared in about 100 cases. There was no difference regarding survival or visceral organ damage. Nevertheless, the duration on the intensive care unit was shorter in the whole-body perfusion group. During whole-body perfusion the cross-clamp time and the total CPB-time is longer [15]. Regarding this data additional effort of whole-body may be discussed controversial.
|
Yan et al. 2013 |
Luehr et al. 2014 |
|
|
Mild hypothermia |
28.1-34 °C |
33-35.9°C |
|
Moderate hypothermia |
20.1-28 °C |
28-32.9°C |
|
Deep hypothermia |
14.1-20°C |
21-27.9°C |
|
Profound hypothermia |
<14°C |
<20.9 |
Table 1: Differences between the definition of hypothermia [7, 8].
|
Temperature (°C) |
Cerebral metabolic rate (% of baseline) |
Safe duration of HCA (min) |
|
37 |
100 |
5 |
|
30 |
56 (52-60) |
9 (8-10) |
|
25 |
37 (33-42) |
14 (12-15) |
|
20 |
24 (21-29) |
21 (17-24) |
|
15 |
16 (13-20) |
31 (25-38) |
|
10 |
11 (8-14) |
45 (36-62) |
Table 2: Safe duration time for brain tissue during hypothermic circulatory arrest [12].
|
Temperature (°C) |
Safe duration of circulatory arrest |
|
37 |
15-20 |
|
32 |
35-50 |
|
28 |
55-75 |
|
20 |
~120 |
Table 3: Safe duration time for spinal cord during hypothermic circulatory arrest [7, 13].
4. Cerebral Perfusion Techniques
In the beginning of aortic arch surgery, the only cerebral protection was based on deep systemic hypothermia. This kind of surgery was associated with 30% of transient neurologic deficits [17-18]. There are two different types of cerebral perfusion during open aortic arch surgery. In the early 90’s the concept of retrograde and antegrade cerebral perfusion was published [17, 19].
4.1 Retrograde cerebral perfusion
Retrograde cerebral perfusion (RCP) is an additional cerebral protection tool during deep hypothermia. During the procedure, the cardiopulmonary bypass pumps blood in a retrograde fashion through the superior vena cava into the brain. The initial intent of this approach was to achieve adequate blood flow even at the capillary level. After clinical introduction of retrograde cerebral perfusion the rate of neurologic deficits dropped down to 5% [17, 18]. It has been shown that during RCP only about 20% of blood flow is drained through the arterial system of the brain while 80% of blood flow is drained through the inferior caval vene into tissues others than the brain [17, 20, 21]. Ehrlich et al. demonstrated in a porcine model in 2010 that arterial backflow might be even lower. In this study only 12% of the retrograde perfusion was measured as a backflow through the aortic arch vessels. Even if the inferior vena cava is occluded the percentage is not increasing at all. The conclusion is that sufficient cerebral capillary perfusion cannot be provided by RCP [22]. In animal models it was also shown that the appearance of cerebral oedema and acidosis was higher during RCP [19]. Especially the intracellular pH-value was lower in the RCP group [23]. The expected benefit of reduced arterial embolism could also not be demonstrated in this studies [24]. It was concluded that the only beneficial effect of RCP may be a better distribution of cerebral cooling [19].
4.2 Antegrade cerebral perfusion
For a physiological-like perfusion of the brain an antegrade perfusion technique is needed. It can be established by direct cannulation or via a prosthetic graft anastomosed to the axillary or carotid artery. Alternatively, the supra-aortic arch vessels can be directly cannulated using balloon occludable perfusion catheters. With this technique the safe time concerning neurologic damage during circulatory arrest is extended even in mild and moderate hypothermia [25, 29]. After invention of antegrade cerebral perfusion (ACP) there was no need for deep systemic hypothermia any longer. This technique provides a physiological like cerebral perfusion. In animal models the metabolic rate was nearly the same as under normal conditions. The rate of acidosis and cerebral oedema is lower compared to deep hypothermic circulatory arrest and RCP. Additional due to collateral backflow at least the higher parts of the spinal cord are perfused as well [19, 23, 30]. Due to this results ACP should be preferred over RCP during open aortic arch procedures. With ACP the limits for aortic arch repair are extended and DHCA is no longer required.
4.3 Uni vs. Bilateral cerebral Perfusion during aortic arch repair
Antegrade cerebral perfusion can be done uni- or bilaterally. There is ongoing controversy between advocates of these two techniques. Most European groups prefer unilateral ACP [9, 31, 32]. Applied via the right axillary or common carotid artery. This way further manipulation of the supra aortic vessels is not required. In more than 1000 cases neurologic outcome was analysed for ACP under moderate hypothermia. Unilateral perfusion was compared with bilateral ACP [9] In conclusion the unilateral perfusion can be performed safely during aortic arch procedure. Although it is necessary to monitor the cerebral oxygen saturation with NIRS (Near infrared spectroscopy). If NIRS value drops below 75% of baseline bilaterally ACP should be considered. A potential limitation of unilateral perfusion is the hyper perfusion of one hemisphere. Theoretically this may cause cerebral oedema resulting in neurologic morbidity after surgery. Nevertheless in a study with over 1000 patients in Germany there was no difference in temporary or permanent neurologic complication between unilateral an bilaterally [31]. Another group was looking for a preoperative assessment for the decision of uni- or bilateral cerebral perfusion. In a study with 99 persons they assessed a computed tomography cerebral angiography to determine the completeness of the circle of Willis. Only 59 had an intact circle of Willis. During the procedure patients were monitored with arterial pressure lines in both radial arteries, electroencephalography, measurement of somatosensory evoked potentials and if possible transcranial Doppler ultrasonography of the middle cerebral arteries. Cerebral cross perfusion during unilateral ACP was independent of the completeness or incompleteness of the circle of Willis [33].
5. Cannulation Techniques
The arterial cannulation technique is also important for temperature management. Due to different cannulation sites the strategy for cooling and cerebral perfusion can be optimized. For a long time, femoral arterial cannulation was most commonly used to establish cardiopulmonary bypass. However retrograde perfusion increases the risk for thromboembolism and leads to cerebral and visceral embolism during the procedure [34]. ACP in the setting of femoral artery cannulation is only possible with additional intubation of the aortic arch vessels. Both common carotid arteries can be cannulated for establishment of cardiopulmonary bypass. The advantage of common carotid artery cannulation is it easy accessibility. Also ACP can easily and safely be established [32, 35]. Villard et al showed back in 1976 that the cannulation of right arteria axillaries is feasible [36]. The axillary artery is usually free of atherosclerosis. The cannulation can be performed directly or in combination with a prothesis in case of dissection. An animal trial showed that the number of micro embolisms could be reduced. The incidence of left hemispheric embolism was reduced by 75% the right side by 40% in comparison to the central arterial cannulation [37]. During central cannulation it is possible that aortic plaque ruptures which leads to embolism into both hemispheres. This won’t appear with cannulation of the axillary artery due to retrograde flow to the aortic arch[37]. Through cannulation of the axillary artery or the left carotid artery the perfusion is close to the physiological state. This results in cell protection, lower risk for oedema , lower cerebral pressure and reduction of acidosis due to metabolic imbalance [19, 23, 30].
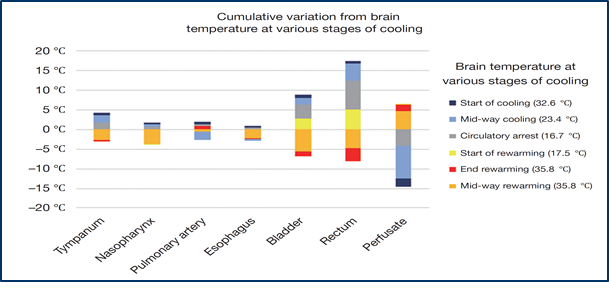
6. Where to Measure?
During hypothermia, a sufficient measurement of the core temperature is mandatory. Core temperature has to be measured as bladder or rectal temperature. A nasopharyngeal temperature probe is additionally used in many centres. During systemic cooling und rewarming temperatures may substantially vary depending on the measurement site.
It is necessary for sufficient protection of the spinal cord and the visceral organs to measure at a core point like the bladder or the rectum. In nasopharyngeal temperature management may have the effect of convection due to the cooled blood from the arterial line through cannulation of the carotid artery or the axillaries artery. Only in the bladder or the rectum the core temperature can be adequately measured, so that the spinal cord and the visceral organs get the expected protection from moderate hypothermia.
7. Moderate to Mild Hypothermia during Aortic Arch Replacement
Until today aortic arch repair still remains as one of the most complex procedures in cardiac surgery. Challenging in this procedure is to keep the circulatory arrest as short as possible. In the beginning of open aortic arch surgery, it was a milestone to establish deep hypothermic circulatory arrest to extend the safe time limit for these procedures.
Over the years it has been shown that deep hypothermic circulatory arrest alone is not sufficient for cerebral protection during aortic arch surgery. With clinical introduction of RCP the neurologic complication could be decreased substantially [17, 39, 40, 41]. Mild to moderate systemic hypothermia in combination with selective ACP set the next milestone in aortic arch surgery. In several studies it has been shown that this strategy helps to prevent severe visceral organ damage. Clinically relevant liver, mesenteric or spinal cord ischemia was reported in less than 1% of patient in elective cases. But complication rates may be higher in the setting of an acute type A dissection [11, 12, 15, 22, 25, 27, 28, 38].
8. Conclusion
Owing the relentless progress in perfusion and temperature management strategies in aortic arch surgery 30-day-mortality in this most complex procedures can be as low as 4-7% [12, 22, 27, 28, 38]. In most European centers selective ACP in comnination with mild to moderate systemic hypothermia is used successfully. Details at ACP such as uni- vs. bilaterally ACP, perfusion pressure during ACP and cerebral perfusate temperature are yet to be defined. Large randomized multicentre trials are necessary to answer these questions. Yet maybe difficult to conduct due to strong local preferences.
References
- LEWIS F J, TAUFIC M. Closure of atrial septal defects with the aid of hypothermia; experimental accomplishments and the report of one successful case. Surgery 33 (1953): 52-59.
- Cooley D A, De Bakey M E. Resection of entire ascending aorta in fusiform aneurysm using cardiac bypass. J. Am. Med. Assoc 162 (1956): 1158-1159.
- R B Griepp, Stinson E B, Hollingsworth J F, Buehler D. Prosthetic replacement of the aortic arch. J. Thorac. Cardiovasc. Surg 70 (1975): 1051-1063.
- Bachet J. What is the best method for brain protection in surgery of the aortic arch? Selective antegrade cerebral perfusion. Cardiol. Clin 28 (2010): 389-401.
- Eugenio Neri, Carlo Sassi, Lucio Barabesi, Massimo Massetti, Giorgio Pula, Dimitrios Buklas, et al. Cerebral Autoregulation After Hypothermic Circulatory Arrest in Operations on the Aortic Arch (2004).
- JHM J D Michenfelder 1. The Relationship Among Canine Brain Temperature, Metabolism, and Function During Hypothermia. Anesthesiology 31 (1989): 305-309.
- Luehr M, Bachet J, Mohra F W, Etza C D. Modern temperature management in aortic arch surgery: The dilemma of moderate hypothermia. Eur. J. Cardio-thoracic Surg 45(2014): 27-39.
- Tristan D Yan, Paul G Bannon, Joseph Bavaria, Joseph S Coselli, John A Elefteriades, Randall B Griepp, et al. Consensus on Hypothermia Clasisfication. Ann Cardiothorac Surg 2 (2013): 163-168.
- Zierer A, El-Sayed Ahmad A, Papadopoulos N, Moritz A, Diegeler A, Urbanski P P. Selective antegrade cerebral perfusion and mild (28°C-30°C) systemic hypothermic circulatory arrest for aortic arch replacement: results from 1002 patients. J. Thorac. Cardiovasc. Surg 144 (2012): 1042-1049.
- Hagl C, Khaladj N, Peterß S, Haverich A. Neuroprotektion in der Aortenbogenchirurgie: Experimentelle Untersuchungen und klinische Analyse. Zeitschrift fur Herz-, Thorax- und Gefasschirurgie 22 (2008): 47-55.
- Qing M, Vazquez-Jimenez J F, Klosterhalfen B, Sigler M, Schumacher K, Duchateau J, et al. Influence of Temperature during Cardiopulmonary Bypass on Leukocyte Activation, Cytokine Balance, and Post-Operative Organ Damage. Shock 15 (2001): 372-377.
- Jock N McCullough, Ning Zhang, David L Reich, Tatu S Juvonen, James J Klein, David Spielvogel, et al. Cerebral metabolic suppression during hypothermic circulatory arrest in humans. Ann. Thorac. Surg 67 (1999): 1895-1899.
- Griepp R B, Di Luozzo G. Hypothermia for aortic surgery. J. Thorac. Cardiovasc. Surg 145 (2013): S56-S58.
- Hiroyuki Kamiya, Christian Hagl, Irina Kropivnitskaya, Dietmar Böthig, Klaus Kallenbach, Nawid Khaladj et al. The safety of moderate hypothermic lower body circulatory arrest with selective cerebral perfusion?: A propensity. J Thorac Cardiovasc Surg 133 (2007): 501-509.
- Christopher L Tarola, Katie L Losenno, Jill J Gelinas, Philip M Jones, Philip Fernandes, Stephanie A Fox, et al. Whole body perfusion strategy for aortic arch repair under moderate hypothermia. Perfusion 33 (2018): 254-263.
- Nota H, Asai T, Suzuki T, Kinoshita T, Ikegami H, Takashima N. Risk factors for acute kidney injury in aortic arch surgery with selective cerebral perfusion and mild hypothermic lower body circulatory arrest. Interact Cardiovasc Thorac Surg 19 (2014): 955-961.
- Usui A, Hotta T, Hiroura M, Murase M, Maeda M, Koyama T, et al. Retrograde cerebral perfusion through a superior vena caval cannula protects the brain. Ann Thorac Surg 53 (1992): 47-53.
- Rita Karianna Milewski, Davide Pacini, William Moser G, Patrick Moeller, Doreen Cowie, Wilson Y Szeto, et al. Retrograde and Antegrade Cerebral Perfusion: Results in Short Elective Arch Reconstructive Times. Ann Thorac Surg 89 (2010): 1448-1457.
- Carlos L Filgueiras, LawrenceRyner, JianYe, LuojiaYang, MauricioEde, JiankangSun, et al. Cerebral protection during moderate hypothermic circulatory arrest: Histopathology and magnetic resonance spectroscopy of brain energetics and intracellular pH in pigs. J. Thorac. Cardiovasc. Surg 112 (1996): 1073-1080.
- Usui A, Oohara K, Murakami F, Ooshima H, Kawamura M, Murase M. Body temperature influences regional tissue blood flow during retrograde cerebral perfusion. J. Thorac. Cardiovasc. Surg 114 (1997): 440-447.
- Ueda Y. Retrograde cerebral perfusion still remains a valuable adjunct for hypothermic circulatory arrest. J. Thorac. Cardiovasc. Surg 156 (2018): 1337-1338.
- Marek P Ehrlich, Christian Hagl, Jock N Mc Cullough, Ning Zhang, Howard Shiang, Carol Bodian, et al. Retrograde cerebral perfusion provides negligible flow through brain capillaries in the pig. J Thorac Cardiovasc Surg 122 (2001): 331-338.
- Carlos L Filgueiras, BeAtrice Winsborrow, JianYe, Jack Scotta, Alexander Aronov, Piotr Kozlowski, et al. A 31P-magnetic resonance study of antegrade and retrograde cerebral perfusion during aortic arch surgery in pigs. J. Thorac. Cardiovasc. Surg 110 (1995): 55-62.
- Midulla P S, Gandsas A, Sadeghi AM, Mezrow CK, Yerlioglu ME, Wang W, et al. Comparison of Retrograde Cerebral Perfusion to Antegrade Cerebral Perfusion and Hypothermic Circulatory Arrest in a Chronic Porcine Model. J. Card. Surg 9 (2008): 560-575.
- Zierer A, Aybek T, Risteski P, Dogan S, Wimmer-Greinecker C, Moritz A. Moderate hypothermia (30 °C) for surgery of acute type A aortic dissection. Thorac. Cardiovasc. Surg 53 (2005): 74-79.
- Zierer A, El-Sayed Ahmad A, Papadopoulos N, Moritz A, Diegeler A, Urbanski P P. Selective antegrade cerebral perfusion and mild (28 C-30 C) systemic hypothermic circulatory arrest for aortic arch replacement: Results from 1002 patients. J. Thorac. Cardiovasc. Surg 144 (2012): 1042-1050.
- Andreas Zierer, Ali El-Sayed Ahmad, Nestoras Papadopoulos, Faisal Detho, Petar Risteski, Anton Moritz, et al. Fifteen years of surgery for acute type A aortic dissection in moderate-to-mild systemic hypothermia †. Eur J Cardiothorac Surg 51 (2017): 97-103.
- El-Sayed Ahmad A, Papadopoulos N, Risteski P, Moritz A, Zierer A. The Standardized Concept of Moderate-to- Mild (‡ 28 C) Systemic Hypothermia During Selective Antegrade Cerebral Perfusion for All-Comers in Aortic Arch Surgery: Single-Center Experience in 587 Consecutive Patients Over a 15-Year Period. Ann. Thorac. Surg 104 (2017): 49-55.
- Zierer A, Risteski P, El-Sayed Ahmad A, Moritz A, Diegeler A, Urbanski P P. The impact of unilateral versus bilateral antegrade cerebral perfusion on surgical outcomes after aortic arch replacement: A propensity-matched analysis. J. Thorac. Cardiovasc. Surg 147 (2014): 1212-1218.
- Christian Hagl, Nawid Khaladj, Sven Peterss, Klaus Hoeffler, Michael Winterhalter, Matthias Karck, et al. Hypothermic circulatory arrest with and without cold selective antegrade cerebral perfusion: Impact on neurological recovery and tissue metabolism in an acute porcine model. Eur. J. Cardio-thoracic Surg 26 (2004): 73-80.
- Zierer A, Risteski P, Ahmad A E, Moritz A, Diegeler A, Urbanski P P. The impact of unilateral versus bilateral antegrade cerebral perfusion on surgical outcomes after aortic arch replacement: A propensity-matched analysis. J. Thorac. Cardiovasc. Surg 147 (2014): 1212-1218.
- Paul P Urbanski, Aristidis Lenos, Juan C Blume, Volker Ziegler, Bernd Griewing, Rainer Schmitt, et al. Cannulation of the left common carotid artery for proximal aortic repair. J. Thorac. Cardiovasc. Surg 126 (2003): 887-888.
- Paul P Urbanski, Aristidis Lenos, Juan C Blume, Volker Ziegler, Bernd Griewing, Rainer Schmitt, et al. Does anatomical completeness of the circle of Willis correlate with sufficient cross-perfusion during unilateral cerebral perfusion?. Eur. J. Cardio-thoracic Surg 33 (2008): 402-408.
- Sabik J F, Lytle B W, McCarthy P M, Cosgrove D M. Axillary artery: An alternative site of arterial cannulation for patients with extensive aortic and peripheral vascular disease. J. Thorac. Cardiovasc. Surg (1995): 885-891.
- Ruggero De Paulis, Martin Czerny, Luca Weltert, Joseph Bavaria, Michael A Borger, Thierry P Carrel, et al. Current trends in cannulation and neuroprotection during surgery of the aortic arch in Europe. Eur. J. Cardio-thoracic Surg 47 (2015): 917-923.
- Villard J, Froment JC, Milleret R, Dureau G, Amouroux C, Boivin J, et al. [Type I, complete, acute aortic dissection. Value of arterial perfusion by the axillary route (author’s transl)]. Ann. Chir. Thorac. Cardiovasc 15 (1976): 133-135.
- Hedayati N, Sherwood J T, Schomisch S J, Carino J L, Markowitz A H. Axillary artery cannulation for cardiopulmonary bypass reduces cerebral microemboli. J. Thorac. Cardiovasc. Surg 128 (2004): 386-390.
- Stone J G, Young W L, Smith C R, Solomon R A, Wald A, Ostapkovich N, et al. Do standard monitoring sites reflect true brain temperature when profound hypothermia is rapidly induced and reversed?. Anesthesiology 82 (1995): 344-351.
- Ergin M A, Uysal S, Reich D L, Apaydin A, Lansman S L, McCullough J N, et al. Temporary neurological dysfunction after deep hypothermic circulatory arrest: A clinical marker of long-term functional deficit. Ann. Thorac. Surg 67 (1999): 1887-1890.
- Dumfarth J, Ziganshin B A, Tranquilli M, Elefteriades J A. Cerebral Protection in Aortic Arch Surgery: Hypothermia Alone Suffices. Rev. Port. Cir. Cardiotorac. Vasc 10 (2003): 109-114.
- Svensson L G, Crawford E S, Hess K R, Coselli J S, Raskin S, Shenaq S A, et al. Deep hypothermia with circulatory arrest. Determinants of stroke and early mortality in 656 patients. J. Thorac. Cardiovasc. Surg 106 (1993): 19-31.
- Ali El-Sayed Ahmad, Nestoras Papadopoulos, Petar Risteski, Theresa Hack, Mahmut Ay, Anton Moritz. Is More than One Hour of Selective Antegrade Cerebral Perfusion in Moderate-to-Mild Systemic Hypothermic Circulatory Arrest for Surgery of Acute Type A Aortic Dissection Safe? Thorac Cardiovasc Surg 66 (2018): 215-221.


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 